Every minute, the world’s population grows, and in order to feed them, crop output and agricultural productivity must be improved by adding crucial microorganisms that boost plant yields in various ways through nitrogen fixation, the secretion of both plant growth regulators and 1-aminocyclopropane 1-carboxylate deaminase, as well as some antimicrobial agents. Numerous endophytic bacteria have recently been used to increase plant yields, and agricultural production in addition to reducing salt stresses. Many scientists have made an effort to clarify and comprehend the processes by which bacteria promote plant growth and production. A vital substance known as 1-aminocyclopropane-1-carboxylate (ACC) deaminase is produced by several bacteria, plants, and fungi to decrease ethylene levels in a plant grown under different environmental stress. The gaseous hormone ethylene (C2H4) is synthesized in plant tissues from the precursor ACC, and it has numerous biochemical roles in plants, such as cells differentiation and tissue development, seedling, root hair, leaf, and flower growth and development in addition to fruit ripening and formation of anthocyanin and volatile compounds. Thus, this critical enzyme had influential roles in plants during their positive interaction with bacteria which increase plant growth due to auxin production and protect plants against different environmental stress like drought, high salts, wilting, high level of heavy metals, contaminants with pesticides, and microbial pathogen infections. Different bacterial genera are highly ACC deaminase-producer, and these bacteria support plant growth and agricultural process. In conclusion, bacteria can replace chemicals in a variety of environmentally benign methods to boost soil fertility and plant productivity. However, much research is required to determine the efficacy of these bacteria before suggesting their use on a broad scale in the field.
ACC, Ethylene, 1-aminocyclopropane- 1 carboxylate, IAA, Phosphate Solubilization
Over the past 50 years, the world’s population has increased and doubled to over 7.5 billion people. As a result, the demand for food is rising quickly, and the food production sector faces significant challenges in providing a potential energy food supply. The main sources of food production are agriculture and fisheries. More than 820 million people experience food scarcity or lack of access to food, as the population suffering from food insecurity has climbed from 15% in 2000 to over 17% recently.1,2 Today, decreased food production led to hunger and malnutrition of babies and adults in poverty, often exacerbated by the conflict that inhibits access to food.
In general, widespread famine was only expected to be avoided by boosting plant growth and output, minimizing environmental stresses on plants, and bringing down global population levels to sustainable levels.3,4 In the middle of the 20th century, farmers and scientists tried their best to boost plant growth and production by managing good practices, creating new fertilizers, pesticides, and plant varieties, using crop rotation and irrigation techniques, as well as applying plant growth-promoting bacteria (PGPB) and helpful microbiomes. Numerous bacteria live in the soil, particularly in the plant rhizosphere, in close proximity to plants, and they employ a wide range of strategies and defense mechanisms to promote plant growth and shield plants from pathogens and all environmental stresses.
Increasing plant growth and output, lowering environmental impacts on plants, and bringing the global population down to a tolerable level were primarily projected to be the main ways to prevent widespread hunger.3,4 Around the middle of the 20th century, farmers and scientists tried their best to boost plant growth and production by managing good practices, creating new fertilizers, pesticides, and plant varieties, using crop rotation and irrigation techniques, as well as applying plant growth-promoting bacteria (PGPB) and helpful microbiomes. Numerous bacteria live in close proximity to plants in the soil, especially in the plant rhizosphere, and they employ a wide range of strategies and defense mechanisms to promote plant growth and shield it from pathogens and environmental stresses.
Different environmental stress that faces plants
Numerous studies have shown that abiotic stresses like drought, extreme soil salinity, and human activities, which reduce soil fertility and increase the extent of saline in agricultural soil, cause an increase in the salt composition of soils worldwide, which reduces plant growth, health, and production, are highly detrimental to plant growth and agricultural productivity.5,6 Salts can enter the soil through natural processes or human actions like irrigation with unfit water. According to reports, one of the major abiotic elements is salt, which seriously impacts plant growth and food production, particularly in soil with high salinity levels that are often simple to detect using the electrical conductivity method. Saline soils are defined as those with electrical conductivity greater than four dS/m or higher, and sodium chloride is the most prevalent soluble salt in soils, followed by calcium and magnesium chlorides.7 Soil salinity detection techniques must be used to combat poor plant growth and increase productivity. According to estimates, salt and other stressors like acidity and alkalinity have an impact on plant growth. Salt accumulation is steadily increased by rain, wind, soil erosion, anthropogenic activity, and irrigation until it reaches a point where crop yield is significantly impacted. To promote plant development under saline circumstances, experts and agriculture authorities advise choosing suitable salt-tolerant crops and controlling soil salinity.8
Another strategy is the use of plant growth promoting bacterial to solve or decrease the salinity impacts and to improve the agricultural economy productivity through decreasing the induction of some materials like amino acids, Ethylene, or sugars, which are with antioxidant enzymes, the primary plant defense mechanisms to overcome salinity and increase the productivity of the plants under the saline conditions.9,10
Evidence shows that utilizing poor irrigation techniques raised the saline level of the soil, which is thought to be one of the major causes of the destruction of the farmed regions and the accumulation of salt around the roots and inside plant cells. The effects of hypersalinity on most grown plants, including osmotic and ionic stress, are well documented.9,11,12 There have been numerous attempts to use plant manipulation to overcome the inhibitory effects of salts on plant growth, such as the creation of salt-resistant plants through genetic transformation and selection of more salt-tolerant varieties, but testing those plants in the environment takes a lot of time and requires numerous lab efforts to be successful. 13,14,15 The application of beneficial plant growth-promoting bacteria related to plants is another strategy. 13,16,17 Additionally, the extraction of mineral resources results in the release of numerous heavy metals into the soil, causing soil pollution, which poses a serious issue for plants as they gradually expand. Increased levels of these heavy substances in the soil resulted in increased plant absorption, accumulation, and, ultimately, limited growth and plant toxicity. Furthermore, these heavy metals may enter a person’s body and result in various health issues. Fortunately, some microorganisms can tolerate and withstand toxic heavy metals, resist them, and have an excellent ability to remove heavy metals efficiently and affordably.18
Saline stress and ethylene synthesis in plants under stress
Osmotic and ionic stress, which affected the majority of critical physiological processes including photosynthesis, were the two main issues that plants encountered under high salinity.12,19 While osmotic pressure causes dehydration and elongation and the growth of the cells and lateral buds is halted due to the accumulation of sodium poisonous in leaves and other plant tissue, ionic stress results in excessive sodium influx, which causes the outflow of potassium ions. Plants produce a lot of reactive oxygen species under stressful conditions like salinity, drought, flooding, or heavy metal contamination,20,21,22 which causes nucleic acid damage like loss of nucleotide bases, mutation, DNA protein linkage, and DNA degradation.23, 24
Moreover, it was noticed that under stress, plants produce Ethylene which has an essential role in the association relationship between plants and bacteria. The interactions of Rhizobium with legumes roots were inhibited, and root nodule formation was decreased, which led to a delay in the nitrogen fixation process.16,25-27 Under saline stress conditions, symbiotic bacteria helped plants adapt, modulate their responses, and survive under stress due to the production of ACC deaminase, volatile metabolites, amino acids, antimicrobial agents, and polysaccharides.23
Typically, Ethylene is identified as a gaseous hormone produced by all higher plants and some microorganisms. This is consistent with the observation reported by Hays et al.28 who reported that Ethylene produced under special conditions, transported freely through plant tissues by passive diffusion and at low concentrations, induced seed germination, root elongation, leaf formation, production of volatile compounds and initiation of flower and fruit stages.29,30
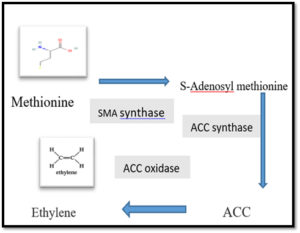
The synthesis of ethylene in plants was documented from methionine and ATP, which form S-adenosyl-methionine (SAM) by the enzyme SAM synthetase. Then, SAM is then transformed to ACC using ACC synthase enzyme, and finally, the ACC produces Ethylene using ACC oxidase enzyme, as summarized in Figure.24,31, 32
Impact of saline soils on the biodiversity of soil microbiota
Salinity stress is a significant factor affecting agriculture productivity, and emerging new varieties, tolerant to different biotic and abiotic stress factors via hybridization and genetic engineering is expensive process and time-consuming but plant growth-promoting microorganisms are more cost-effective and environmentally friendly to ease stress effects. Delivery of ACC-deaminase and plant growth regulators production via beneficial bacteria mainly affected Ethylene and abscisic acid-dependent signaling positively, facilitating plant growth and alleviating stressful conditions positively. It should be noted that microbial communities are found mainly in all types of soils, and their Biodiversity is high, and their numbers are high in the Rhizosphere, and the number of bacteria decreases as we move away from the root zone, which is rich in nutrients. Moreover, the bacterial counts and types were affected by both abiotic and biotic factors such as soil temperature, humidity, type, salinity, pH, composition, and fertility.6 These factors played more significant roles in transforming the soil microbiota, which forms varied interactions and relationships with plants and increases growth, development, production, and protection against fungal and bacterial pathogens. In addition, the soil microbiota played a significant role in minerals, agricultural and organic waste cycles, and soil fertility degradation. Therefore, soil salinity mainly affects soil microbiota diversity, abundance, and their potential to interact with plants, and it allows the survival of halo-tolerant bacterial and fungal strains in addition to mycorrhizae. Yaish et al.33,34 evaluated the endophytic microbiota associated with Medicago truncatula plants, and they added that soil salinity affects 70 % of endophytic microbiota detected using sequencing of 16S rDNA genes. They added that the isolated endophytic bacteria might pose plant growth-promoting strategies like ACC deaminase, ammonia and IAA production, Zn+2 and PO4-3 solubilization, and secondary product secretion. Similar results were obtained for date palm seedlings, whereas salt stress differed the endophytic microbiota associated with the root system and significantly decreased the genera Rhizobium, Enterobacter, and Pseudomonas.33,34,35
Plant growth-promoting bacteria
Genera Achromobacter, Bacillus, Brevibacterium, Citrobacter, Enterobacter, Leclercia, Ochrobactrum, Parastrephia, and Serratia were reported by many authors to improve plant growth.11,36-41 Under salinity stress, some growth-promoting bacteria could program plants to overcome stress and grow well by producing ACC deaminase and IAA that significantly increase sugar, organic acids, amino acids, and proteins. Many future critical studies are needed to guide us on using plant growth-promoting bacteria (PGPB) to manage plant growth in the field, wherever salinity is the primary constraint.
Under extreme conditions, many promising novel microorganisms within the genera Bacillus, Nocardia, and Streptomyces are present, and they are undoubtedly prominent producers of putative new bioactive agents with excellent plant-promoting activities. Soil bacteria are often described as chemical gold, considered excellent sources of new natural products, and a treasure for plants and humans. Over 500,000 living microbial species, divided into many different families, were isolated from the soil, and new isolates are still to be discovered. The soil surrounding the plant roots or root rhizosphere is a rich place with PGPB, which use soil nutrients and plant root exudate to grow, and their numbers increased in the rhizosphere area due to the presence of a high quantity of root exudate compared to soil far from the plant root area. Root area is a rich source of nutrients, and root exudates contain high amounts of vitamins, soluble carbohydrates, simple proteins, amino and organic acids, small molecules, and organic polymers. Root exudate reaches about a third of the fixed carbon by the plant. Thus, the region around the root becomes the richest with the microbes of the Rhizosphere. Each plant has its endophytes microbiome, which can be isolated from the root system, leaves parts (endosphere), flowers, or fruit tissues, but other microbe inhabits the leaf or stems surfaces (phyllosphere).42 The presence of large numbers of the microbiome is due to the presence of nutrients exuded by the roots of the plant.13 Typical soils contained 107– 108 cfu/g of bacteria, 103-104cfu/g of actinomycetes, and 104– 105 spore/g of fungi, and these numbers decreased in poor or stressed soils.43 These microorganisms enhance soil fertility and plant growth and make plants more tolerant to salt stress, heavy metals, and toxic pollutants and pathogens.44,45,46 The most active bacterial isolates belong to the genus Bacillus.37,38, 40
According to studies by Siddikee et al.,11 Upadhyay et al.,39 Saeed et al.,47 and others, numerous microbial species including Aeromonas, Pseudomonas, Bacillus, Azotobacter, and Azospirillum have been discovered as plant growth-promoting bacteria. Also connected to the nodules on the roots of Medicago sativa were Bacillus megaterium and Enterobacter cloacae, while Pseudomonas monilia was identified from Solanum lycopersicum. All of these microorganisms promote growth. Numerous studies have demonstrated that whereas rhizosphere soil is rich in nutrients because of root exudates that allow significant bacteria to grow and prosper, non-agricultural soils, poor soil, and soil with harsh circumstances including salt, dryness, and absence of critical nutrients have low microbiota. Furthermore, compared to other plant growth-promoting bacteria, halophilic and halotolerant bacteria have distinct advantages in saline environments which enable them to grow and survive in saline environments. Thus many studies aimed for a safe, eco-friendly, and efficient strategy to significantly increase plant growth and production under stress conditions.
The most active bacterial genera that promote plant growth
Leclercia adecarboxylata MO1, which produces the halotolerant IAA, is extensively distributed in a variety of environmental sources and can support tomato growth and plant resistance to salinity stress. According to Tamura et al.48, Kelemu et al.49, Sun et al.50, Verma et al.51, and Shahzad et al,52 the preceding isolate, known initially as Escherichia adecarboxylata, may be isolated from a variety of plant components, the rhizosphere, soil, and water. As a solute in osmatic alterations and excess cation balance during salinity stress, rhizosphere microorganisms can drive organic acid metabolism in plants when stressed. Increases in organic acids following the injection of helpful bacteria have been observed under osmotic pressure. These acids decrease nutrient deficiencies and stress tolerance because they can solubilize phosphorus from insoluble complexes to make it available for plants. Furthermore, the organic acids and exopolysaccharides produced by the soil microbiota operate as active components for bacterial quorum sensing during the development of a biofilm on the root surface and the colonization of the rhizosphere by bacteria. By eliminating biotic and abiotic stresses, these bacteria produce a high number of soluble amino acids that act as the building blocks for secondary metabolites that raise plant tolerance. It has been demonstrated that PGPB actively contributes to stress tolerance by increasing IAA production and promoting enhanced plant growth in inoculated plants.
The genus Bacillus is recognized as a sporulating bacterium that exhibits ubiquitous prevalence in virtually any habitat and under adverse conditions. In this regard, Bacillus species are able to withstand different environmental stresses through a variety of direct and indirect actions that support plant growth. These actions include lengthening roots and aerial structures through the production of enzymes, antibiotics, and plant growth regulators, as well as growth under various stress conditions due to the ability of the vegetative cells to sporulate.6 For many years, authors have claimed that the genus Bacillus is magical and that its species are ideal candidates for possessing a wide range of biological functions with advantageous processes and serve as important bio-inoculants, bio-stimulants, bio-fertilizers, or biocontrol agents. In the field and under salt stress conditions, to combat adverse conditions like salt, drought, and nutrient deficiency, Bacillus cells are successfully used as soil inoculums.13 Particular, saline-tolerant bacillus cells were isolated, purified, characterized, and identified from the soil, and these isolates showed an excellent broad capacity to promote the growth of diverse plant species, and their interactions with plants had unlimited benefits.12,23,53-57 It is unknown whether environmental microorganisms increase their salinity tolerance or decrease soil salinity to support plant growth when conditions are salty.
The use of traditionally and genetically engineered microorganisms to increase plant growth
Using traditionally and genetically engineered plant growth-promoting microorganisms is an excellent method to improve plant growth under saline conditions.58,59 According to a study, the use of beneficial microorganisms for plants grown under stress improved plant tolerance. These microorganisms included nitrogen-fixing bacteria, which have a close relationship with plant roots and produce hormones that will enhance nutrient uptake, secrete siderophore, solubilize phosphate, and prevent the growth of plant pathogens. Bacteria that produce IAA and ACC deaminase play crucial roles in plant growth as well as in defending plants from disease and environmental stress. Root exudates were transformed by rhizosphere bacteria into plant growth regulators like IAA, which promote plant growth and trigger the transcription and production of the enzyme ACC synthase. Environmental stress has been observed to cause ACC deaminase to produce ethylene from ACC.60
Roles of soil microbiota and plant growth-promoting bacteria
In the removal of heavy metals, nutrient recycling, and degradation of agricultural wastes
Rhizosphere is rich with free-living saprophytic plant growth-promoting bacteria (PGPB) that live in association with plant roots to enhance plant growth either directly or indirectly.61,62 All significant bacterial isolates had a metabolic activity that made them resistant to different concentrations of heavy metals. Bacteria adapted to low levels of heavy metals and can resist high levels over time. Thus, they can be used in the bioremediation process of these environmental pollutants at the lowest costs and best effects. The bacterial genera Bacillus, Enterobacter, Nocardiopsis, and Pseudomonas are considered important genera resistant to heavy metals from contaminated environments and have high bioremediation potential. Bakran et al.63 isolated two bacterial isolates, Streptomyces toxytricini and Streptomyces sp., with an excellent ability to remove lead (Pb++) from polluted industrial wastewater. The highest biosorption rate (99%) was at pH eight and temperature 37°C. Jafarzade et al.64 reported that Serratia sp. WPRA3 showed high tolerances to heavy metals like Ni, Co, Cr, Pb, and Zn and can be used to clean polluted soil. Similarly, Cimermanova et al.65 identified nine isolates of the genus Streptomyces that can survive in toxic environments and were highly resistant to Pb, Zn, Cu, and Ni. Afzal et al.66 reported that high concentrations of heavy metals are adsorbed on the bacterial cell walls, affect cell membranes, and form pores in them, and these effects differ with the metal type.
Bacteria use many techniques to protect themselves from unfavorable conditions like heavy metals and salinity. They had physiological systems with different safeguard protocols to protect themselves against contaminants, like the Efflux protocol, which exports toxic metal ions to the outside of the cell, and they are precise to a particular heavy metal ion, accumulation, and complex formation protocol leading to the formation of metallothioneins or cysteine-rich proteins which prevent the exposure of essential cellular components to the toxic metal. Reduction protocol which degrades the contaminant using enzymatic reduction to less toxic materials released outside the cells, and finally, alteration of cellular components protocol which decreases the cell sensitivity and adapts themselves to the presence of the toxic metal ions through mutations or genetic transformation67-69 Some bacteria could accumulate the toxic metals on their cell walls (biosorption process, complex formation, degradation through reduction, oxidation, or precipitation) and inhibit their passage through the cell membrane.
In the environment, microorganisms play essential roles in recycling different nutrients, primarily carbon, nitrogen, and mineral, in addition to energy which moves among living and nonliving things. As a result of the complete nutrient recycling process, soil quality was enhanced, crop yield was increased, and the used quantity of chemical fertilizers was decreased. In addition, microorganisms enhanced plant growth using different protocols like secretion of secondary metabolites and bioactive agents, increased iron and soluble phosphate viability, and purified the soil from chemical hydrocarbons, phenol, heavy metals, and agricultural wastes by different biodegradation processes. Thus, they can be used for various industrial and biotechnological applications.
In the production of 1-aminocyclopropane-1-carboxylate deaminase
The enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase is recorded in some microorganisms and act as plant growth-promoting enzyme which cleaves ACC, the immediate precursor of the plant hormone ethylene producing ammonia and a ketobutyric acid, which diminishes the bad properties of high levels of Ethylene.70 Under biotic and abiotic stresses, Ethylene is produced by plants as a vital signaling product which negatively affects plant growth.71 Presence of some microorganisms may reduce the effects and lower the concentration of ethylene hormone in plants. Previous studies have demonstrated that inoculation of plants with ACC producing bacteria declined ethylene levels, resulting from decreasing resistance of plant growth under biotic and abiotic stresses.72 Some plant growth-promoting bacteria secrete a varied range of ACC deaminase which reached to approximately e ≥20nmol α-ketobutyrate mg-1h-1, allowing the bacterium to grow on ACC and act as a plant bio-fertilizer. It was reported that one bacterial isolates grew well on a medium supplemented with ACC, and this isolate had the highest level of ACC deaminase activity, ranging between 0.005 to 0.107 mmol. α-ketobutyrate mg-1h-1. Thus, it was reported as an excellent and effective bacterium for promoting plant growth, while some other isolates had low amounts of ACC deaminase. From the previous results, this isolate may be used to promote the development of plants, particularly under stressful conditions such as phytopathogens, and this activity could be utilized for enhancing plant growth through production-resistant plants.36
Moreover, Bacillus species produce 1-amino cyclopropane-1-carboxylic acid deaminase, which enables it to have synergistic stress tolerance activities with plants, such as the accumulation of trehalose, and this phenomenon is discussed and highlighted.46 In some cases, stress conditions in Bacillus act as inducers to some plant growth regulators and protective agents against some plant diseases. Thus, finding such bacterial species in saline agroecosystems is not exceptional, given that ACC deaminase activity is one of the primary mechanisms to fulfill this beneficial function in the interacting of PGPB with plants. For example, Heydarian et al.73 detected that plants that contained the bacterial acdS gene showed increased salinity tolerance due to the production of reactive oxygen species to avoid cellular injury. In addition, some critical bacteria associated with plant roots secreted ACC deaminase to improve salinity tolerance and decrease the induction of Ethylene and abscisic acid under stress conditions.
Under salt or other environmental stresses, plants produce an increased amount of ACC synthase and ACC oxidase, which leads to the formation of Ethylene from the direct precursor ACC but the presence of PGP bacteria that produce ACC deaminase enzyme act decreases and degradation of ACC to α-ketobutyrate and ammonia which lower plant inhibitory ethylene levels.74,75 It was reported that the quantity of Ethylene was decreased by the presence of ACC deaminase-producing bacteria, which only reduced the quantity of Ethylene produced but did not prevent its synthesis completely. Thus, generally, PGP bacteria that contain ACC deaminase promote plant growth, reduce the damage due to stress, and help plants adapt and survive.
In this regard, the ACC deaminase from PGPB acts as an essential sink for ACC in the plant, lowers its level, and prevents its accumulation in large amounts, but the produced bacterial indole-3-acetic acid (IAA) facilitates plant growth and may enhance ACC synthase, and the synthesized amount of Ethylene may inhibit IAA production and plant growth and so no. However, a PGPB containing the ACC deaminase enzyme decreases feedback inhibition.16,25,76
It is also consistent with the observation that Leclercia adecarboxylate is a plant growth-promoting bacteria because it produced significant amounts of ACC deaminase and IAA, which enhanced plant growth and improved its ability to tolerate salt stress. Meanwhile, many bacterial isolates have been reported to increase metal bioremediation by some plants due to the synthesis of both ACC deaminase and IAA.75
At present, many rapidly unpredictable climatic changes and environmental stresses are due to the growing global population and their anthropogenic activities, which negatively affect plant growth and production in addition to food security which is needed to be maintained in a sustainable and eco-friendly way. Among the various and significant environmental stresses that threaten life on the earth is climate change which causes many substantial stress factors like salinity stress which badly influences more than 77 million hectares of agriculture production.
The microbiome of plants, which live in stressful conditions, frequently contains ACC deaminase. Timmusk et al.43 conducted research on the significance of this enzyme and its value to plants. It is preferable to identify and describe rhizobacteria in microenvironments like the rhizosphere because they have important functions and produce the enzyme ACC deaminase, which aids in plant growth.77-79 In vitro bacterial ACC-deaminase has been linked directly to root growth.38,79 However, the relationship between bacteria and plants is intricate, and a bacterium’s impact can alter as its environmental conditions do.29 The outcomes obtained by Glick et al.74 demonstrate the significance of bacterial ACC deaminase in enhancing plant growth.
Finding ACC deaminase activity in PGPB and screening for the acids gene are two separate approaches. This activity has a wide distribution in many bacterial genera, according to searches for the acdS gene in the genomes of soil microorganisms and endophytic bacteria.80, 81 Additionally, the actinomycetes like Deinococcus, Proteobacteria, and Formicates bacterial groupings all showed the phylogeny of the acdS gene. Along with Lrp-like regulatory proteins called AcdR which control acdS gene expression in proteobacteria.82 The beneficial plant fungus Trichoderma asperellum, which similarly secretes ACC deaminase, is another class of non-bacterial microorganisms which benefit the plant and also secrete ACC deaminase, and has a role in phytopathogenic biocontrol and plant growth promotion.83
The aforementioned instance is one of the scant number of studies83,84,85 that have documented the activity of ACC deaminase. ACC deaminase has been found in a variety of rhizobia before.86 According to reports,26, 27, 76 Arthrobacter protophormiae contains ACC deaminase and is linked to other advantageous microorganisms that promote rhizobium nodulation and mycorrhizal infection, resulting in the induction of salt stress tolerance in Pisum sativum plants. The enzyme was previously only known to exist in free-living bacteria, yeast, and fungi. Studying the bacteria, which work in symbiosis with legume plants to fix atmospheric nitrogen is important. Later, the presence of ACC deaminase has been reported not only in the genus Rhizobium but also in several genera of family Rhizobiaceaelike Sinorhizobium and Agrobacterium and family Phyllobacteriaceae like genera Phyllobacterium and Mesorhizobium in addition to Azospirillum.87, 88 Many genera of PGPB bacteria also exhibited ACC deaminase activities, like Aneurini bacillus, Pseudomonas, Ralstonia, Micrococcus, and Arthrobacter.
By creating mutant strains, isolating the target gene, and expressing it in heterologous hosts, one can examine the precise function of a gene. In the case of Pseudomonas sp. UW4’s acids gene, Shah et al.89 work involved the isolation and expression of this gene in hosts like Escherichia coli DH5, Pseudomonas putida ATCC 17399, and P. fluorescens ATCC 17400, none of which naturally contain ACC deaminase, allowing the transformed strains to grow in minimal medium.
A similar approach was used by Brgido et al.90 to express the acdS gene in two strains of Mesorhizobium cicero, one of which is salt sensitive and the other salt tolerant. Additionally, M. ciceri is a bacterium that coexists with chickpea roots and fixes nitrogen. According to their findings, Mesorhizobium cells underwent a considerable metamorphosis that improved the symbiotic relationship when compared to the wild type.
By producing ACC deaminase in mutants of the genus Pseudomonads that lack it, Ali et al.84 were able to further demonstrate the beneficial role of the bacterial acdS gene during the interaction of plants with their essential bacteria under saline conditions. This improved tomato growth, increased fresh and dry biomass, and increased chlorophyll contents.
Plants would naturally create ACC under stressful growth conditions, which would impede growth and development. It is important to note that numerous manuscripts25, 35, 41,91 have reported or suggested that the presence of ACC deaminase activity in PGPB is one of the key mechanisms that is involved in the bacterial promotion of plant growth. The list, as was previously stated, is enormous and keeps expanding as fresh original works are consistently released with new bulk soil, rhizospheric, and endophytic strains that promote plant development after the generation of ACC deaminase. 70,92,93,94
Likewise, plants produce Ethylene under stress, which regulates plant responses to biotic and abiotic stresses.70,95,96,97 However, in response to biotic and abiotic stresses, the plant frequently significantly increases endogenous ethylene production, which has detrimental effects on plant growth and is thought to be the cause of senescence in plants.70,84,98,99 Under ambient conditions, plants produce the necessary levels of ethylene, which confer beneficial effects on plant growth and development. It’s interesting to note that the PGPB also has the enzyme ACC deaminase,84,73,100 which can convert the plant ethylene precursor ACC to ammonia and α-ketobutyrate, lowering the level of ethylene under different biotic and abiotic stresses,73 like salt stress,38, 101,102 flooding stress,103 drought stress,38 heavy metal stress,104,105 and pathogen attack.36 As a result, bacteria that produce ACC deaminase lower the level of ACC in stressed plants, restrict the generation of ethylene, and so halt plant damage. Because plants are frequently subjected to conditions that produce the production of ethylene, it is advised that these bacteria be helpful for plant growth. The most active and widely dispersed bacteria in soil were luminous Pseudomonas isolates. They produced many enzymes and secondary metabolites, showed high catabolic flexibility, and had outstanding root colonization abilities. These earlier actions help plants withstand a variety of biotic and abiotic stressors. 38,106,107 Additionally, using 16S rDNA sequence analysis, the most active bacterial isolate in ACC deaminase production was identified as Pseudomonas fluorescens REN1, and this isolate significantly increased root elongation and growth of rice seedlings compared to control seedlings. They attributed this increase to the production of indole-3-acetic acid and ACC deaminase more than siderophore production or phosphate solubilization.108
Tiwari et al.35 isolated thirty-seven bacterial isolates from twenty-five soil samples collected from India. All isolates contained ACC deaminase, which enabled them to use ACC in a culture medium as a nitrogen source. They added that Bacillus licheniformis and B. subtilis both had the highest levels of ACC breakdown (45.36% and 45.03%, respectively). Comparing the two isolates to other isolates under stress conditions improved plant growth, cell wall polymers and quantity of protein, phenolic contents, and chlorophyll. The two isolates also played key roles in producing IAA, siderophore and HCN, phosphate solubilization, removal salt, and polyethylene glycol stress. Elephant grass (Pennisetum purpureum) and model grass (Brachypodium distachyon) both grew better under salt and drought stress than control plants, and the composition of the plant cell walls returned to nearly normal following the application of helpful endophytic bacteria.109, 110 Additionally, the plants treated with rhizobacteria which produce ACC deaminase, preserve the composition of the plant’s cell wall under stress closer to typical plants.
Additionally, one of the main agricultural issues affecting crop output in the majority of the world’s arid and semiarid regions is drought stress. This type of abiotic stress impacts the interactions between plants and water at both the cellular and systemic levels, leading to both particular and generalized reactions and damage. Exopolysaccharide (EPS), which bacteria produce, protects microorganisms from water stress by improving water retention and controlling the diffusion of organic carbon sources.111-114 This allows bacteria to survive under stressful conditions. Due to the participation of a network of fibrillar material that firmly bonds the bacteria to the root surface, EPS also aids microorganisms in irreversibly attaching and colonizing the roots.115,116 Plants living in arid or semiarid locations may be more resilient to drought if native, advantageous bacteria are used to inoculate the plants with ACC deaminase. In order to best aid plants under drought stress, we thus made an effort to extract and characterize EPS and ACC deaminase from drought-tolerant Pseudomonas strains from cropped soils of various arid and semiarid natural settings.
A series of morphological, physiological, biochemical and molecular changes in plants are induced by abiotic stress which negatively affecting the plant growth and productivity and the mechanisms essential for plant survival are in conjunction with significant changes in the patterns of metabolites and proteins, hence the use of certain bacteria in order to increase stress tolerance and to develop plant protectants against stress is needed. Also, plants create ACC under stressful growth conditions, which inhibits plant growth and development. However, the presence of ACC deaminase activity in bacteria that promote plant growth is thought to be the primary mechanism underlying the bacterial stimulation of plant growth. To face the challenges caused by global population growth and climate change, plant growth-promoting bacteria are among the main interacting factors of stress tolerance and are often a magic solution to enhance plant growth through the increasing of nutrient uptake, secreting siderophore, solubilizing phosphate, and preventing the growth of plant pathogens. Thus, a priority task is to explore the activity of different bacteria with wide stress tolerance to enhance plant growth and development.
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Grote U, Fasse A, Nguyen TT, Erenstein O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front Sustain Food Syst. 2021;4:617009.
Crossref - FAO. The state of the world’s land and water resources for food and agriculture – systems at breaking point (Rome, Italy: FAO). 2022.
Crossref - Bene C, Prager SD, Achicanoy HAE, et al. Understanding food systems drivers: a critical review of the literature. Glob Food Sec. 2019;23(4):149-159.
Crossref - Bene C, Fanzo J, Prager SD, et al. Global drivers of food system (un) sustainability a multi-country correlation analysis. PLoS ONE. 2020;15(4):e0231071.
Crossref - Lamz-Piedra A, Gonzalez-Cepero MC. La salinidadcomoproblema en la agricultura: la mejora vegetal una solucion inmediata. CultivosTropicales. 2013;34(4):31-42.
- Santoyo G, Pacheco CH, Salmeron JH, Leon RH. The role of abiotic factors modulating the plant-microbe-soil interactions: toward sustainable agriculture. A review. Span J Agric Res. 2017;15(1):e03R01
Crossref - Sairam RK, Tyagi A, Chinnusamy V. Salinity tolerance: cellular mechanisms and gene regulation. Plant-environment Interactions. 2016:137-191.
- Carrera-Villacres DV, Crisanto-Perrazo T, Ortega-Escobar H, et al. Qualitative and Quantitative Salinity of the Santa Maria-Verde River Hydrographic System, Mexico. Tecnologia y ciencias del agua. 2015;6(2):69-83.
- Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004; 55(396):307-319.
Crossref - Rengasamy P. Soil processes affecting crop production in salt-affected soils. Funct Plant Biol. 2010;37(7):613-620.
Crossref - Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol. 2010;20(11):1577-1584.
Crossref - Horie T, Karahara I, Katsuhara M. Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice. 2012;5(1):11.
Crossref - Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012.
Crossref - James K, Bradshaw K. Detecting plant species in the field with deep learning and drone technology. Methods Ecol Evol. 2020;11:1509–1519.
Crossref - Roy SJ, Negrדo S, Tester M. Salt resistant crop plants. Curr Opin Biotechnol. 2014;26:115-124.
Crossref - Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169(1):30-39.
Crossref - Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda Mdel C, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res Feb. 2016;183:92-99.
Crossref - Jiang J, Pan C, Xiao A, Yang X, Zhang G. Isolation, identification, and environmental adaptability of heavy-metal-resistant bacteria from ramie rhizosphere soil around mine refinery. 3 Biotech. 2017;7(1).
Crossref - Prasad MNV (Ed.). Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. Springer, New York. 2020:395-412.
- Bharti N, Barnawal D. Amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity. PGPR Amelioration in Sustainable Agriculture. 2019:85-106.
Crossref - Covarrubias SA, Cabriales JJP. Contaminacion nambiental por metales pesados en Mexico: Problematica y Estrategias de Fitorremediacion. Rev Int Contam Ambient. 2017;33:7-21.
Crossref - Forni C, Duca D, Glick BR. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410(1-2):335-356.
Crossref - Sntoyo, F., González, A.E., Terrón, M.C., 24Ramírez, L. and Pisabarro, A.G. (2008) Quantitative Linkage Mapping of Lignin-Degrading Enzymatic Activities in Pleurotus ostreatus. Enzyme and Microbial Technology, 43, 137-143.
Crossref - Yang Y, Guo Y. Unraveling salt stress signalling in plants. J Int Plant Biol. 2018;60(9):796-804.
Crossref - Kang SM, Shahzad R, Bilal S, et al. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019;19(1):80.
Crossref - Nascimento FX, Brigido C, Rossi MJ, Glick BR. The role of rhizobial ACC deaminase in the nodulation process of leguminous plants. Int J Agron. 2016b;2016:1369472.
Crossref - Nascimento FX, Rossi MJ, Glick BR. Ethylene and 1-Aminocyclopropane-1- carboxylate (ACC) in plant-bacterial interactions. Front Plant Sci. 2018;9:114.
Crossref - Hays DB, Do J, Mason RE, Morgan G, Finlayson SA. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Science. 2007;172(6):1113-1123.
Crossref - Lynch JM, de Leij, F Rhizosphere. In eLS; Key Concepts; John Wiley & Sons, Ltd.: Chichester, UK, 2012.
Crossref - Choudhary DK. In: Varma, A., Tuteja, N. (Eds.), Plant-Microbe Interaction: an Approach to Sustainable Agriculture. Springer, New Delhi, India. 2017.
Crossref - Liang W, Ma X, Wan P, Liu L. Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun. 2018;495(1):286-291.
Crossref - Liu L, Kloepper JW, Tuzun S. Induction of systemic resistance in cucumber against bacterial angualar leaf spot by plant growth promoting rhizobacteria. Phytopathology. 1995;85:843-847.
Crossref - Yaish MW, Al-Harrasi I, Alansari AS, Al-Yahyai R, Glick BR. The use of high throughput DNA sequence analysis to assess the endophytic microbiome of date palm roots grown under different levels of salt stress. Int Microbiol. 2016;19(3):143-155.
- Yaish MW, Al-Lawati A, Jana GA, Patankar HV, Glick BR. Impact of soil salinity on the structure of the bacterial endophytic community identified from the roots of caliph medic (Medicagotruncatula). PLoS One. 2016;11(7):e0159007.
Crossref - Kong Z, Mohamad OA, Deng Z, Liu X, Glick BR, Wei G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ Sci Pollut Res. 2015;22:12479-12489.
Crossref - Wang C, Knill E, Glick BR, Defago G. Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol. 2000;46(10):898-907.
Crossref - Ghosh S, Penterman JN, Little RD, Chavez R, Glick BR. Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of canola, Brassica campestris. Plant Physiol Biochem. 2003;41(3):277-281.
Crossref - Viterbo A, Landau U, Kim S, Chernin L, Chet I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol Lett. 2010;305(1):42-48.
Crossref - Upadhyay SK, Singh DP, Saikia R. Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr Microbiol. 2009;59(5):489-496.
Crossref - Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015;22(2):123-131.
Crossref - Maxton A, Singh P, Masih SA. ACC deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J Plant Nutr. 2018;41(5):574-583.
Crossref - Arshad M, Frankenberger Jr WT. Plant growth-regulating substances in the Rhizosphere: microbial production and functions. Adv Agrono. 1997;62:45-151.
Crossref - Timmusk S, Paalme V, Pavlicek T, et al. Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS One. 2011;6(3):e17968.
Crossref - Dodd IC, Perez-Alfocea F. Microbial amelioration of crop salinity stress. J Exp Bot. 2012;63(9):3415-3428.
Crossref - Orozco-Mosqueda MC, Rocha-Granados MC, Glick BR, Santoyo G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol Res. 2018;208:25-31.
Crossref - Orozco-Mosqueda M, Duan J, DiBernardo M, et al. The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front Microbiol. 2019;10:1392.
Crossref - Saeed Q, Xiukang W, Haider FU, et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int J Mol Sci. 2021;22(19):10529.
Crossref - Tamura K, Sakazaki R, Kosako Y, Yoshizaki E. Leclercia adecarboxylata gen. Nov., comb. Nov., formerly known as Escherichia adecarboxylata. Curr Microbiol. 1986;13:179-84.
Crossref - Kelemu S, Fory P, Zuleta C, Ricaurte J, Rao I, Lascano C. Detecting bacterial endophytes in tropical grasses of the Brachiaria genus and determining their role in improving plant growth. African J Biotechnol. 2011;10:965-976.
- Sun K, Liu J, Gao Y, Jin L, Gu Y, Wang W. Isolation, plant colonization potential, and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6-gfp. Sci Rep. 2014;4:5462.
Crossref - Verma P, Yadav AN, Khannam KS, et al. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol. 2015;65:1885-1899.
Crossref - Shahzad R, Waqas M, Khan AL, et al. Indoleacetic acid production and plant growth promoting potential of bacterial endophytes isolated from rice (Oryza sativa L.) seeds. ActaBiol Hung. 2017;68(2):175-186.
Crossref - Tahir M, Ahmad I, Shahid M, Shah GM, Farooq ABU, Akram M, Zakir A. Regulation of antioxidant production, ion uptake and productivity in potato (Solanum tuberosum L.) plant inoculated with growth promoting salt tolerant Bacillus strains. Ecotoxicol Environ Saf. 2019;178:33-42.
Crossref - Vilchez JI, Tang Q, Kaushal R, Chen S, Liu R, Zhang H. Genome sequence of Bacillus cereus strain TG1-6, a plant-beneficial rhizobacterium that is highly salt tolerant. Genome Announ. 2018;6(19):e00351-18.
Crossref - Chinnaswamy A, Coba de la Pena T, Stoll A, et al. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and alleviates salt stress in alfalfa plants. Ann Appl Biol. 2018;172(3):295-308.
Crossref - Abd_Allah EF, Alqarawi AA, Hashem A, et al. The endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating plant defense mechanisms. J Plant Interact. 2018;13(1):37-44.
Crossref - Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopory SK. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem. 2013;66:1-9.
Crossref - Zaidi SSeA, Mahas A, Vanderschuren H, Mahfouz MM, . Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020;21:289.
Crossref - Yiting L, Jing F, Hangcheng P, Xiuwei Z, Yunlei Z. Genetically engineered bacterium: Principles, practices, and prospects. Front Microbiol. 2022;13.
Crossref - Gamalero E, Glick BR. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015;169(1):13-22.
Crossref - Kloepper JW, Beauchamp CJ. A review of issues related to measuring colonization of plant roots by bacteria. Can J Microbiol. 1992;38(12):1219-1232.
Crossref - Sairam RK, Tyagi A, Chinnusamy V. Salinity tolerance: cellular mechanisms and Gene Regulation. In Plant-Environment Interactions, CRC Press, Boca Raton, FL, USA. 2016:121-175.
- Bakran FM, Aly MM, Zabermawi NMO. Removal of Some Heavy Metals from Industrial Wastewater by Actinomycetes Isolated From Contaminated Soil, IOSR Journal Of Pharmacy And Biological Sciences, 2019;14(5):58-69.
- Jafarzade M, Mohamad S, Usup G, Asmat A. Heavy-metal tolerance and antibiotic susceptibility of Red pigmented bacteria isolated from marine environment. Natural Resources. 2012;3(4):171-174.
Crossref - Cimermanova M, Pristas P, Piknova M. Biodiversity of actinomycetes from heavy metal contaminated technosols. Microorganisms. 2021;9(8):1635.
Crossref - Afzal AM, Rasool MH, Waseem M, Aslam B. Assessment of heavy metal tolerance and biosorption potential of Klebsiella variicola isolated from industrial effluents. AMB Express. 2017;7(1):184.
Crossref - Ianieva OD. Mechanisms of bacteria resistance to heavy metals. Mikrobiolohichny-zhurnal. 2009;71(6):54-65.
- Timkova AI, Sedlakova-Kadukova J, Prista P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations. 2018;5(4):54.
Crossref - Todorovic B, Glick BR. The interconversion of ACC deaminase and D-cysteine desulfhydrase by directed mutagenesis. Planta. 2008;229(1):193-205.
Crossref - Biswas R, Halder U, Kabiraj A, Mondal A, Bandopadhyay R. Overview on the role of heavy metals tolerance on developing antibiotic resistance in both Gram-negative and Gram-positive bacteria. Arch Microbiol. 2021;203(6):2761-2770.
Crossref - Abeles FB, Morgan PW, Saltveit MEJr. Ethylene in Plant Biology, 2nd ed., Academic Press, New York. 1992.
- Farwell AJ, Vesely S, Nero V, et al. The use of transgenic canola (Brassica napus) and plant growth-promoting bacteria to enhance plant biomass at a nickel-contaminated field site. Plant and Soil. 2007;288(1-2):309-318.
Crossref - Heydarian Z, Yu M, Gruber M, Glick BR, Zhou R, Hegedus DD. Inoculation of soil with plant growth promoting bacteria producing 1-Aminocyclopropane-1-Carboxylate deaminase or expression of the corresponding acdS gene in transgenic plants increases salinity tolerance in Camelina sativa. Front Microbiol. 2016;7.
Crossref - Glick BR, Penrose DM, Li J. A model for lowering plant ethylene concentrations by plant growth promoting rhizobacteria. J TheorBiol. 1998;190(1):63-68.
Crossref - Glick BR. Using soil bacteria to facilitate phytoremediation. Biotechnol Adv. 2010;28(3):367-374.
Crossref - Nascimento FX, Tavares MJ, Franck J, Ali S, Glick BR, Rossi MJ. ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha- and Betaproteobacte riarhizobial strains. Arch Microbiol. 2019;201:817-822.
Crossref - Vicente-Hernandez A, Salgado-Garciglia R, Valencia-Cantero E, et al. Bacillus methylotrophicus M4-96 stimulates the growth of strawberry (Fragaria x ananassa ‘Aromas’) plants in vitro and slows Botrytis cinerea infection by two different methods of interaction. J Plant Growth Regul. 2018;38:1-13.
Crossref - Wu Y, Zhou J, Li C, Ma Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen. 2019;8(8):e813.
Crossref - Shaharoona B, Arshad M, Zahir Z. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vignaradiasa L.). Lett Appl Microbiol. 2006;42(2):155-159.
Crossref - Blaha D, Prigent-Combaret C, Mirza MS, Moenne-Loccoz Y. Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol. Ecol. 2006;56(3):455-470.
Crossref - Bruto M, Prigent-Cobaret C, Muller D, Moenne-Loccoz Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci Rep. 2014;4:6261.
Crossref - Nascimento FX, Rossi MJ, Soares CR, McConkey BJ, Glick BR. New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS One. 2014;9(6):e99168.
Crossref - Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A. ACC deaminase containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol. 2014;171(11):884-894.
Crossref - Ali S Z, Sandhya V, Rao LV. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann Microbiol. 2013;64:493-502.
Crossref - Contreras-Cornejo HA, Macias-Rodriguez L, del-Val E, Larsen J. Interactions of Trichoderma with plants, insects, and plant pathogen microorganisms: chemical and molecular bases. Co-Evol Secondary Metab. 2018:1-28.
Crossref - Ma W, Sebestianova SB, Sebestian J, Burd GI, Guinel FC, Glick BR. Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobium spp. Antonie Van Leeuwenhoek. 2003;83(3):285-291.
- Acuna JJ, Campos M, de la Luz Mora M, Jaisi DP, Jorquera MA. ACCD producing rhizobacteria from an Andean Altiplano native plant (Parastrephia quadrangular) and their potential to alleviate salt stress in wheat seedlings. Appl Soil Ecol. 2019;136:184-190.
Crossref - Mayak, S, Tirosh T, Glick BR. Plant growth promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 2004;42(6):565-572.
Crossref - Duan J, Jiang W, Cheng Z, Heikkila JJ, Glick BR. The complete genome sequence of the plant growth-promoting bacterium Pseudomonas sp. UW4. PLoS One. 2013;8(3):e58640.
Crossref - Brigido C, Nascimento FX, Duan J, Glick BR, Oliveira S. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Mesorhizobium spp. reduces the negative effects of salt stress in chickpea. FEMS Microbiol Lett. 2013;349(1):46-53.
Crossref - Akhgar AR, Arzanlou M, Bakker PAHM, Hamidpour M. Characterization of 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase-containing Pseudomonas spp. in the Rhizosphere of salt-stressed canola. 2014;24(4):461-468.
Crossref - Tiwari G, Duraivadivel P, Sharma S, Hariprasad P. 1-Aminocyclopropane-1- carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci Rep. 2018;8(1):1-12.
Crossref - Frankenberger WTJ, Arshad M. Phytohormones in soil. Marcel Dekker Inc., New York. 1995;35-71.
- Spaink HP. Ethylene as a regulator of Rhizobium infection. Trends Plant Sci. 1997;2:203-204.
Crossref - Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1-18.
Crossref - Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139(3):1393-1409.
Crossref - O’Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274(5294):1914-1917.
Crossref - Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defense in gene in Arabidopsis. Plant Cell. 1998;10(12):2103-2113.
Crossref - Woltering EJ, van Doorn WG. Role of Ethylene in senescence of petals morphological and taxonomical relationships. J Exp Bot. 1988;39(11):1605-1616.
Crossref - Nayani S, Mayak S, Glick BR. The effect of plant growth promoting rhizobacteria on the senescence of flower petals. Ind J ExpBiol. 1998;36(8):836-839
- Shah S, Li J, Moffatt BA, Glick BR. ACC deaminase genes from plant growth promoting rhizobacteria. Plant growth-promoting rhizobacteria. Present status and future prospects. Organization for Economic Cooperation and Development, Paris, 1997; 320-324
- Cheng Z, Park E, Glick BR. 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can J Microbiol. 2007;53(7):912-918.
Crossref - Zahir AZ, Ghani U, Naveed M, Nadeem SM, Asghar HN. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under saltstressed conditions. Arch Microbiol. 2009;191:415-424.
Crossref - Grichko VP, Glick BR. Amelioration of flooding stress by ACC deaminase containing plant growth-promoting bacteria. Plant Physiol Biochem. 2001;39(1):11-17.
Crossref - Stearns JC, Saleh S, Greenberg BM, Dixon DG, Glick BR. Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol Biochem. 2005;43(7):701-708.
Crossref - Belimov AA, Hontzeas N, Safronova VI, et al. Cadmium-tolerant plant growth promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil BiolBiochem. 2005;37(2):241-250.
Crossref - Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001;20(1):1-11.
Crossref - Vivekananthan R, Ravi M, Ramanathan A, Samiyappan R. Lytic enzymes induced by Pseudomanas fluorescens and other biocontrol organisms mediate defense against the anthracnose pathogen in mango. World J MicrobBiot. 2004;20:235-244.
Crossref - Etesami H, Hosseini HM, Alikhani HA. Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol Mol Biol Plants. 2014;20(4):425-434.
Crossref - Li XX, Geng X, Xie R, et al. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of hybrid Pennisetum. Biotechnol Biofuels. 2016;9:190.
Crossref - Gagne-Bourque F, Mayer BF, Charron J-B, Vali H, Bertrand A, Jabaji S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonization by Bacillus sbutilis B26. Plos One. 2015;10(6):e0130456.
Crossref - Wilkinson JF. The extracellular polysaccharides of bacteria. Bacteriol Rev. 1958;22(1):46-73.
Crossref - Hepper CM. Extracellular polysaccharide of soil bacteria. In N walker (Ed.) soil microbiology. Butterworth’s, London. 1975; 93-110.
- Roberson EB, Firestone MK. Relationship between desiccation and exopolysaccharide production in soil Pseudomonas sp. Appl Environ Microbiol. 1992;58(4):1284-1291.
Crossref - Vikram P, Debiec-Andrzejewska K, Fiodor A, Lyzohub M, Ajijah N, Singh S, Pranaw K. Plant Growth-Promoting Bacteria (PGPB) integrated phytotechnology: A sustainable approach for remediation of marginal lands. Front Plant Sci. 2022;13:999866.
Crossref - Chenu C, Roberson EB. Diffusion of glucose in microbial extracellular polysaccharide as affected by water potential. Soil Biol Biochem. 1996;28(7):877-884.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.