ISSN: 0973-7510
E-ISSN: 2581-690X
Biodeterioration plays a significant role in the damage and loss of monuments including gypsum work. Bacteria and fungi are mainly responsible for the biodeterioration of gypsum work. Thus, the present study aimed to evaluate the effects of essential oils (Thyme, clove, cinnamon, garlic, castor, and olive) on the growth of the main fungi and bacteria which isolated from the Nujoumi Dome which is also called the Dome of the Toshka Martyrs and is located in Aswan, Egypt. Microbial swabs were taken from these infected objects, and the isolated microorganisms were characterized. The next genera were identified: four fungal isolates were isolated and identified as Aspergillus japonicas, Aspergillus terrus, Penicillium commune, and Cladosporium elatum while two species of bacteria were isolated, identified as Bacillus cereus and Listeria monocytogenes. Garlic oil had the best effect on all isolates, showing 35 and 33 mm inhibition of growth of Bacillus cereus and Listeria monocytogenes, respectively, while 28 mm inhabitation of growth of Penicillium commune, 25 mm inhibition of Aspergillus japonicas, and finally 20 mm inhibition of both Aspergillus terrus and Cladosporium elatum. In conclusion, garlic oil could be an effective natural product for controlling the biodeterioration.
Archaeological dome, gypsum, microbial deterioration, antibacterial, antifungal, essential oil, thyme, garlic, castor, clove, cinnamon and olive oils
Biodeterioration is defined as any undesirable change in a material brought about by the vital activities of organisms.1 Bacteria, actinomycetes, and fungi are constantly causing problems in the conservation of gypsum windows because of their bio-deteriorative potential. Conservation can be defined as the process of understanding and management architectural heritage in order to safely hand it over to future generations.2 The building materials have been suffering from several deterioration factors. In historic buildings, moisture is essential for microbes to grow on surfaces. In addition to moisture, several other issues influence microorganism growth, such as temperature, pH, nitrogen, and other nutrients.3
Growths, microorganisms, and lichens are additionally found on painted artworks in chapels, giving in and even acting as biodeteriogens of design faces and stone landmarks in outside conditions.4,9 The most significant things experiencing genuine contagious assaults are rock craftsmanship like the caverns of Lascaux in France.10 The battle of Toshka was led by Nujoumi and the British forces, which were supported by the Egyptian army, and which the Egyptian and English army won in 1889. This dome was established by King Farouk the First, in order to remember those who were martyred in 1303-1306 AH, and 1886-1889 AD. Despite the importance of the historical, architectural, and archaeological dome, it was registered only 10 years ago in the Egyptian Ministry of Antiquities as one of the most important archaeological domes. Fig. (1, 2).
The dome is considered one of the most essential architectural elements in the architecture of mosques. Domes are among the most famous architectural elements; they are found in the ancient ruins (which may date back more than five thousand years ago), and increase the charm of the place.11 It is realized that the vault was affected by Byzantine engineering at its start. The continued of vaults in Islamic design in its few structures prompted the improvement of arches’ idea among Muslim planners to become one of the main engineering components in Islamic design.12
This dome in our study (the Najoumi dome) is considered one of the most luxurious archaeological domes remaining in Aswan in the Mamluk style, as it is characterized by its enormity and height, as the height of the building is 17 meters on the ground, and it is characterized by various stucco windows with several hollow decorations. Some of the decorations are vegetal, and some are starry dishes Fig. 7. The door is also above the decorative Qamariyat (Fig. 2). Qamariyat is an Islamic form of the window and is one of the prominent elements in the Islamic archaeological buildings. It is employed to find a relationship that combines the aesthetic and utilitarian value, and its functions are to prevent insects that infiltrate from outside the building to the inside. Qamariyat is made of gypsum and colored glass of the very magnificence It is surmounted by a foundation slab of white marble with the foundation text on it (Fig. 3, 4).
Fig. 3. Shows that the building is square and then turns from a square shape to an octagonal shape through corner bends as a transition stage to the neck of the circular dome. It has 16 open windows with semi-circular arches.
Fig. 4. Shows the main entrance a large wooden door, consisting of two sashes, and each door has decorative geometric shapes.
The Najoumi dome contains a number of stained-glass stucco windows decorated with a variety of decorations, which show a special beauty (Fig. 5, 6). These stucco windows suffer from various damage factors and microbiological damage. These factors not only deform the plaster, but also may lead, with their persistence and negligence in treatment and restoration, to the permanent loss of the gypsum decorative elements. In monumental domes, gypsum is damaged by various types of fungi and bacteria attached to the surfaces of the stucco decoration (Fig. 7, 10) which requires modern methods of treatment and the use of appropriate antifungal treatments. Also we need examination as SEM-EDX to suggest best treatment (Fig. 11, 13).
Fig. 8. Shows Deterioration of gypsum window and Qamariyat. and some parts of the decorative elements were lost.
The bio decay seen on materials of social legacy is controlled by certain elements: (A) the compound design and nature of the actual material, (B) the climate and openness of the item, and (C) the style and routineness of surface cleaning. Microorganisms are primarily responsible for the general crumbling marvels observed on stone and other structural materials.5 On building stone, presented to the climate, parasites might be the most significant bio deteriorative life forms.13
The growth of fungi and bacteria on wall paintings is manifested most commonly by deterioration of the surfaces. The microbial population causes discoloration of pigments, physical decay when fungal hyphae grow either on or below the surfaces, and chemical decay of paintings through their metabolites and enzyme production like gluconic, citric, oxalic, and malic.14,15 Ancient building stones and plaster can be colonised by various collections of microorganisms. Residents existing in a stone substratum are frequently the effects of successive colonisation by different microorganisms that have taken place over many years.16
Fig. 10. Show Accumulation of dust, which encourages the increase of microbiological damage, from inside the dome and around the gypsum windows.
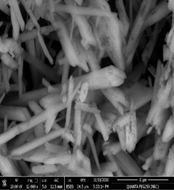
Fig. 11. Scanning electron micrograph of the efflorescence spots and fungi which were detected on the gypsum from wall of Nujoumi dome.
Essential oils with antimicrobial activities extracted from plants are generally used in many industries, such as medicine, perfumery, cosmetics, and food preservation. About 3000 different essential oils are recognized, but only 300 EOs are commercially applied in the flavor and perfume market.17 Their use in the control of fungal growth on archaeological objects was reported by.18 The essential oils (EOs) and their components have been shown to have a broad-range of various properties, including antibacterial, antifungal,19 antiparasitic, antiviral,20 antioxidant,21 antitumor,22 insecticidal23 properties. Moreover, they also play a role in growth enhancers for animals,24 and have antiseptic and medicinal properties (analgesic, anti-inflammatory, anti-carcinogenic, local anesthetic, sedative and spasmolytic).19 Several types of essential oils have been used for the treatment of various human diseases.25
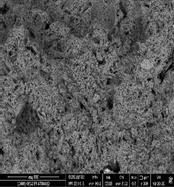
Fig. 12. Scanning electron micrograph of the efflorescence spots and fungi which were detected on the gypsum from gypsum window.
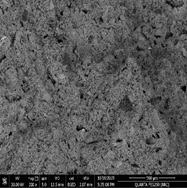
Fig. 13. Scanning electron micrograph of the fungi spots which were detected on the gypsum from gypsum window.
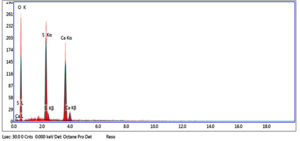
Fig. 14. EDX spectra results of the elongated crystals show the appearance of (K), (S) and (O) elements which may confirm that it is potassium sulphate salt elements which may confirm that it is potassium sulphate salt.
Biodegradation is a genuine danger to social legacy, which needs the use of a successful method to recognize the microorganisms associated with this cycle and to survey their biodegradation.23 Their organic assault occurs at an ideal temperature and relative humidity for the development of microorganisms and spores on the base. Every colonizer specialist has various ways of giving and taking the construction in capacity of the substrates.26 In this study, we evaluated six essential oils, namely; Thyme (Thymus vulgaris), clove (Syzygium aromaticum), and cinnamon (Cinnamomum zeylanicum), garlic (Allium sativum), castor (Ricinus communis), olive (Olea europaea) for controlling the biodegradation.
Samples and site description
Samples for the study were taken from different parts of the archaeological gypsum window according to scientific and non-destructive methods. The sample was taken from the Qmarea under study, which consists of two rectangular openings crisscrossed by a refracted arch and topped by a hexagonal opening. The Qmarea holes from the inside have stained glass intertwined in gypsum (Fig. 2, 8).
Also, Scanning Electron Microscopy (SEM-EDX) was used with samples of gypsum. The morphology and microstructure of the mineral constituents in the gypsum samples were recorded with a SEM (Model Quanta FEG 250 attached with EDX unit; Energy Dispersive X-ray Analyses). Deteriorated gypsum sample was taken from a gypsum window inside the Nujoumi dome. Scanning electron micrograph showing damage to gypsum crystals and loss of bonding materials in addition to found fungi. Infection between grains, fungi between minerals crystals of gypsum, and fungal hyphae was found between the minerals crystals. Results from the gypsum samples studied are presented in Fig. 11, 13 and Table 1. In addition to degradation observed in gypsum crystals, loss of the binding materials, we detected an increase in the size of stone pores, cracks, and loss in the interior structure as a consequence of internal pressures among the mineral crystals due to re-crystallization of the salt inside stone pores. A fungal infection was also observed. Samples showed that they essentially consist of calcium (Ca), silicon (Si), aluminium (Al), carbon (C) and oxygen (O) carbon (Fig. 14). From simple walls and domes through decorative architectural pieces, complex decorations, and gypsum windows to the most magnificent figurative models, gypsum has been decorating buildings for centuries.27
Table (1):
The identification minerals: analysis table of gypsum window.
Element |
Weight % |
Atomic % |
Net Int. |
Error % |
|---|---|---|---|---|
O K |
61.74 |
78.52 |
80.58 |
11.35 |
S K |
16.22 |
10.29 |
132.47 |
4.07 |
CaK |
22.04 |
11.19 |
113.8 |
4.14 |
Samples were collected from Nujoumi Dome in Aswan showing signs of microbial infection, including fragments from gypsum windows and Interior wall plaster. Sampling was carried out by two methods: (a) taking fragments by sterilized cotton swabs from the surface of infected gypsum windows under aseptic conditions, kept in a sterile bag until used directly for inoculation. (b) using a sterile spatula to scratch the surface of infected plaster and transferring it directly onto prepared agar media.
The organic examples were taken by sterile cotton trade and brooded for 7 days; the secluded growths were distinguished by morphological and spore structures.28
Isolation of Fungi and bacterial isolates from Nujoumi Dome
Four isolates of fungi and two of bacteria were collected from different sites of archaeological gypsum windows were studied on samples taken from the Nujoumi Dome of Aswan,in March 2019 by using the sterile cotton swap method. Samples were taken from gypsum and also fragments collapsed from wall surfaces in the investigation. After serial dilution of samples, they were inoculated on nutrient agar and potato dextrose agar and incubated for five days at 30°C.purified of isolated colonies were kept at 4°C for identification in the identification unit of the Regional Center of Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt.
Essential oils
Six essential oils were purchased from a commercial store for agricultural seeds, spices, and medical plants in Cairo, Egypt: thyme (Thymus vulgaris), clove (Syzygium aromaticum), cinnamon (Cinnamomum zeylanicum), garlic (Allium sativum), castor (Ricinus communis), and olive (Olea europaea). The six essential oils were directly used without any kind of extraction or treatments. Different concentrations of each essential oil were prepared and applied for their antimicrobial activity against fungal and bacterial isolates in both Potato Dextros Agar (PDA) and Nutrient Agar (NA) media, respectively. Media and cultivation conditions were prepared as described before.29
Potato dextros agar PDA and Tween-20
PDA (Sigma-Aldrich, pH; 5.6±0.2 at 25°C, agar; 15 g/L, dextrose; 20 g/L, potato extract; 4 g/L) was used as a medium for germinating the isolated fungi.
Nutrient agar (NA)
NA (Sigma-Aldrich, used for bacterial isolates. A nutrient agar medium was used for bacterial growth [Beef Extract, 3.0g; Bacteriological Peptone, 5.0g; Agar, 20.0g]. The pH was adjusted to 6.2 ± 0.2 at 25 (±2)°C. Tween-20 ((Sigma-Aldrich, Germany, lauric acid; ≥40% ‘‘balance primarily myristic, palmitic, and stearic acids’’; impurities; ≤3.0% water, CMC; 0.06 mM’’20-25°C’’, refractive index; n20/D 1.468 lit., density; 1.095 g/mL at 25°C, lit., acid number; ≤2.2 mg/g, hydroxyl value; 96 108 mg/g, transition temp; cloud point of 76°C, HLB; 16.7 to dissolve the plant oil in the media.
Preparation of disc for antimicrobial activities
The sterile blotting paper disc (5 mm) was soaked in the essential oils. The prepared disc was dried at a controlled temperature to remove the excess of solvent. The modified paper disc diffusion30 was employed to determine the antimicrobial activity of the aqueous extract of the herbal preparations.
An inoculum was spread over the agar plate using a sterile cotton swab in order to obtain uniform microbial growth. Then the prepared antimicrobial discs were kept over the lawn and pressed slightly along with control. Griseofulvin and Streptomycin 10 μg/discs were used as positive controls (slandered) for antifungal and antibacterial purposes, respectively. The plates were incubated for 48 h at 37°C. The antimicrobial activity was evaluated, and the diameter of the inhibition zones was measured. The experiment was carried out in triplicate and the average diameter of the zone of inhibition was recorded. The antibacterial activity was classified as highly active (>10 mm), mildly active (7-10 m) and slightly active (6-7 mm), and less than 6 mm was taken as inactive.31
Four fungal species were isolated and identified as Aspergillus japonicas, Aspergillus terrus, Penicillium commune, and Cladosporium elatum, while two species of bacteria were isolated and identified as Bacillus cereus and Listeria monocytogenes, isolated from the gypsum samples of Nujoumi Dome.
Effect of essential oils on microbial strains
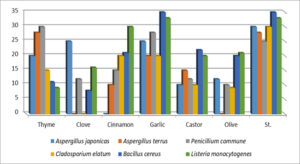
The results of the antifungal and antibacterial activities of the six essential oils on all isolates were reported in table (2 A. japonicas in agar plates demonstrated the high efficacy of clove and garlic oil (25 mm inhibition zone diameter), followed by them (20 mm), olive (12 mm), and castor oil (10 mm), whereas cinnamon oil did not inhibit the growth of A. japonicas when compared to the standard (Griseofulvin), which showed 30 mm inhibition of fungal growth.
Table (2):
Microbial inhibition zone (mm) of plant essential oils.
Thyme |
Clove |
Cinnamon |
Garlic |
Castor |
Olive |
St. |
|
|---|---|---|---|---|---|---|---|
Aspergillus japonicas |
20 |
25 |
00 |
25 |
10 |
12 |
30 |
Aspergillus terrus |
28 |
00 |
10 |
20 |
15 |
00 |
28 |
Penicillium commune |
30 |
12 |
15 |
28 |
12 |
10 |
25 |
Cladosporium elatum |
15 |
00 |
20 |
20 |
10 |
09 |
30 |
Bacillus cereus |
11 |
08 |
21 |
35 |
22 |
20 |
35 |
Listeria monocytogenes |
09 |
16 |
30 |
33 |
20 |
21 |
33 |
The maximum growth inhibition was 28 mm in the case of thyme oil against A. terrus with the same results as griseofulvin, followed by garlic, castor, and cinnamon oils, which recorded 20, 15, and 10 mm inhibition, respectively, as compared to clove and olive oil, which did not show any ability to inhibit the growth of the same fungus species.
P.Commune growth was the most sensitive to thyme and garlic oils (30 and 28 mm inhibition zones, respectively), and these results were better than griseofulvin 25 mm inhibition (Table 2).15 mm inhibition of P. commune, followed by 12 mm inhibition by clove and castor oils, and 10 mm inhibition by olive oils.
The results of both oils of garlic and cinnamon showed the same effect on the growth of Cladosporium elatum (20 mm inhibition zone), while 15, 10, and 9 mm diameters of inhibition of C. elatum were recorded by oils of thyme, castor, and olive, respectively, as compared to 30 mm inhibition by standard griseofulvin. While clove oil did not show any effect on the C. elatum growth (Fig. 15).
Table 2 showed great antibacterial potency of garlic oil against two bacterial isolates used, whereas 35 mm inhibition of Bacillus cereus was observed, which is the same effect as Streptomycin a Standard as antibiotic. On the other hand, castor, cinnamon, olive, thyme, and clove recorded 22, 21, 20, 11, and 08 mm inhibition of bacterial growth.
The same garlic oil showed the same effect as streptomycin on the growth of Listeria monocytogenes (33 mm inhibition zone). followed by cinnamon oil, which showed a 30 mm inhibition. While 21, 20, 16, and 09 mm inhibited the growth of Listeria monocytogenes by olive, castor, clove, and thyme oils, respectively (Table 2 and Fig. 15).
Many researchers have used essential plant oils as antimicrobials, such as32 that the components of essential oils can be used in prevailing microbial growth due to their biodegradability, cost-effectiveness, environmental compatibility, non-toxicity, antimicrobial, antifungal, and antioxidant properties. Essential oils (EOs) were an excellent alternative to commercial biocides, with significant potential in the field of cultural heritage conservation.19 EOs have been tested in vitro against fungal species isolated from royal tomb paintings at Tanis, Egypt,17 documentary heritage, Cuban and Argentine.26 Some unexplored or less explored plants also offer some antibacterial, bactericidal, and antioxidant properties. The antibacterial and bactericidal effects of extracted essential oils (EEOs) were tested in comparison with standard antibiotics.33 It has been reported that the essential oils of Pimpinella anisum, Origanum vulgare, and Allium sativum showed the best antifungal efficiency against four fungal strains (Aspergillus clavatus, A. niger, Penicillium sp. and Fusarium sp.) isolated from Argentine and Cuban documentary heritage; whereas, Origanum vulgare oil suppresses mycelia growth and sporulation of Aspergillus, Penicillium, and Fusarium sp.34
Likewise, it has been reported that O. vulgare EO can inhibit the growth of conidia of Aspergillus and Penicillium species isolated from the divider works of art of Gradac Cloister, Serbia.35 Past work recorded the high antifungal action of five EOs in Regal Burial Chamber Paint at Tanis, Egypt.36 Past studies thought about the antifungal activities of fundamental oil in contrast to seven Aspergillus species secluded from an alternate substrate (stone, block, silk, and paper) of social legacy objects in Serbia.37 They tracked down that the EO displayed more grounded against Aspergillus movement with morpho-physiological changes (absence of sporulation, depigmentation of conidiogenous contraption and conidia, and the presence of deviant contagious constructions). The advancement of safe strains of parasites against fundamental oils might be more uncertain for what it’s worth for some manufactured fungicides. Given that few dynamic parts are consistently present in EOs, and synergistic connections between the various parts of the oils may exist.38
Previous work investigated the antimicrobial efficiency of cinnamon, peppermint, clove, lavender, and thyme essential oil against Streptomyces rochei and Bacillus safensis which were isolated from deteriorated archaeological limestone from seven historic Egyptian sites (Giza pyramid complex and related tombs, Seti I tomb at Luxor, Senusret I obelisk of Al Mattaryia district, store of the National Museum of Egyptian Civilization, Roman Amphitheatre of Alexandria, Mosque of Elkadi Abd El Basset, and Ismailia Museum of Antiquities), Egypt.39 Plant EOs antimicrobial efficacy was due to the presence of bioactive constituents.36
Identifying the active antimicrobial components of essential oils is complicated due to the fact that essential oils contain complex mixtures of up to 45 different constituents, whereas the composition of a certain EO may change depending on the methods used to evolve the oil, the season of harvest, and the environmental conditions (weather, day length, water, sunlight, nutrients, pressure, soil type, nutrients, and diurnal fluctuation). Essential oil components are multiple families of low molecular weight organic compounds with different variations in antimicrobial activities.38
Previous work reported that Cinnamomum cassia Presl, Thymus mongolicus Ronn, and Cymbopogon martini exhibited stronger inhibitory effects and lower minimal inhibitory concentrations (MICs) against Aspergillus carbonarius, Aspergillus flavus, and Penicillium viridicatum due to the active compounds of EOs (trans-cinnamaldehyde, d-limonene, citral, citronellal, trans-geraniol, and carvacrol).40 Some deoxy-glucosides of borneol, carvacrol, citronellal, fenchol, menthol, thymol, and s-perillyl alcohol exhibited remarkable antifungal properties against Fusarium oxysporum, Aspergillus flavus, and A. ochraceus. Previous work isolated Aspergillus niger from a Roman mural painting in Italy and evaluated the essential oil constituents as preservation products. Thymol, eugenol, and cinnamaldehyde, which are the major component of thyme, clove, and cinnamon oil, respectively, provided a constant and strong inhibition effect.19
The constituents of EOs can be used for middle and long-term protection applications. This makes them available for use in the future development of long-lasting preservation products. The essential oils and their components have distinct targets, including the membrane and cytoplasm, which perfectly change the morphology of the cells.37 The antimicrobial efficiency of Eos might be caused by the attributes of terpenes/terpenoids which are highly lipophilic in nature and low molecular weight, and control the germination and sporulation of fungi, causing cell death.41 The antifungal agents may deactivate the fungal cells by disrupting the function and structure of membranes or organelles of the fungal cell and suppressing the nuclear material or protein creation.42 Most of the tested plants contain medicinal values that are utilised in artificial medicines and to cure emerging diseases.33
The phenolic constituents of EOs can be related to their antimicrobial efficiency. The terpenoids are dynamic compounds against a diverse spectrum of microorganisms and the most active monoterpenoids are carvacrol and thymol.43 The antimicrobial capacity of terpenoids was evaluated against Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, and Listeria monocytogenes, whereas the most active component was carvacrol, followed by thymol.41
Thyme oil (Thymus vulgaris)
The most efficient compounds in thyme oil are terpenoids. Terpenoids can be split into epoxides, alcohols, esters, aldehydes, ethers, ketones, and phenols. Terpenoids such as carvacrol, thymol, linalyl acetate, linalool, citronellal, menthol, geraniol, and piperitone showed antimicrobial activities.44 The antimicrobial effectiveness of terpenoids is depends on their functional groups, the presence of delocalized electrons and the hydroxyl group of phenolic terpenoids. A previous study found that thyme oil has antibacterial activity at a very low concentration (0.5%) against rock colonizing bacteria that were isolated from different archaeological limestone objects, Egypt. Thymol, a phenolic monoterpenoid, is the major ingredient of thyme oil.20 Thymol’s structure is very similar to that of carvacrol, which has the hydroxyl group in an inconstant position on the phenolic ring.
The antimicrobial activities of phenolic compounds (thymol and carvacrol) were due to the structural and functional damage of the cytoplasmic membrane. The primary mode of action of thymol is considered to participate in inner and outer membrane disruption as well as interaction with intracellular targets and membrane proteins. When thymol collaborates with cell membranes, it affects membrane permeability, which leads to a lack of membrane potential with outflows of Adenosine 5′-triphosphate (ATP), carboxyfluorescein and potassium ions. The potency of cells to improve after exposure to thymol is very low because thymol affects critical energy-generating processes. Thymol damages ergosterol biosynthesis as well as disrupted vesicles and cell membranes in Candida strains because ergosterol regulates membrane asymmetry and fluidity.45
Clove oil (Syzygium aromaticum)
Previous study found that clove oil has antibacterial and antifungal activities when used against six bacterial species (Bacillus polymyxa, Bacillus cereus, Bacillus thuringiensis, Bacillus sp., Enterobacter agglomerans, and Streptomyces sp.) and four fungal species (Aspergillus niger, Aspergillus clavatus, Penicillium sp., and Fusarium sp.) which isolated from different documentary supports and indoor environments of repositories of the National Archive of the Republic of Cuba and Historical Archive of the Museum of La Plata, Argentina.34 The antibacterial activity of clove oil has been shown against pathogenic bacteria including Campylobacter jejuni, Escherichia coli, Salmonella enteritidis, and Staphylococcus aureus.46 The clove oil exhibited potent antibacterial efficacy against Staphylococcus aureus, and Bacillus cereus.47 Clove oil exhibited significant inhibition against 32 multi-resistant Staphylococcus epidermidis strains.48
Past work showed that the most minimal centralization of eugenol as the principal constituent of clove oil has a huge hindrance against a wild strain of Aspergillus niger that was gathered from a painting saved in the cellar of the Ariadne House (Archaeological Site of Pompeii), Italy.19 It has been shown that clove oil displays a solid fungicidal action by diminishing organisminoculum size.49 Clove oil and its principal part, eugenol, have been considered as regular fungicides against Botrytis cinerea, Penicillium expansum, Aspergillus niger and A. flavus.50
Garlic oil (Allium sativum)
It has been observed that garlic oil shows a highly inhibitory effect against Escherichia coli.51 Previous work suggested that possible use of natural products from plants in the control of biodeterioration of documentary heritage, where the garlic oils showed the best antifungal activity against Aspergillus niger, Aspergillus clavatus, Penicillium sp., and Fusarium sp., while the highest antibacterial activity was recorded against Enterobacter agglomerans, Bacillus polymyxa, and Streptomyces sp., which were isolated from repositories air and documents of the National Archive of the Republic of Cuba and the Historical Archive of the Museum of La Plata, Argentina.52
Previously, it was found that the pathogenic fungal and bacterial species were inhibited by garlic bulb extracts (Allium sativum) which is mainly contain allicin that is used as a therapeutic panacea. Several changes are observed in microorganisms (membrane permeability and protein leakage) due to the effect of garlic extract. The antimicrobial activity of allicin may be due to the devastation of the structural solidity of cell membranes, leading to cell death.53 Allicin has a strong smell of garlic and exhibits antiviral, antifungal, antiparasitic, and antibacterial properties.54 Allicin displayed antibacterial efficiency against Gram-negative and Gram-positive bacteria.51
Olive oil (Olea europaea)
Previous work examined the antifungal activity of olive essential oil against Aspergillus niger, Penicillium commune (moulds), Coniophora puteana (brown rot), Trametes versicolor (white rot) and Chaetomium globosum (soft rot), which were isolated from archaeological wood, whereas the olive oil showed a slight to strong growth inhibition, particularly with the brown-rot species (Coniophora puteana).55 Also, it was found that olive oil is composed of a high concentration of monounsaturated fatty acid (oleic acid) with minor compounds (phenolic compounds) which possess antioxidant and antimicrobial activities.56 A previous study showed that olive oil can limit the growth of Pseudomonas fluorescens and Enterobacteriaceae due to the presence of polyphenols that have antimicrobial activities.57
Castor oil (Ricinus communis)
Castor oil is the fatty substance of ricinoleic corrosive, which contains receptive auxiliary hydroxyl bunches.58 It displays a critical microbial hindrance towards the human pathogenic microorganisms. Castor oil was perceived as the best vegetable oil because of its inborn biodegradability, sustainability, minimal expense, and plentiful accessibility.59 Previous research found that Castor EO had a significant inhibitory effect against Bacillus subtilis (Gram-positive microorganisms).17 Likewise, the concentrate of castor created optional metabolites (phenolics, diminishing specialists) and displayed cell reinforcement property.
Cinnamon oil (Cinnamomum zeylanicum)
Cinnamon oil is antimicrobial against both fungal and bacterial species.60 Also, cinnamaldehyde is the major component (50-90%) of cinnamon bark oil, with other minor constituents including eugenol, linalool, and a-pinene.61 It has been shown that cinnamaldehyde inhibited the growth of brown-rot fungus, which is isolated from wood and stone religious artefacts.62
Cinnamaldehyde showed remarkable inhibitory effects against a wild strain of Aspergillus niger that was collected from a mural painting in Pompeii, Italy.19 Cinnamaldehyde is an aromatic aldehyde, and it has been suggested that the carbonyl groups of aldehydes can bind to metal ions, sulfhydryl groups, amino groups, and proteins of the microbial cell.62 Previous studies showed that cinnamaldehyde was effective at its lowest concentration against all bacterial and fungal strains, where it inhibited cells division of fungal cell and inhibited the cell wall synthesizing enzymes in Saccharomyces cerevisiae.63,64
Most of the tested essential oils showed a positive effect against the isolated bacteria and fungi, which can be applied in the future to the treatment of archaeological antiques. The most effective essential oil was garlic essential oil.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All the authors listed have made a considerable direct and intellectual contribution to the work, and accepted it for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during the study are included in the manuscript.
- Allsopp D. Worldwide wastage: the economics of biodeterioration. Microbiol Tod. 2011;38:150-153.
- Forsyth M. Structures and Construction in Historic Building Conservation. Wiley-Blackwell. 2007.
Crossref - Eriksson KEL, Blanchette RA, Ander P. Microbial and enzymatic degradation of wood and wood components. Springer Science & Business Media. 2012.
- Ettenauer J, Pinar G, Sterflinger K, Gonzalez-Munoz MT, Jroundi F. Molecular monitoring of the microbial dynamics occurring on historical limestone buildings during and after the in situ application of different bio-consolidation treatments. Sci Total Environ. 2011;409(24):5337-5352.
Crossref - Pinar G, Sterflinger K. Microbes and building materials. In: Cornejo DN, Haro JL (eds) Building materials: properties, performance and applications. Nova Science Publishers, New York. 2009:163-188.
- Saarela M, Alakomi HL, Suihko ML, Maunuksela L, Raaska L, Mattila-Sandholm T. Heterotrophic microorganisms in air and biofilm samples from Roman catacombs, with special emphasis on actinobacteria and fungi. Int Biodeterior Biodegrad. 2004;54(1):27-37.
Crossref - Steiger M, Charola AE, Sterflinger K. Weathering and deterioration. In: Siegesmund S, Snethlage R (eds) Stone in architecture. Springer, Berlin. 2011:291-304.
Crossref - Sterflinger K. Fungi as geologic agents. Geomicrobiol J. 2000;17(2):97-124.
Crossref - Urzi C. Microbial deterioration of rocks and marble monuments in the Mediterranean basin: a review. Corros Rev. 2004;22(5-6):441.
Crossref - Bastian F, Alabouvette C. Lights and shadows on the conservation of a rock art cave: the case of Lascaux cave. Int J Speleol. 2009;38(1):55-60.
Crossref - Elkhateeb AA. Domes in the Islamic Architecture of Cairo City: A Mathematical Approach. Nexus Netw J. 2012;14(1):151-176.
Crossref - Tarrad M, Matrouk M. The Dome in Islamic Architecture and The Contemporary Orientations to The Design of Mosques’ Domes. in Proceedings of the International Congress. Domes in The World. 2012.
- Scheerer S, Ortega-Morales O, Gaylarde C. Microbial deterioration of stone monuments-an updated overview. Adv Appl Microbiol. 2009;66:97-139.
Crossref - Pepe O, Sannino L, Palomba S, et al. Hetrotrophic microorganisms in deteriorated medival wall painting in southern Italian churches. Microbiol Res. 2010;165(1):21-33.
Crossref - Garg KL, Mishra KK, Jain A. Role of fungi in the deterioration of wall paintings. Sci Total Environ. 1995;167(1-3):255-271.
Crossref - Macedo MF, Miller AZ, Dionisio A, Saiz-Jimenez C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin. Microbiology. 2009;155(11):3476-3490.
Crossref - Burt S. Essential oils: their antimicrobial properties and potential application in foods-a review. Int J Food Microbiol. 2004;94(3):223-253.
Crossref - Gomez-Alarcon G, Munoz ML, Flores M. Excretion of organic acids by fungal strains isolated from decayed sandstone. Int Biodeter Biodegr. 1994;34(2):169-180.
Crossref - Veneranda M, Blanco-Zubiaguirre L, Roselli G, Di Girolami G, Castro K, Madariaga JM. Evaluating the exploitability of several essential oils constituents as a novel biological treatment against cultural heritage biocolonization. Microchemical Journal. 2018;138:1-6.
Crossref - Cattelan MG, de Castilhos MBM, Sales PJP, Hoffmann FL. Antibacterial activity of oregano essential oil against foodborne pathogens. Nutr Food Sci. 2013;43(2):169-174.
Crossref - Villa F, Giacomucci L, Toja F, et al. Degradation of nitrocellulose-based paint by Desulfovibrio desulfuricans ATCC 13541. Biodegradation. 2012;23(5):705-716.
Crossref - Abed el Hamid H, Darwish SS, Bahaa A. Microbial studies to evaluate biodeterioration of oil painting and its prevention. Journal of the General Union of Arab Archaeologists. 2010;11:102-128.
- Kim MK, Ingremeau F, Zhao A, Bassler B, Stone H. Local and global consequences of flow on bacterial quorum sensing. Nat Microbiol. 2016;1:15005.
Crossref - Akthar M S, Degaga B, Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms. A review. Biological Sciences and Pharmaceutical Research. 2014;2(1):001-007.
- Ali M, Ali M, Darwish S, et al. Investigation and conservation of El-Shenawy palace photographic collection in Mansoura, Egypt. Mediterranean Archaeology and Archaeometry. 2015;15(3):165-185.
- Borrego S, De Saravia SG, Valdes O, Vivar I, Battistoni P, Guiamet P. Biocidal activity of two essential oils on Fungi that cause biodeterioration of paper documents. International Journal of Conservation Science. 2016;7(2)369-380.
- Ireland R. Conserving Decorative Plaster. The Building Conservation Directory. Cathedral Communications Limited. 2005:1-11.
- Khalaphallah R, El-Derby A. The effect of nano-TiO2 and plant extracts on microbial strains isolated from Theban ancient Egyptian royal tomb painting. Afr J Microbiol Res. 2015;9(21):1424-1430.
Crossref - Atlas RM. Handbook of microbilology media. In Laurence C. and Parks, L.c. eds. Paks, CRC press. 1993:278,538,785.
- Delignette-Muller ML, Flandrois JP. An accurate diffusion method for determining bacterial sensitivity to antibiotics. Antimicrob Chemother. 1994;3(1):73-81.
Crossref - Chandra R, Dwivedi V, Shivam K, Jha KA. Detection of antimicrobial activity of Oscimum sanctum (Tulsi) and Trigonella foenum graecum (Methi) against some selected bacterial and fungal strains. Res J Pharm Biol Chem Sci. 2011;2(4):809-813.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils -a review. Food Chem Toxicol. 2008;46(2):446-475.
Crossref - Zaman, Gaffar Sarwar, et al. Screening of the antioxidant and antibacterial effects of extracted essential oils from Thunbergia coccinea, Acacia polyacantha, Polygonum micrpcephallum, Abies spectabilis and Clerodendrum colebrookianum. Cell Mol Biol. 2021;67(4).
Crossref - Borrego S, Valdes O, Vivar I, et al. Essential Oils of Plants as Biocides against Microorganisms Isolated from Cuban and Argentine Documentary Heritage. International Scholarly Research. 2012;2012:826786.
Crossref - Stupar M, Grbic MLJ, Dzamic A, et al. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. South African Journal of Botany. 2014;93:118-124.
Crossref - Stupar M, Grbic ML, Simic GS, Jelikic A, Vukojevic J, Sabovljevic M. A sub-aerials biofilms investigation and new approach in biocide application in cultural heritage conservation: Holly Virgin Church (Gradac Monastery, Serbia). Indoor and Built Environment. 2014;23(4):584-593.
Crossref - Helmi F, Elmitwalli H, Rizk M, Hagrassy A. Antibiotic extraction as a recent biocontrol method for Aspergillus Niger and Aspergillus Flavus fungi in ancient Egyptian mural paintings. Mediterranean Archaeology and Archaeometry. 2011;11(2)1-7.
- Elsayed Y, and Shabana Y. The effect of some essential oils on aspergillus niger and Alternaria alternata infestation in archaeological oil paintings. Mediterranean Archaeology and Archaeometry. 2018;18(3):71-87.
- Sterflinger K. Fungi: Their role in deterioration of cultural heritage. Fung Biol Rev. 2010;24(1-2):47-55.
Crossref - Wang W, Ma Y, Ma X, et al. Diversity and seasonal dynamics of airborne bacteria in the Mogao Grottoes, Dunhuang, China. Aerobiologia. 2012;28(1):27-38.
Crossref - Ma Y, Zhang H, Du Y, et al. The community distribution of bacteria and fungi on ancient wall paintings of the Mogao Grottoes. Sci Rep. 2015;5:7752.
Crossref - Noshyutta W, Osman E, Mansour M. An investigation of the biological fungicidal activity of some essential oils used as preservatives for a 19th century Egyptian Coptic cellulosic manuscript. International Journal of Conservation Science. 2016;7(1):41-56.
- Ogbulie JN, Obiajuru IO. Microbial deterioration of surface paint coatings. Global Journal of Pure and Applied Sciences. 2004;10(4):485-490.
Crossref - Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological efects of essential oils – a review. Food Chem Toxicol. 2008;46(2):446-475.
Crossref - Dal Sasso M, Culici M, Braga PC, Guffanti EE, Mucci M. Thymol inhibitory activity on Escheichia coli and Stphylococcus aureus adhesion to human vaginal cells. J Essent Oil Res. 2006;18(4):455-461.
Crossref - Chaib K, Hajlaoui H, Zmantar T, et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae).: a short review. Phythoter Res. 2007;21(6):501-506.
Crossref - Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308-316
Crossref - Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complementary and Alternnative Medicine. 2006;6:39.
Crossref - Nunez L, D’Aquino M, Chirife J. Antifungal properties of clove oil (Eugenia caryophylata) in sugar solution. Braz J Microbiol. 2001;32(2):123-126.
Crossref - Pathirana HNKS, Wimalasena SHMP, Silva BCJ, Hossain S, Gang JH. Antibacterial activity of clove essential oil and eugenol against fish pathogenic bacteria isolated from cultured olive flounder (Paralichthys olivaceus). Slov Vet Res. 2019;56(1):31-38.
Crossref - Pehlivan M, Sevindik M. Antioxidant and antimicrobial activities of Salvia multicaulis. Turkish Journal of Agriculture Food Science and Technology. 2018;6(5):628-631.
Crossref - Karuppiah P, Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac J Trop Biomed. 2012;2(8):597-601.
Crossref - Chen TI, Kuo, CM, Hong JW, Chou RL, Lee YH, Guo JJ. The effects of garlic-supplemented diets on antibacterial activities against Photobacterium damselae subsp. piscicida and Streptococcus iniae and on growth in Cobia. Rachycentron Canadum Aquaculture. 2015;435:111-115.
Crossref - Lanzotti V, Barile E, Bonanomi G, Antignani V, Scala F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera Phytochemistry. 2012;78:126- 134.
Crossref - Medina E, de Castro A, Romero C, Brenes M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J Agric Food Chem. 2006;54(14):4954-4961.
Crossref - Thielmann J, Kohnen S, Hauser C. Antimicrobial activity of Olea europaea Linne extracts and their applicability as natural food preservative agents. Int J Food Microbiol. 2017;251:48-66.
Crossref - Khan I, Bahuguna A, Kumar P, Bajpai VK, Kang S C. Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front Microbiol. 2017;8:2421.
Crossref - Hussain AI, Anwar F, Sherazi STH, Przybylski R. Chemical composition: Antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108(3):986-995.
Crossref - Zarai Z, Ines BC, Riadh BM, et al. Essential oil of the leaves of Ricinus communis L.: In vitro cytotoxicity and antimicrobial properties. Lipids in Health and Disease. 2012;11:102.
Crossref - Mith H, Dure’ R, Delcenserie V, Zhiri A, Daube G, Clinquart A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci Nutr. 2014;2(4):403-416.
Crossref - Frankova A, Marounek M, Mozrova V, Weber J, Kloucek P, Lukesova D. Antibacterial Activities of Plant-Derived Compounds and Essential Oils toward Cronobacter sakazakii and Cronobacter malonaticus. Foodborne Pathog Dis. 2014;11(10):795-779.
Crossref - Chaudhari LK, Jawale BA, Sharma S, Sharma H, Kumar CD, Kulkarni PA. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J Contemp Dent Pract. 2012;13:71-74.
Crossref - Gupta A, Duhan J, Tewari S, et al. Comparative evaluation of antimicrobial efficacy of Syzygium aromaticum, Ocimum sanctum and Cinnamomum zeylanicum plant extracts against Enterococcus faecalis. A preliminary study. Int Endod J. 2013;46(8):775-783.
Crossref - Miller AB, Cates RG, Lawrence M, et al. The antibacterial and antifungal activity of essential oils. Pharm Biol. 2015;53(4):548-554.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.