Antibiotic overuse in animal and human healthcare has led in the accumulation of potentially hazardous antibiotic residues, known as emerging contaminants. These residues contaminate animal products including meat, milk, and eggs, which humans then ingest. Furthermore, antibiotic residues from pharmaceutical firms, hospitals, and households reach wastewater treatment plants, providing an environment conducive to bacterial growth and dissemination. This, in turn, can result in the spread of antibiotic resistance genes (ARGs) among bacterial cells, posing serious threats to both human health and the environment. In the case of ARGs, conventional approaches for eliminating antibiotic residues from wastewater and aquatic habitats have proven ineffective. Recent study, however, has shown that the adsorption technique, particularly when low-cost and environmentally acceptable bioadsorbents such as sawdust, prawn shell waste, algae, and fungi are used, is highly successful in removing antibiotic residues. Bioadsorbents Microalgae, Terminalia catappa leaf, and siris seed pods, in particular, have shown outstanding removal efficiency for antibiotics such as tetracycline, dicloxacillin, and nitromidazole, reaching up to 98.74%. These investigations have shed insight on the fundamental principles of the adsorption process, revealing its ability to target ARGs and antibiotic-resistant bacteria as well as remove antibiotic residues. As a result, addressing the issue of antibiotic residues in the environment has become critical in order to protect human health and prevent the spread of antibiotic resistance. Adsorption, particularly when bioadsorbents are used, appears to be a promising and efficient method of combating antibiotic residues and limiting the spread of antibiotic resistance genes and antibiotic-resistant bacteria in aquatic settings.
Antibiotic Residues, Aquatic Environment, Bio Adsorbents, Adsorption, Emerging
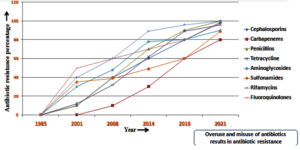
Antibiotics play a crucial role in preventing and treating infectious diseases in both humans and animals, while also being widely present in the environment. Their use as growth promoters in livestock is well-documented.1-4 The most commonly prescribed antibiotic classes, including Beta-lactams, fluoroquinolones, tetracyclines, macrolides, sulfonamides, and cephalosporins, have been identified.5,6 Notably, there has been a significant 91% increase in antibiotic consumption from 1985 to 2021, with India and China exhibiting the highest consumption rates, primarily involving cephalosporins and tetracyclines7-10 (Figure 1). This surge can be attributed to population growth, rapid urbanization, and the emergence of infectious diseases.11 Tetracyclines are particularly prevalent in global animal production.12,13 Furthermore, the majority of administered antibiotics are excreted, contributing to their release and potential environmental impact. Proper disposal of unused or expired antibiotics is crucial to mitigate the environmental antibiotic burden.12
Antibiotic residues are found in various environmental hotspots such as sewage, hospitals, livestock farms, aquaculture farms, and pharmaceutical industries.14 These residues are detected in municipal waste effluents, surface water, groundwater, drinking water, soil, sediments, and even in sewage sludge and manure-filled soils.15 Residual antibiotics are also detected in sewage sludge; animal manure and manure-filled soils.15 The presence of antibiotics in the environment contributes to the development of antibiotic-resistant bacteria and genes, posing risks to human and animal health.16,17 Antibiotic-resistant strains are increasingly prevalent.18 Remediation of antibiotic residues in wastewater is crucial, and adsorption processes using specific adsorbents or bioadsorbents have been considered highly efficient for removing antibiotics from aqueous environments.19,20 Factors like pH, ionic strength, temperature, and organic matter influence the adsorption process, and the structure and functional groups of antibiotics play a significant role.19,21 Adsorption mechanisms involve intermolecular forces and interactions, making it a simpler and less time-consuming method for remediation.22,23 While adsorption is widely recognized as an important mechanism for antibiotic removal, further in-depth analysis is needed.23 Overall, understanding the adsorption of antibiotics and their residues can provide valuable insights into their interactions with adsorbents and bioadsorbents, contributing to effective wastewater treatment and pollutant reduction.
Occurrences of Antibiotic Residues
Pharmaceutical, municipal, and hospital wastewater contain high concentrations of antibiotic residues.24-26 Antibiotics are essential components of modern medications used to treat infections caused by diverse bacteria.27 Overuse, improper usage, and discharge of antibiotics contribute to adverse environmental effects by facilitating their release into the environment.
Antibiotic residues, including both mother compounds and metabolites, can accumulate in various cells, tissues, organs, and edible products, posing risks to human and animal health.28-30 These residues are often detected in wastewater treatment plants (WWTPs) and subsequently discharged into water bodies, contributing to the dissemination of antibiotic-resistant bacteria and genes in the environment Sulfamethoxazole and ciprofloxacin, Diclofenac were the highest resistant antibiotics present in the municipal WWTP. 31,32,33 Environmental risk assessments have indicated that fluoroquinolones and macrolides pose potential risks to the environment and the development of antibiotic resistance.34 The accumulation of antibiotics and antibiotic-resistant microorganisms in plants, water, and the human gut is a concern associated with WWTPs.35,36 Antibiotic residues in food and water can lead to various adverse effects, including the transmission of antibiotic-resistant bacteria, autoimmune diseases, cancer, reproductive disorders, and hepatotoxicity.37,38
To address these issues, the development of new technologies and techniques for the efficient remediation of antibiotic residues in wastewater is necessary.36,39 Increased awareness, education, and responsible use of antibiotics are essential in preventing the spread of antibiotic residue pollution.40
Rapid screening processes and the utilization of adsorption techniques can aid in monitoring and removing antibiotic residues from wastewater.41 Overall, comprehensive strategies are needed to mitigate the environmental impact of antibiotic residues and promote safe water and food consumption.
Impact of antibiotic residues on environment, human & animal health
Antibiotics, whether synthetic, natural, or semi-synthetic, possess the ability to kill bacteria or impede their growth.42 However, the presence of antibiotic residues in the environment, even at low concentrations, has raised concerns about the transmission of antibiotic resistance and adverse health effects, especially for vulnerable populations. 43,44
Antibiotic residues in wastewater are classified as F-listed and K-listed pollutants, originating from pharmaceutical industries, hospitals, municipalities, and veterinary sources. 17,45 These residues can accumulate in edible plant tissues, as plants lack excretory systems, potentially surpassing maximum residue limits,45 therefore antibiotic residues accumulate in edible plant tissues and can exceed the normal Maximum Residue Limit (MRL) value.
Research has indicated that antibiotics possess genotoxic properties, as demonstrated by animal and microbial assays such as the SOS chromotest on Escherichia coli and the Ames test on Salmonella species.46 Higher plants and animal models like zebrafish have been employed to assess genotoxicity, revealing effects such as chromosomal aberrations, sister chromatid exchange, and micronucleus formation.34,47 Infact animal models such as zebrafish has also been used to test the genotoxicity of amoxicillin on the model animal zebra fish.48 Another research study has been conducted to determine the concentration of ceftriaxone antibiotic in raw and pasteurized cow milk and its toxicity on zebrafish model.49 In genotoxicity of antibiotic test some chromosomal aberrations, sister chromatid exchange, micronucleus formation and many more are clearly observed.48 For a well evidence, Florfenicol have shown growth inhibition in Lemna minor and Scenedesmus vacuolatus.50 Chloramphenicol and Rifampicin have shown delayed cell growth of human stem cell.51 Ceftriaxone and doxycycline have shown genotoxic as well as cytotoxic effect on human peripheral blood lymphocytes. Penicillin have shown lipid metabolism dysfunction in mice model.52
Development of Antibiotic Resistance
Antibiotics are known as the wonder discoveries of the 20th century. The first discovery of wonder antibiotic penicillin has inspired many scientists for further studies and discoveries of more antibiotics for the treatment and prevention of bacterial diseases. But, now we all are wondering about the antibiotic resistance development in hospitals, the environment and in communities. Antibiotic-resistant bacteria are difficult or more precisely they are impossible to prevent and are becoming increasingly more common and thereby causing a global health crisis.53 New resistance genes are constantly identified and transmitted from one bacterial cell to another by exploiting new resistance mechanisms day by day.53 In recent studies, some researchers reported that human gut microbiome are the ultimate reservoir for potential dissemination of resistance genes from normal flora to pathogens and are termed as gut resistome.54 But the question is how this antibiotic resistance problem is rising day by day in the environment. Conventional mechanical and biological wastewater treatment are not able to remediate all pollutants completely, and therefore these pollutants enter into the surface water bodies along with treated wastewater. Consumption of these water contaminated with antibiotic resistance genes (ARGs), antibiotic resistance bacteria (ARB), antibiotic residues are ultimately transmitted within human and animal bodies.54-56 These ARGs might be diffused extensively by Horizontal gene transfer (HGT)57 as shown in Figure 2. Transmission of these genes impacts the phenotype of bacteria and leads to the failure of drug treatments, thus threatening human health. Previous studies have revealed several kinds of ARGs in livestock farming such as, tetA, tetM, tetG (tetracycline resistance genes)58,59 (,sul1 and sul2 (sulfonamide resistance genes),59 ermB, ermF and mefA (macrolide resistance genes).53 These ARGs, are excreted with livestock feces, flow into the WWTP (wastewater treatment plant) and are finally discharged into the environment.60
Figure 2. Antibiotic resistance genes transmission mechanism among microorganisms via horizontal gene transfer
Remediation of different of antibiotic residues by Bioadsorbents
Several adsorbents and bioadsorbents have been studied for the effective removal of harmful antibiotic residues from wastewater. Different antibiotics exhibit varying adsorption mechanisms due to the involvement of distinct intermolecular interactions (Figure 3).
Figure 3. General Mechanism of bioadsorption process for antibiotic residue remediation from aquatic environment
Tetracycline
Tetracycline, a wide-spectrum antibiotic effective against both gram-positive and gram-negative bacteria, poses a significant concern as its residues are frequently detected in various water systems, including surface water, groundwater, drinking water, and wastewater. Due to its incomplete metabolism and absorption in humans and animals, tetracycline residues persist in aquatic environments.
Coagulation, flocculation, and biodegradation are not efficient methods for eliminating oxytetracycline (OTC).61 Pumice stone was found to have a maximum adsorption capacity of 37.09 mg/g at pH 3, with surface complexation and cation exchange identified as the primary adsorption mechanisms.62 Water hyacinth roots exhibited a removal efficiency of 58.9-84.6%.63 Ceramsite derived from bentonite, red mud, and pine sawdust demonstrated an adsorption capacity of 2.13 mg/g, attributed to electrostatic interaction, hydrophobic interaction, and hydrogen bonding .64 Electrostatic interaction, hydrophobic interaction, hydrogen-bonding were the main reasons for the adsorption mechanism in this study.64 Shrimp shell waste (SSW) as a bioadsorbent exhibited a maximum adsorption capacity of 229.98 mg/g at 55°C, with hydrogen bonds and pi-bonds formed between the antibiotic and SSW bioadsorbent at pH 3.3.65 Iron (III)-loaded cellulose nanofibers showed a maximum adsorption capacity of 294.12 mg/g at pH 7, with surface complexation and interactions such as hydrogen bonding, electrostatic interactions, and Van der Waals forces.66 There are many more adsorbents and adsorbents involved in the remediation of tetracycline from the aqueous environment (Figure 4, Table 1).
Figure 4. Tetracycline adsorption from wastewater by using shrimp shell waste biomass as bioadsorbent
Table (1):
Bioadsorbents for tetracycline remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Tectona grandis Linn. |
62.5 mg/g |
302.27 mg/g |
– |
104 |
Zirconium-modified |
77.2 mg/g |
87.7% |
92.3% |
|
wheat straw |
Hydrogen |
105 |
||
chloride |
||||
(HCl) |
||||
Pennisetum sinese Roxb |
36.161 mg/g |
80% |
44.86 |
106 |
mg/g HCl |
||||
Fe-modified oyster shell |
92% |
89.9% |
HCl |
107 |
Pomelo peel derived |
476.19 mg/g |
454.56 mg/g |
KOH |
98 |
biochar |
||||
Rice husk ash |
8.37 mg/g |
60.93% |
– |
108 |
Scenedesmus almeriensis |
27.09 mg/g |
98.7% |
89% |
109 |
microalgae-bacteria |
NaOH |
|||
consortium |
||||
Spirulina sp. (microalgae)- |
147.9mg/g |
137.8 mg/g |
61% |
110 |
derived biochars |
||||
Water hyacinth |
202.62 mg/g |
210.45 mg/g |
– |
111 |
Human-hair derived high |
210.18 mg/g |
78.94% |
– |
112 |
surface area porous carbon |
||||
material |
Dicloxacillin
Dicloxacillin, a beta-lactam antibiotic belonging to the penicillin class, is widely used for treating infections caused by gram-positive bacteria by inhibiting bacterial cell wall synthesis. Tannin, a low-cost and suitable adsorbent, was employed for the adsorption of dicloxacillin from pharmaceutical wastewater. Tannin, a water-soluble polyphenolic compound with a molecular weight range of 500 to several thousands dalton, was isolated from Terminalia catappa L. leaf samples67 as shown in Figure 5. The study focused on the remediation of dicloxacillin residues, and tannin exhibited a maximum adsorption capacity of 17.28 mg/g at pH 6.0 within 24 hours of contact time.68 Currently, only one study has been conducted on the adsorption of dicloxacillin, indicating the need for further research on the remediation of this antibiotic using other adsorbents(Figure 5, Table 2).68,69 So there is a need for further research on remediation of dicloxacillin by other adsorbents.
Table (2):
Bioadsorbents for dicloxacillin remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Natural zeolite |
1.072 mg/g |
96.7% |
– |
113 |
Tannin from Terminalia catappa |
17.28 mg/g |
98.7% |
HCl |
68 |
leaf |
Ciprofloxacin
Ciprofloxacin is a bactericidal antibiotic belonging to the subclass of Fluoroquinolones. It is used for the treatment of urinary tract infections, sexually transmitted diseases, skin infections, and bone infections, as approved by the FDA.70 These components are present in wastewaters due to the incomplete delivery to the consumer. Everyday, almost 84% of these residues get discharged as incomplete metabolic products in the mentioned wastewaters.70
Remediation studies have investigated the use of different chemical and biological adsorbents. For example, activated carbon derived from banana stalk, an environmentally friendly bioadsorbent, exhibited a maximum monolayer adsorption capacity of 49.7 mg/g at pH 4.5 and 323K through a physical adsorption mechanism71 as shown in (Figure 6, Table 3).
Table (3):
Bioadsorbents for ciprofloxacin remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Dialium guineense seed waste |
120.34 mg/g |
87.6% |
– |
114 |
Wheat bran |
– |
75% |
– |
115 |
Corn cob |
13.76 mg/g |
56.3% |
– |
116 |
Rice husk |
2.33 mg/g |
59.7% |
– |
117 |
photocatalytic hydrogel layer |
93.5 mg/g |
– |
– |
118 |
supported on alkali modified |
||||
straw fibers |
||||
Enteromorpha prolifera |
21.7 mg/g |
35.4% at 2.0 g/L |
– |
119 |
bioadsorbent |
||||
dosage |
||||
Gibberella fujikuroi |
39.17 mg/g |
53.74% |
76.57% |
120 |
NaCl |
Meropenem
Meropenem is a novel antibiotic used to treat severe infections of the skin, stomach, bacterial meningitis, pneumonia, sepsis, and intra-abdominal infections.72 Administered via intravenous infusion, meropenem is classified as an intravenous beta-lactam antibiotic and has been approved by the FDA for the prevention and treatment of complicated urinary tract infections.73,74,75 However, extensive use of meropenem has led to the development of resistance in most bacteria, posing challenges for its complete removal from wastewater treatment plants. As a result, residues find their way into rivers, lakes, seas, and ultimately into drinking water and food sources.73,76 Another research study found that ligno-cellulosic bioadsorbents derived from sawdust waste exhibited a 92% removal efficiency for meropenem, which increased to 96% after post-treatment (Figure 7, Table 4).77 Further research is needed due to the importance of meropenem as the last line of defense for treating severe bacterial infections.
Figure 7. Meropenem adsorption from wastewater by using chemically modified saw dust as bio adsorbent
Table (4):
Bioadsorbents for meropenem remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Lignocellulosic bioadsorbent |
231.29 mg/g |
96% |
92.4% |
121 |
derived from sawdust waste |
||||
Rice husk functionalized with |
43.5 mg/g |
– |
– |
122 |
Mg/Fe-layered double hydroxides |
Ceftazidime
Ceftazidime is a broad-spectrum bactericidal antibiotic from the third generation of cephalosporins, effective against many gram-negative and some gram-positive bacteria. The presence of residues of ceftazidime and other antibiotics in aquatic environments has been linked to excessive production and usage, leading to concerns about the persistence and emergence of antibiotic resistance genes. Studies have identified the presence of ceftazidime in pharmaceutical, hospital, and municipal wastewaters, highlighting the need for effective removal methods. Research has shown that the ceftazidime-tolerant green algae Chlorella pyrenoidosa can act as an efficient bioadsorbent, achieving a maximum adsorption capacity of 98.34%.43,78 The functional groups on the surface of the algal cells, such as amino, hydroxyl, and carboxyl groups, play a role in the adsorption mechanism. The dead algal cells exhibited a high removal efficiency of 99.20%, with electrostatic interactions and hydrogen bonding contributing to the process (Figure 8, Table 5).78
Figure 8. Ceftazidime adsorption from wastewater by using microalgal biomass of Chlorella pyrenoidosa as bio adsorbent
Table (5):
Bioadsorbents for ceftazidime remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Chlorella pyrenoidosa |
98.4% |
– |
87.6% NaOH |
78 |
Moringa oleifera |
121.95 mg/g |
87.65% |
– |
123 |
Sulfonamide
Sulfonamides are generally the structural analogues of PABA (para-aminobenzoic acid) having distinct solubility level, excretion and absorption features.79
Chitosan has been identified as a reliable adsorbent for sulfonamide remediation due to its stability in high temperatures and pH ranges.80 Adsorption mechanisms involve ionic-pi bonding, pi-pi interactions, and hydrogen bonding.81 Pine bark has shown good affinity for sulfadiazine, sulfamethazine, and sulfachloropyridazine, with up to 95% adsorption within 24 hours.80 Carbonaceous materials like powdered activated carbon, wood-based granular activated carbon, and graphene exhibit 90-95% adsorption capacity within 5 hours at pH 4.0.82 Diatom Chaetoceros and arthropod Artemia have demonstrated adsorption of sulfonamides within 24 hours and 5 hours of contact time, respectively, at a temperature of 25°C and pH range of 5.0-8.083 as shown in Figure 9. Maximum adsorption capacities were 88% and 90%, respectively. Spent coffee grounds based on biochar and hydrochar showed adsorption capacities of 121.5μg/g and 130.1μg/g for biochar and 82.2μg/g and 85.7μg/g for hydrochar at 25°C.84 Carboxyl-functionalized biochar derived from walnut shells exhibited 99% removal efficiency for sulfonamide, with involvement of hydrogen bonding and pi-pi interactions.85 Bioadsorbents demonstrated efficient adsorption of sulfonamide (Figure 9, Table 6).86
Table (6):
Bioadsorbents for sulfonamide remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Fe3O4-assisted extracellular |
77.93% (SMX) |
67.12% |
– |
124 |
polymeric substances (EPS) |
74.13% (SM1) |
|||
65.62% (SM2) |
||||
56.64% (SDZ) |
||||
Fiber industry wastes |
24.06 mg/g |
48% |
– |
125 |
sulfonated coffee waste |
256 mg/g |
– |
– |
126 |
Pectin derived from orange |
120 mg/g |
92.2% |
– |
127 |
peel biomass |
||||
Discarded biodiesel waste- |
206.2 mg/g |
138.8 mg/g |
65.5%NaOH |
128 |
derived lignocellulosic biomass |
||||
Corncobs |
– |
48% |
– |
116 |
Nitroimidazole
Nitroimidazole antibiotics are commonly used to treat anaerobic bacterial and protozoan infections, but they are frequently detected in wastewater treatment plants (WWTPs), drinking water, fish-farm waters, and industrial effluents.87 These antibiotics are challenging to degrade due to their high polarity and are considered potentially carcinogenic and mutagenic.88 They can contribute to the dissemination and proliferation of antibiotic resistance genes (ARGs) in the environment, posing risks to human and animal health. Consequently, the remediation of nitroimidazole antibiotic residues has become a significant concern for researchers. To facilitate practical application and separation, waste biomass-based adsorbents have been found to be more suitable than powdered biochar, as they are easier to recover and regenerate.89
The adsorption mechanisms between the adsorbents and nitroimidazole antibiotics involve hydrogen bonding, pi-pi dispersion, and micropore filling87 as shown in Figure 10. A study assessed the combined use of microorganisms and activated carbon for nitroimidazole adsorption, demonstrating a maximum adsorption capacity of 2.04 mmol/g. However, the researchers observed that the microorganisms used in the biological stage of a wastewater treatment plant did not degrade nitroimidazoles. Nonetheless, the presence of microorganisms during the adsorption process enhanced the adsorption on activated carbon. Pi-pi dispersion interactions between carbon graphene layers and nitroimidazole aromatic rings played a crucial role, while electron-activating groups in both the adsorbent and adsorbate initiated the adsorption process, and pH had no significant effect (Figure 10, Table 7).90
Figure 10. Metronidazole adsorption from wastewater by using Chlorella vulgaris microalgal biomass as bioadsorbent
Table (7):
Bioadsorbents for nitromidazole remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Siris seed pods |
180.74 mg/g |
98.74% |
– |
129 |
Prosopis juliflora |
13.55 mg/g |
17.45 mg/g |
– |
130 |
Rice husk |
4.79 mg/g |
96.4% |
– |
131 |
Cephalosporin 7 ACA
Cephalosporin antibiotics are commonly used to treat bacterial diseases, but their residues in wastewater pose environmental risks. 7-amino cephalosporanic acid (7-ACA) is an intermediate residue in cephalosporin synthesis, exhibiting antibacterial activity due to its beta-lactam ring.91
Guo et al. found that three microalgal strains isolated from Southern Taiwan (Chlorella sp., Chlamydomonas sp., and Mychonastes sp.) had adsorption capacities of 4.74 mg/g, 3.09 mg/g, and 2.95 mg/g, respectively, at pH 7.5 and 26°C.92,93 The adsorption mechanism involved monolayer and multilayer adsorption on the microalgae’s heterogeneous surface. Activated carbon, previously used as an adsorbent, has regeneration issues. Cephalosporin 7-ACA showed very good removal efficiency by bio adsorbents (Figure 11 and Table 8).94
Figure 11. Cephalosporin-7-ACA adsorption from wastewater by using Chlorella sp. biomass as bio adsorbent
Table (8):
Bioadsorbents for cephalosporin 7-ACA remediation
Bioadsorbents/ adsorbents |
Maximum adsorption efficiency |
Removal efficiency |
Desorption efficiency & desorbing agents |
Reference |
|---|---|---|---|---|
Lipid-accumulating Chlorella sp., |
4.74 mg/g, |
– |
– |
92 |
Chlamydomonassp., and |
3.09 mg/g |
|||
Mychonastes sp |
2.95 mg/g |
|||
Pseudomonas putida |
109.5 mg/g |
more than |
89.7% HCl |
132 |
50% |
||||
Activated olive stones |
40.71 mg/g |
65% |
– |
133 |
Mechanism of Bio adsorption
The adsorption process, traditionally considered exothermic, has been found to exhibit both exothermic and endothermic characteristics in recent research articles.95,96 Adsorption can occur through two types: physisorption (physical adsorption) and chemisorption (chemical adsorption). When bioadsorbents such as microalgae, macroalgae, fungi, bacteria, and medicinal plants are exposed to antibiotic-containing solutions, they exhibit various responses to survive and remediate the harmful antibiotic residues. 95 Among remediation techniques, adsorption is considered a reliable method for removing emerging contaminants from wastewater by binding the antibiotic residues to solid materials, such as adsorbents or bioadsorbents.65 Antibiotic adsorption by adsorbents like biochar, activated carbon, and nanomaterials primarily occurs through hydrogen bonds, hydrophobic bonds, electrostatic attraction, and Van der Waals forces.64
The adsorption mechanism involves ion exchange, pi-pi bond interactions, functional groups and H-bond interactions, electrostatic interactions, pore filling, and intra-particle diffusion.97 Ion exchange maintains electrical neutrality between liquid and solid phases, while intra-particle diffusion and pore filling depend on specific surface area and pore size of the adsorbent.98 Surface adsorption, electrostatic interactions, hydrogen bonds, and hydrophobic interactions play significant roles in the adsorption of antibiotics.99 Various modifications of biochar, bacteria, plants, fungi, algae, and agricultural wastes can enhance their adsorption capabilities. Tetracycline is a extensively studied antibiotic, known for its broad-spectrum feasibility, polar functional groups (carboxyl and acyl-amino), while ciprofloxacin possesses non-polar functional groups.100
The adsorption process offers unique advantages for the removal of antibiotic residues from wastewater. Tetracycline has been extensively studied in the research on remediation of antibiotics and their residues using adsorption techniques. The reason for focusing on tetracycline is its favorable response to various types of adsorbents and bioadsorbents. Adsorption technology provides several benefits, including low energy consumption, easy operation, and no production of by-products or secondary pollutants. This process effectively eliminates harmful antibiotic residues, antibiotic resistance genes, and antibiotic-resistant bacteria present in wastewater. Numerous researchers have reported that the use of bioadsorbents makes the adsorption process more eco-friendly and cost-effective.19,101,102 The adsorption process demonstrates a short remediation period and has been proven to be the most efficient and effective method for removing antibiotic residues from wastewater due to its stability (Figure 4-11, Table 1-8).22 Bioadsorbents such as banana peel, Moringa oleifera, Pseudomonas putida, Saccharomyces cerevisiae, and other agricultural wastes have been identified as suitable options for adsorbing antibiotic residues from wastewater. These bioadsorbents are used in a dried form, eliminating the need for additional nutrients.96,98,103 Further studies are required to explore additional advantages of using this process for the remediation of antibiotic residues from wastewater.
In conclusion, the use of bioadsorbents for removing antibiotic residues from wastewater has shown promising results. Different bioadsorbents, including raw shrimp shell waste, mussel shell, pine bark, oak ash, and tannin from Indian almond leaf, algae, fungi, bacteria have exhibited excellent adsorption capacities for specific antibiotic residues. The interactions between the functional groups of bioadsorbents and antibiotic residues, such as Van der Waals forces and hydrogen bonds, play a crucial role in the adsorption process.
This review highlights the need for further research and evaluation of various bioadsorbents to address antibiotic residues in wastewater. The adoption of eco-friendly adsorption techniques for treating wastewater from pharmaceutical, hospital, and municipal sources is gaining momentum. The development of new adsorbents and bioadsorbents holds significant promise, providing comprehensive, economic, social, and environmental benefits in water pollution control.
Overall, this review serves as a valuable resource for researchers and practitioners engaged in the study and application of adsorption processes for the removal of antibiotic residues from wastewater. Continued exploration and advancement of adsorbent materials and techniques will contribute to more efficient and sustainable solutions in the future.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Gao FZ, He LY, He LX, et al. Untreated swine wastes changed antibiotic resistance and microbial community in the soils and impacted abundances of antibiotic resistance genes in the vegetables. Sci Total Environ. 2020;741:140482.
Crossref - Hedberg N, Stenson I, Nitz Pettersson M, et al. Antibiotic use in Vietnamese fish and lobster sea cage farms; implications for coral reefs and human health. Aquaculture. 2018;495:366-375.
Crossref - Turnidge J. Antibiotic use in animals–prejudices, perceptions and realities. J Antimicrob Chemother. 2003;53(1):26-27.
Crossref - Witte W. Ecological impact of antibiotic use in animals on different complex microflora: environment. Int J Antimicrob Agents. 2000;14(4):321-325.
Crossref - Manjunath SV, Singh Baghel R, Kumar M. Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chem Eng J. 2020;381:122713.
Crossref - Wu M, Zhao S, Jing R, et al. Competitive adsorption of antibiotic tetracycline and ciprofloxacin on montmorillonite. Appl Clay Sci. 2019;180:105175.
Crossref - Bacanlı M, Başaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462-466.
Crossref - Liu X, Lu S, Guo W, Xi B, Wang W. Antibiotics in the aquatic environments: A review of lakes, China. Sci Total Environ. 2018;627:1195-1208.
Crossref - Shao Y, Wang Y, Yuan Y, Xie Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci Total Environ. 2021;798:149205.
Crossref - Van TTH, Yidana Z, Smooker PM, Coloe PJ. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J Glob Antimicrob Resist. 2020;20:170-177.
Crossref - Liu S, Wang P, Wang C, Wang X, Chen J. Anthropogenic disturbances on antibiotic resistome along the Yarlung Tsangpo River on the Tibetan Plateau: Ecological dissemination mechanisms of antibiotic resistance genes to bacterial pathogens. Water Res. 2021;202:117447.
Crossref - Ghosh S, LaPara TM. The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J. 2007;1(3):191-203.
Crossref - Girmatsion M, Mahmud A, Abraha B, et al. Rapid detection of antibiotic residues in animal products using surface-enhanced Raman Spectroscopy: A review. Food Control. 2021;126:108019.
Crossref - Nnadozie CF, Kumari S, Bux F. Status of pathogens, antibiotic resistance genes and antibiotic residues in wastewater treatment systems. Rev Environ Sci Biotechnol. 2017;16(3):491-515.
Crossref - Ekwanzala M, Lehutso R, Kasonga T, Dewar J, Momba M. Environmental Dissemination of Selected Antibiotics from Hospital Wastewater to the Aquatic Environment. Antibiotics. 2020;9(7):431.
Crossref - Li J, Cheng W, Xu L, Strong PJ, Chen H. Antibiotic-resistant genes and antibiotic-resistant bacteria in the effluent of urban residential areas, hospitals, and a municipal wastewater treatment plant system. Environ Sci Pollut Res. 2015;22(6):4587-4596.
Crossref - Zainab SM, Junaid M, Xu N, Malik RN. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020;187:116455.
Crossref - Merker M, Tueffers L, Vallier M, et al. Evolutionary Approaches to Combat Antibiotic Resistance: Opportunities and Challenges for Precision Medicine. Front Immunol. 2020;11:1938.
Crossref - Singh S, Kumar V, Anil AG, et al. Adsorption and detoxification of pharmaceutical compounds from wastewater using nanomaterials: A review on mechanism, kinetics, valorization and circular economy. J Environ Manage. 2021;300:113569.
Crossref - Wang Y, Jiao WB, Wang JT, Liu GF, Cao HL, Lü J. Amino-functionalized biomass-derived porous carbons with enhanced aqueous adsorption affinity and sensitivity of sulfonamide antibiotics. Bioresour Technol. 2019;277:128-135.
Crossref - Shi H, Ni J, Zheng T, Wang X, Wu C, Wang Q. Remediation of wastewater contaminated by antibiotics. A review. Environ Chem Lett. 2020;18(2):345-360.
Crossref - Ahmed MB, Zhou JL, Ngo HH, Guo W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci Total Environ. 2015;532:112-126.
Crossref - Minale M, Guadie A, Li Y, Meng Y, Wang X, Zhao J. Enhanced removal of oxytetracycline antibiotics from water using manganese dioxide impregnated hydrogel composite: Adsorption behavior and oxidative degradation pathways. Chemosphere. 2021;280:130926.
Crossref - Rafraf ID, Lekunberri I, Sànchez-Melsió A, Aouni M, Borrego CM, Balcázar JL. Abundance of antibiotic resistance genes in five municipal wastewater treatment plants in the Monastir Governorate, Tunisia. Environ Pollut. 2016;219:353-358.
Crossref - Bengtsson-Palme J, Milakovic M, Švecová H, et al. Industrial wastewater treatment plant enriches antibiotic resistance genes and alters the structure of microbial communities. Water Res. 2019;162:437-445.
Crossref - Xu J, Xu Y, Wang H, et al. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere. 2015;119:1379-1385.
Crossref - Zafar R, Bashir S, Nabi D, Arshad M. Occurrence and quantification of prevalent antibiotics in wastewater samples from Rawalpindi and Islamabad, Pakistan. Sci Total Environ. 2021;764:142596.
Crossref - Griboff J, Carrizo JC, Bonansea RI, Valdés ME, Wunderlin DA, Amé MV. Multiantibiotic residues in commercial fish from Argentina. The presence of mixtures of antibiotics in edible fish, a challenge to health risk assessment. Food Chem. 2020;332:127380.
Crossref - Gros M, Rodríguez-Mozaz S, Barceló D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A. 2013;1292:173-188.
Crossref - Liu X, Lv Y, Xu K, Xiao X, Xi B, Lu S. Response of ginger growth to a tetracycline-contaminated environment and residues of antibiotic and antibiotic resistance genes. Chemosphere. 2018;201:137-143.
Crossref - Spataro F, Ademollo N, Pescatore T, Rauseo J, Patrolecco L. Antibiotic residues and endocrine disrupting compounds in municipal wastewater treatment plants in Rome, Italy. Microchem J. 2019;148:634-642.
Crossref - Harrabi M, Varela Della Giustina S, Aloulou F, Rodriguez-Mozaz S, Barceló D, Elleuch B. Analysis of multiclass antibiotic residues in urban wastewater in Tunisia. Environ Nanotechnol Monit Manag. 2018;10:163-170.
Crossref - Chianese S, Iovino P, Leone V, Musmarra D, Prisciandaro M. Photodegradation of Diclofenac Sodium Salt in Water Solution: Effect of HA, NO3 − and TiO2 on Photolysis Performance. Water Air Soil Pollut. 2017;228(8):270.
Crossref - Rodriguez-Mozaz S, Vaz-Moreira I, Varela Della Giustina S, et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ Int. 2020;140:105733.
Crossref - Ávila C, García-Galán MJ, Borrego CM, Rodríguez-Mozaz S, García J, Barceló D. New insights on the combined removal of antibiotics and ARGs in urban wastewater through the use of two configurations of vertical subsurface flow constructed wetlands. Sci Total Environ. 2021;755(pt 2):142554.
Crossref - Santos L, Ramos F. Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: A review. Trends Food Sci Technol. 2016;52:16-30.
Crossref - Huygens J, Daeseleire E, Mahillon J, et al. Presence of Antibiotic Residues and Antibiotic Resistant Bacteria in Cattle Manure Intended for Fertilization of Agricultural Fields: A One Health Perspective. Antibiotics. 2021;10(4):410.
Crossref - Peterson JW, Petrasky LJ, Seymour MD, Burkhart RS, Schuiling AB. Adsorption and breakdown of penicillin antibiotic in the presence of titanium oxide nanoparticles in water. Chemosphere. 2012;87(8):911-917.
Crossref - Ji K, Kho Y, Park C, et al. Influence of water and food consumption on inadvertent antibiotics intake among general population. Environ Res. 2010;110(7):641-649.
Crossref - Elbalkiny HT, Yehia AM. Artificial networks for spectral resolution of antibiotic residues in bovine milk; solidification of floating organic droplet in dispersive liquid-liquid microextraction for sample treatment. Spectrochim Acta A Mol Biomol Spectrosc. 2022;266:120449.
Crossref - Hounfodji JW, Kanhounnon WG, Kpotin G, et al. Molecular insights on the adsorption of some pharmaceutical residues from wastewater on kaolinite surfaces. Chem Eng J. 2021;407:127176.
Crossref - Lu ZY, Ma YL, Zhang JT, Fan NS, Huang BC, Jin RC. A critical review of antibiotic removal strategies: Performance and mechanisms. J Water Process Eng. 2020;38:101681.
Crossref - Ben Y, Hu M, Zhang X, et al. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water. Water Res. 2020;175:115699.
Crossref - Finley RL, Collignon P, Larsson DGJ, et al. The Scourge of Antibiotic Resistance: The Important Role of the Environment. Clin Infect Dis. 2013;57(5):704-710.
Crossref - Gosset A, Wiest L, Fildier A, et al. Ecotoxicological risk assessment of contaminants of emerging concern identified by “suspect screening” from urban wastewater treatment plant effluents at a territorial scale. Sci Total Environ. 2021;778:146275.
Crossref - Ben Y, Fu C, Hu M, Liu L, Wong MH, Zheng C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ Res. 2019;169:483-493.
Crossref - Batt AL, Bruce IB, Aga DS. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environ Pollut. 2006;142(2):295-302.
Crossref - Chowdhury J, Mandal TK, Mondal S. Genotoxic impact of emerging contaminant amoxicillin residue on zebra fish (Danio rerio) embryos. Heliyon. 2020;6(11):e05379.
Crossref - Chowdhury J, Mukherjee R, Dutta D, Mandal TK, Basu T, Mondal S. Evaluation of Antibiotic Residue in Cow Milk and Its Impact on Danio Rerio. In Review; 2021.
Crossref - Sharma L, Siedlewicz G, Pazdro K. The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art. Plants. 2021;10(3):591.
Crossref - Limbu SM, Zhou L, Sun SX, Zhang ML, Du ZY. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ Int. 2018;115:205-219.
Crossref - Kim DB, Song NE, Nam TG, Jung YS, Yoo M. Investigation and human health risk assessment of multi-class veterinary antibiotics in honey from South Korea. J Food Compos Anal. 2021;102:104040.
Crossref - Fernandez JE, Perreten V, Schwendener S. The novel macrolide resistance genes mef (F) and msr (G) are located on a plasmid in Macrococcus canis and a transposon in Macrococcus caseolyticus. J Antimicrob Chemother. 2021;76(1):48-54.
Crossref - Hu Y, Jiang L, Sun X, et al. Risk assessment of antibiotic resistance genes in the drinking water system. Sci Total Environ. 2021;800:149650.
Crossref - Baquero F, Martínez JL, Cantón R. Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol. 2008;19(3):260-265.
Crossref - Bergeron S, Boopathy R, Nathaniel R, Corbin A, LaFleur G. Presence of antibiotic resistant bacteria and antibiotic resistance genes in raw source water and treated drinking water. Int Biodeterior Biodegrad. 2015;102:370-374.
Crossref - Oyedemi BOM, Kotsia EM, Stapleton PD, Gibbons S. Capsaicin and gingerol analogues inhibit the growth of efflux-multidrug resistant bacteria and R-plasmids conjugal transfer. J Ethnopharmacol. 2019;245:111871.
Crossref - Zhu Y, Wang C, Schwarz S, et al. Identification of a novel tetracycline resistance gene, tet (63), located on a multiresistance plasmid from Staphylococcus aureus. J Antimicrob Chemother. 2021;76(3):576-581.
Crossref - Zou Y, Xiao Y, Wang H, Fang T, Dong P. New insight into fates of sulfonamide and tetracycline resistance genes and resistant bacteria during anaerobic digestion of manure at thermophilic and mesophilic temperatures. J Hazard Mater. 2020;384:121433.
Crossref - Li H, Song R, Wang Y, et al. Simultaneous removal of antibiotic-resistant bacteria and its resistance genes in water by plasma oxidation: Highlights the effects of inorganic ions. Sep Purif Technol. 2022;278:119672.
Crossref - Liu H, Zhu X, Zhang X, Wang Z, Sun B. Photodegradation of Oxytetracycline in the Presence of Dissolved Organic Matter and Chloride Ions: Importance of Reactive Chlorine Species. Water Air Soil Pollut. 2019;230(10):235.
Crossref - Guler UA, Sarioglu M. Removal of tetracycline from wastewater using pumice stone: equilibrium, kinetic and thermodynamic studies. J Environ Health Sci Eng. 2014;12(1):79.
Crossref - Lu X, Tang B, Zhang Q, Liu L, Fan R, Zhang Z. The Presence of Cu Facilitates Adsorption of Tetracycline (TC) onto Water Hyacinth Roots. Int J Environ Res Public Health. 2018;15(9):1982.
Crossref - Wang Y, Gong S, Li Y, Li Z, Fu J. Adsorptive removal of tetracycline by sustainable ceramsite substrate from bentonite/red mud/pine sawdust. Sci Rep. 2020;10(1):2960.
Crossref - Chang J, Shen Z, Hu X, et al. Adsorption of Tetracycline by Shrimp Shell Waste from Aqueous Solutions: Adsorption Isotherm, Kinetics Modeling, and Mechanism. ACS Omega. 2020;5(7):3467-3477.
Crossref - Lu L, Liu M, Chen Y, Luo Y. Effective removal of tetracycline antibiotics from wastewater using practically applicable iron(III)-loaded cellulose nanofibres. R Soc Open Sci. 2021;8(8):210336.
Crossref - Pizzi A. Tannins medical / pharmacological and related applications: A critical review. Sustain Chem Pharm. 2021;22:100481.
Crossref - Sunsandee N, Ramakul P, Phatanasri S, Pancharoen U. Biosorption of dicloxacillin from pharmaceutical waste water using tannin from Indian almond leaf: Kinetic and equilibrium studies. Biotechnol Rep. 2020;27:e00488.
Crossref - Barral-Martinez M, Fraga-Corral M, Garcia-Perez P, Simal-Gandara J, Prieto MA. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods. 2021;10(8):1793.
Crossref - Cardoso AR, Carneiro LPT, Cabral-Miranda G, Bachmann MF, Sales MGF. Employing bacteria machinery for antibiotic detection: Using DNA gyrase for ciprofloxacin detection. Chem Eng J. 2021;409:128135.
Crossref - Agboola OS, Bello OS. Enhanced adsorption of ciprofloxacin from aqueous solutions using functionalized banana stalk. Biomass Convers Biorefinery. 2020:5463-5478.
Crossref - Oliva A, Curtolo A, Volpicelli L, et al. Synergistic Meropenem/Vaborbactam Plus Fosfomycin Treatment of KPC Producing K. pneumoniae Septic Thrombosis Unresponsive to Ceftazidime/Avibactam: From the Bench to the Bedside. Antibiotics. 2021;10(7):781.
Crossref - Marathe NP, Pal C, Gaikwad SS, Jonsson V, Kristiansson E, Larsson DGJ. Untreated urban waste contaminates Indian river sediments with resistance genes to last resort antibiotics. Water Res. 2017;124:388-397.
Crossref - Raza A, Ngieng SC, Sime FB, et al. Oral meropenem for superbugs: challenges and opportunities. Drug Discov Today. 2021;26(2):551-560.
Crossref - Proia L, Anzil A, Borrego C, et al. Occurrence and persistence of carbapenemases genes in hospital and wastewater treatment plants and propagation in the receiving river. J Hazard Mater. 2018;358:33-43.
Crossref - Voigt AM, Ciorba P, Döhla M, et al. The investigation of antibiotic residues, antibiotic resistance genes and antibiotic-resistant organisms in a drinking water reservoir system in Germany. Int J Hyg Environ Health. 2020;224:113449.
Crossref - Altamirano Briones A, Cóndor Guevara I, Mena D, et al. Degradation of Meropenem by Heterogeneous Photocatalysis Using TiO2/Fiberglass Substrates. Catalysts. 2020;10(3):344.
Crossref - Yu Y, Zhou Y, Wang Z, Torres OL, Guo R, Chen J. Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment. Sci Rep. 2017;7(1):4168.
Crossref - Chaturvedi P, Singh A, Chowdhary P, Pandey A, Gupta P. Occurrence of emerging sulfonamide resistance (sul1 and sul2) associated with mobile integrons-integrase (intI1 and intI2) in riverine systems. Sci Total Environ. 2021;751:142217.
Crossref - Conde-Cid M, Cela-Dablanca R, Ferreira-Coelho G, et al. Sulfadiazine, sulfamethazine and sulfachloropyridazine removal using three different porous materials: Pine bark, “oak ash” and mussel shell. Environ Res. 2021;195:110814.
Crossref - Liu Y, Nie P, Yu F. Enhanced adsorption of sulfonamides by a novel carboxymethyl cellulose and chitosan-based composite with sulfonated graphene oxide. Bioresour Technol. 2021;320:124373.
Crossref - Luo B, Huang G, Yao Y, An C, Zhang P, Zhao K. Investigation into the influencing factors and adsorption characteristics in the removal of sulfonamide antibiotics by carbonaceous materials. J Clean Prod. 2021;319:128692.
Crossref - Matsuura R, Kanehara R, Kadoya A, Suzuki S. Adsorption of sulfonamides to marine diatoms and arthropods. Environ Toxicol Pharmacol. 2021;82:103557.
Crossref - Zhang X, Zhang Y, Ngo HH, et al. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci Total Environ. 2020;716:137015.
Crossref - Geng X, Lv S, Yang J, Cui S, Zhao Z. Carboxyl-functionalized biochar derived from walnut shells with enhanced aqueous adsorption of sulfonamide antibiotics. J Environ Manage. 2021;280:111749.
Crossref - Braschi I, Blasioli S, Gigli L, Gessa CE, Alberti A, Martucci A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J Hazard Mater. 2010;178(1-3):218-225.
Crossref - Sun L, Wan S, Yuan D, Yu Z. Adsorption of nitroimidazole antibiotics from aqueous solutions on self-shaping porous biomass carbon foam pellets derived from Vallisneria natans waste as a new adsorbent. Sci Total Environ. 2019;664:24-36.
Crossref - Carrales-Alvarado DH, Leyva-Ramos R, Rodríguez-Ramos I, Mendoza-Mendoza E, Moral-Rodríguez AE. Adsorption capacity of different types of carbon nanotubes towards metronidazole and dimetridazole antibiotics from aqueous solutions: effect of morphology and surface chemistry. Environ Sci Pollut Res. 2020;27(14):17123-17137.
Crossref - Saldarriaga JF, Montoya NA, Estiati I, Aguayo AT, Aguado R, Olazar M. Unburned material from biomass combustion as low-cost adsorbent for amoxicillin removal from wastewater. J Clean Prod. 2021;284:124732.
Crossref - Rivera-Utrilla J, Prados-Joya G, Sánchez-Polo M, Ferro-García MA, Bautista-Toledo I. Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J Hazard Mater. 2009;170(1):298-305.
Crossref - Salgado-Caxito M, Moreno-Switt AI, Paes AC, et al. Higher Prevalence of Extended-Spectrum Cephalosporin-Resistant Enterobacterales in Dogs Attended for Enteric Viruses in Brazil Before and After Treatment with Cephalosporins. Antibiotics. 2021;10(2):122.
Crossref - Guo WQ, Zheng HS, Li S, et al. Removal of cephalosporin antibiotics 7-ACA from wastewater during the cultivation of lipid-accumulating microalgae. Bioresour Technol. 2016;221:284-290.
Crossref - Ribeiro AR, Sures B, Schmidt TC. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environ Pollut. 2018;241:1153-1166.
Crossref - Dutta M, Baruah R, Dutta NN, Ghosh AC. The adsorption of certain semi-synthetic cephalosporins on activated carbon. Colloids Surf Physicochem Eng Asp. 1997;127(1-3):25-37.
Crossref - Aziz NA, Jayasuriya N, Fan L, Al-Gheethi A. A low-cost treatment system for underground water using Moringa oleifera seeds and Musa cavendish peels for remote communities. J Chem Technol Biotechnol. 2021;96(3):680-696.
Crossref - Viotti PV, Moreira WM, Santos OAA dos, Bergamasco R, Vieira AMS, Vieira MF. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J Clean Prod. 2019;219:809-817.
Crossref - Saremi F, Miroliaei MR, Shahabi Nejad M, Sheibani H. Adsorption of tetracycline antibiotic from aqueous solutions onto vitamin B6-upgraded biochar derived from date palm leaves. J Mol Liq. 2020;318:114126.
Crossref - Cheng D, Ngo HH, Guo W, et al. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci Total Environ. 2020;720:137662.
Crossref - Iervolino G, Vaiano V, Palma V. Enhanced removal of water pollutants by dielectric barrier discharge non-thermal plasma reactor. Sep Purif Technol. 2019;215:155-162.
Crossref - Tang X, Huang Y, He Q, Wang Y, Zheng H, Hu Y. Adsorption of tetracycline antibiotics by nitrilotriacetic acid modified magnetic chitosan-based microspheres from aqueous solutions. Environ Technol Innov. 2021;24:101895.
Crossref - Conde-Cid M, Fernández-Sanjurjo MJ, Ferreira-Coelho G, et al. Competitive adsorption and desorption of three tetracycline antibiotics on bio-sorbent materials in binary systems. Environ Res. 2020;190:110003.
Crossref - Radmehr S, Hosseini Sabzevari M, Ghaedi M, Ahmadi Azqhandi MH, Marahel F. Adsorption of nalidixic acid antibiotic using a renewable adsorbent based on Graphene oxide from simulated wastewater. J Environ Chem Eng. 2021;9(5):105975.
Crossref - Orlewska K, Piotrowska-Seget Z, Cycoń M. Use of the PCR-DGGE Method for the Analysis of the Bacterial Community Structure in Soil Treated With the Cephalosporin Antibiotic Cefuroxime and/or Inoculated With a Multidrug-Resistant Pseudomonas putida Strain MC1. Front Microbiol. 2018;9:1387.
Crossref - Srivastava A, Dave H, Azad SK, et al. Iron Modification of Biochar Developed from Tectona grandis Linn. F. for Adsorptive Removal of Tetracycline from Aqueous Solution. Anal Chem Lett. 2021;11(3):360-375.
Crossref - Wang J, Liu X, Yang M, et al. Removal of tetracycline using modified wheat straw from solution in batch and column modes. J Mol Liq. 2021;338:116698.
Crossref - Sun J, Cui L, Gao Y, He Y, Liu H, Huang Z. Environmental application of magnetic cellulose derived from Pennisetum sinese Roxb for efficient tetracycline removal. Carbohydr Polym. 2021;251:117004.
Crossref - Yang XG, Fu X, Cui Y. Removal of tetracycline from wastewater by Fe-modified oyster shell. Ferroelectrics. 2021;581(1):73-81.
Crossref - Chen Y, Wang F, Duan L, Yang H, Gao J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J Mol Liq. 2016;222:487-494.
Crossref - Zambrano J, García-Encina PA, Hernández F, Botero-Coy AM, Jiménez JJ, Irusta-Mata R. Removal of a mixture of veterinary medicinal products by adsorption onto a Scenedesmus almeriensis microalgae-bacteria consortium. J Water Process Eng. 2021;43:102226.
Crossref - Choi YK, Choi TR, Gurav R, et al. Adsorption behavior of tetracycline onto Spirulina sp. (microalgae)-derived biochars produced at different temperatures. Sci Total Environ. 2020;710:136282.
Crossref - Qu J, Wang S, Jin L, et al. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr(Ⅵ) and tetracycline adsorption from water. Bioresour Technol. 2021;340:125692.
Crossref - Ahmed MJ, Islam MdA, Asif M, Hameed BH. Human hair-derived high surface area porous carbon material for the adsorption isotherm and kinetics of tetracycline antibiotics. Bioresour Technol. 2017;243:778-784.
Crossref - Alvarez-García S, Ramírez-García JJ. Sorption Behavior of Dicloxacillin in Zeolites Modified with a Cationic Surfactant at Different pH. Water Air Soil Pollut. 2021;232(4):152.
Crossref - Ezekoye OM, Akpomie KG, Eze SI, Chukwujindu CN, Ani JU, Ujam OT. Biosorptive interaction of alkaline modified Dialium guineense seed powders with ciprofloxacin in contaminated solution: central composite, kinetics, isotherm, thermodynamics, and desorption. Int J Phytoremediation. 2020;22(10):1028-1037.
Crossref - Khokhar TS, Memon FN, Memon AA, et al. Removal of ciprofloxacin from aqueous solution using wheat bran as adsorbent. Sep Sci Technol. 2019;54(8):1278-1288.
Crossref - Peñafiel ME, Matesanz JM, Vanegas E, Bermejo D, Ormad MP. Corncobs as a potentially low-cost biosorbent for sulfamethoxazole removal from aqueous solution. Sep Sci Technol. 2020;55(17):3060-3071.
Crossref - Peñafiel ME, Vanegas E, Bermejo D, Matesanz JM, Ormad MP. Organic residues as adsorbent for the removal of ciprofloxacin from aqueous solution. Hyperfine Interact. 2019;240(1):71.
Crossref - Huang X, Wu S, Tang S, Huang L, Zhu D, Hu Q. Photocatalytic hydrogel layer supported on alkali modified straw fibers for ciprofloxacin removal from water. J Mol Liq. 2020;317:113961.
Crossref - Wu S, Li Y, Zhao X, et al. Biosorption Behavior of Ciprofloxacin onto Enteromorpha prolifera: Isotherm and Kinetic Studies. Int J Phytoremediation. 2015;17(10):957-961.
Crossref - Akar T, Sayin F, Celik S, Tunali Akar S. Attached culture of Gibberella fujikuroi for biocomposite sorbent production and ciprofloxacin sequestration applications. J Chem Technol Biotechnol. 2021;96(9):2610-2619.
Crossref - Shukla P, Giri BS, Mishra RK, Pandey A, Chaturvedi P. Lignocellulosic biomass-based engineered biochar composites: A facile strategy for abatement of emerging pollutants and utilization in industrial applications. Renew Sustain Energy Rev. 2021;152:111643.
Crossref - M-Ridha MJ, Hasan YR, Ibrahim MA. Adsorption kinetics and mechanisms for meropenem antibiotic removal in batch mode via rice husk functionalized with Mg/Fe-layered double hydroxides. Sep Sci Technol. 2021;56(16):2721-2733.
Crossref - Menezes Santos T, Valdo da Silva J, Francisco da Silva G, Magalhães Pontes LA. Development of a Low-Cost Adsorbent Obtained from Moringa Oleifera and Functionalized with Iron Nanoparticles for Removal of Oil from Produced Water. Biointerface Res Appl Chem. 2021;11(5):13214-13231.
Crossref - Pi S, Li A, Cui D, Su Z, Zhou L, Ma F. Enhanced adsorption performance and regeneration of magnetic Fe3O4 nanoparticles assisted extracellular polymeric substances in sulfonamide-contaminated water. Environ Sci Pollut Res. 2020;27(5):4866-4875.
Crossref - Fernandez-Sanroman A, Acevedo-García V, Pazos M, Sanromán MA, Rosales E. Removal of sulfamethoxazole and methylparaben using hydrocolloid and fiber industry wastes: Comparison with biochar and laccase-biocomposite. J Clean Prod. 2020;271:122436.
Crossref - Ahsan MdA, Islam MdT, Imam MA, et al. Biosorption of bisphenol A and sulfamethoxazole from water using sulfonated coffee waste: Isotherm, kinetic and thermodynamic studies. J Environ Chem Eng. 2018;6(5):6602-6611.
Crossref - Kadam AA, Sharma B, Saratale GD, et al. Super-magnetization of pectin from orange-peel biomass for sulfamethoxazole adsorption. Cellulose. 2020;27(6):3301-3318.
Crossref - Ganesan S, Karthick K, Namasivayam C, et al. Discarded biodiesel waste–derived lignocellulosic biomass as effective biosorbent for removal of sulfamethoxazole drug. Environ Sci Pollut Res. 2020;27(15):17619-17630.
Crossref - Ahmed MJ, Theydan SK. Microwave assisted preparation of microporous activated carbon from Siris seed pods for adsorption of metronidazole antibiotic. Chem Eng J. 2013;214:310-318.
Crossref - Manjunath SV, Kumar M. Evaluation of single-component and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from Prosopıs julıflora. Chem Eng J. 2018;346:525-534.
Crossref - Van Tran S, Nguyen KM, Nguyen HT, Stefanakis AI, Nguyen PM. Food processing wastes as a potential source of adsorbent for toxicant removal from water. In: Circular Economy and Sustainability. Elsevier; 2022:491-507.
Crossref - bozorginia S, Jaafari J, Taghavi K, Ashrafi SD, Roohbakhsh E, Naghipour D. Biosorption of ceftriaxone antibiotic by Pseudomonas putida from aqueous solutions. Int J Environ Anal Chem. 2023;103(9):2067-2081.
Crossref - León G, Saura F, Hidalgo AM, Miguel B. Activated Olive Stones as a Low-Cost and Environmentally Friendly Adsorbent for Removing Cephalosporin C from Aqueous Solutions. Int J Environ Res Public Health. 2021;18(9):4489.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.