ISSN: 0973-7510
E-ISSN: 2581-690X
The goals of the present study were to use silver nitrate (AgNO3) solution to synthesize plant-mediated silver nanoparticles (AgNPs) using Boerhavia procumbens extract, to evaluate the antimicrobial potential of crude B. procumbens extracts as well as the antimicrobial potential of synthesized AgNPs. The antimicrobial activity was tested against ten pathogenic bacterial strains including Klebsiella pneumonia, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Citrobacter braakii, Providentia spp., Salmonella typhi, Salmonella para typhi, Vibrio cholera, and Proteus vulgaris and seven fungal species; Rhizopus stolonifer, Candida albican, Alternaria alternata, Aspergillus flavus, Verticillium chlamydosporium, Penicillium chrysogenum, and Aspergillus oryzae. The methanol extract was fractionated using several solvents and subjected to phytochemical analysis along with FTIR. Phytochemical analyses revealed flavonoids, tannins, saponins, steroids, quinones, and phenols in the crude plant extract. AgNPs were synthesized using B. percumbens extract and characterized by UV-Vis, Fourier-transform infrared spectroscopy (FTIR), and Scanning Electron Microscopy (SEM). Synthesized AgNPs were spherical, with 20-80 nm diameter. The absorption peak of synthesized AgNPs was observed at 392 nm. AgNPs have significant antimicrobial potential against selected pathogenic bacterial and fungal species as compared to different fractions of crude B. procumbens extract. The current study suggests that green synthesis is a useful technique and can be used as an alternative to antimicrobial agents against pathogenic organisms.
Boerhavia procumbens, Nyctaginaceae, scanning electron microscopy, FTIR, silver nanoparticles, Quinones
Medicinal plants have been widely used and considered the most common sources of medicine in the pharmaceutical industry (Ramadan 2008; Ramadan et al. 2008; Mohdaly et al., 2015; Abo El-Maati et al. 2016; Shakya, 2016; Rauf et al., 2018). These plants produced a wide range of bioactive compounds (Bajguz, 2007; Cushnie et al., 2014). The composition of plant secondary metabolites depends on the species, association with microbes, and soil type (Zhao et al., 2011; Morsy, 2014). These bioactive compounds have potential against a wide range of bacterial infections and the best source for the formulation of drugs, declared by the World Health Organization (Iwu et al., 1999; WHO Traditional Medicine Strategy, 2002).
Nanotechnology or nanoscale technology is a strong field of novel research dealing with design, manipulation, and synthesis of particles with 1-100 nm (Sultana et al., 2019). Nanoparticles (NPs) have several uses in cosmetics, health care, food and feed, mechanics, optics, environmental health, drug-gene delivery, biomedical sciences, electronics, space industries, chemical industries, optoelectronics, energy science, catalysis, single-electron transistors, nonlinear optical devices and photo-electrochemical applications (Iravani et al., 2014). Nanotechnology finds its importance by generating nano-products and NPs with novel and size-related physicochemical traits in contrast to bigger molecules (Chouhan, 2018).
The most important branch of nanotechnology is plant-mediated green synthesis. Due to the environmentally friendly and practical cost, it has gained more importance (Chanel et al., 2017). Green synthesis has many advantages, i.e., less toxicity, cost-effective and straightforward, less energy consumption, perform activities under moderate conditions, and combined the effect of nanoparticles and plant bioactive constituents. Furthermore, green synthesized silver nanoparticles possess more biological potential than nanoparticles synthesized by chemical methods (Choudhury et al., 2016).
Silver NPs (AgNPs) are of interest due to their novel chemical, physical, and biological traits compared to their macro-counterparts (Sultana et al., 2019). AgNPs possess distinct physicochemical properties, comprising a high thermal and electrical conductivity, surface- chemical stability, enhanced Raman scattering, catalytic activity, and non-linear optical properties (Khattak et al., 2019). Those characteristics are responsible for their role in the formation of inks, in medical imaging and microelectronics. Besides, AgNPs reveal wide-spectrum fungicidal and bactericidal potential, making them highly accessible in functional products (Tran and Le, 2013).
Boerhavia procumbens belong to the family Nyctaginaceae, nearby termed Biskhpara/Jangli, and spreading hogweed. Leaves are large, broad, and rounded beneath with a vigorous shape. Its branches (about 3 ft.) are glabrous and slender. The branches are long, glabrous, pubescent, stout, or viscous, with 1-2 inches leaves. They are oval, dull-witted, acute, and ordinarily cordate green beneath with panicle inflorescence (Fig. 1). Its habitat Africa, Pakistan, the USA, and India. In Pakistan, it could be found in Peshawar, Attock, Hazara, Sind, Baluchistan, Multan, Thal to Kurram, and Rawalpindi. Its color is purplish-red, and the blooming period is January to August (Hussain et al., 2012). Boerhavia procumbens has a lot of pharmaceutical and health-related benefits. Medicinal applications of Boerhavia procumbens include diaphoretic, diuretic, expectorant, emetic, laxative, stomachic, rejuvenates and purgative (Khalil et al., 2017; Wajid et al., 2017; Khan et al., 2020). It is mentioned to be effective against cardiac disorders, asthma, hemorrhoids, edema, eye diseases, insomnia, jaundice, kidney stone, nervous system related disorders, cough, against snake venom, urethritis, skin related diseases, intestinal, anemia, and colic kidney disorders (Hussain et al., 2012; Ali et al., 2019).
The purpose of the current investigation was to carry out green synthesize of AgNPs using aqueous extract of Boerhavia procumbens and to study the antimicrobial potential of different fractions and synthesized AgNPs against pathogenic bacteria and fungal species.
Plant collection
B. procumbence was collected from the botanical garden of the Medicinal Botanic Centre of Pakistan Council for Scientific and Industrial Research (PCSIR), Peshawar, Pakistan. The plant was identified in the Department of Botany, University of Peshawar. The leaves having a voucher name BP554 was submitted for future correspondence. The freshly collected plant was washed carefully, then air-dried for 15 days under the shed (Pirtarighat et al., 2019). The dried plant was grounded to make a coarse powder using an electric grinding machine.
Preparation of plant extract
The crude extracts were prepared with different solvents, including methanol, water, ethanol, ethyl acetate, chloroform, and n-hexane. All solvents were taken into flasks, and plant material was soaked in flasks for three days. The solvents were decanted, and the procedure was repeated four times for each solvent. All extracts were concentrated using a rotary evaporator at 45°C under vacuum. The extracts were kept in the refrigerator for further analysis (Pirtarighat et al., 2019).
Moisture determination
Dried powdered B. procumbens (5 g) was pre-weighed in the Petri dish and kept at 105-110˚C for three h. Moisture (%) was calculated regarding the air-dried sample (Pirtarighat et al., 2019).
% Moisture = [ Wight of moisture in g / Wight of plant material (g) before heating ] X 100
Determination of extractive value
The total weight of 5 g of each dried powder of plant material was extracted for about 24 h with 100 mL of respective solvent (methanol, ethanol, ethyl acetate, chloroform, and n-hexane). The flasks were shaken, and the mixture allowed to stand overnight, followed by recovery of solvents by decantation and filtration. The filtrates were dried, and the extract was weighted in percentage (Singh et al., 2013).
% Extract = [Wight of the extract (g)/ Wight of plant material (g)] X 100
Determination of ash
The total weight of 5 g of dried powder of B. procumbens was burnt at 600˚C in a furnace for three h followed by cooling. The ash was weighed, and the percentage calculated regarding the air-dried B. procumbens sample (Premnath et al., 2012).
% Ash = [ Wight of plant material after burning / Wight of plant material before burning ] X 100
Phytochemical analyses
B. Procumbens fractions were analyzed for the presence of phytochemical constituents, according to Singh et al. (2013).
Preparation of silver nanoparticles (AgNO3)
Ten g of the plant powder was boiled with 100 mL deionized distilled water. The resultant aqueous solution was filtered using Whatman filter paper three times to remove all the insoluble material. This extract was added to a 10-3 M silver nitrate (AgNO3) solution. Analytical grade AgNO3 (Molecular weight 169.87 g/mol) was purchased from Merck (Darmstadt, Germany). The solution was heated for three h in a water bath at 60°C. The solution color changed from light yellow to brown-reddish. It showed the reduction of pure Ag (I) ions. The reduced ions were monitored and measured using UV-visible spectra of the mixture of plant extract and AgNO3 solution at regular intervals. Peaks were measured on UV-Vis spectra from 200 nm and 700 nm (Shakeel et al., 2015).
Evaluation of antimicrobial activity
Antimicrobial activities were performed using well diffusion method for both B. procumbens crude extracts and AgNPs. The ethyl acetate, methanol, ethanol, water, chloroform, and n-hexane extracts of the plant and AgNPs solutions of B. procumbens were applied against ten pathogenic bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Citrobacter braakii, Proteus vulgaris, Salmonella Typhi, Salmonella Para typhi, Vibrio cholerae, and Providencia stuartii) and five fungal strains (Candida albicans, Alternaria alternata, Aspergillus flavus, Aspergillus niger, and Aspergillus oryzae). The agar media was prepared then autoclaved and dispensed into sterilized plates in Laminar flow hood (LFH) and left for the solidification. The test microorganism uniform lawn was arranged on the surface of nutrient agar media in Petri plates with the help of a sterile swab. Uniform wells of 6 mm were bored in each plate, with sterile metallic borer. Ten mg of test samples (fraction and AgNPs) were prepared in 1.0 mL of DMSO (10%) and added into particular wells. Petri dishes were incubated for 16 h at 37°C. The zones were determined after the incubation period from the width of the wells. The formula documented the percent inhibition:
% Inhibition = [ Zone of inhibition of test sample / Zone of inhibition of standard] X 100
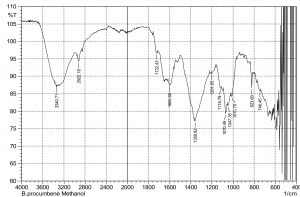
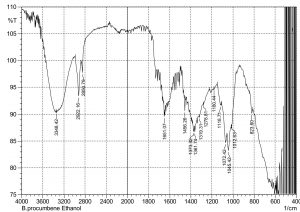
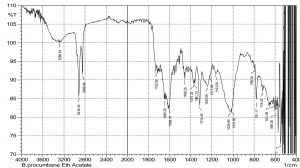
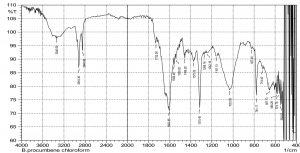
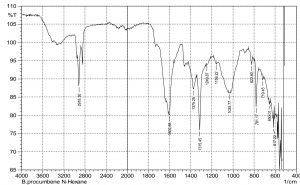
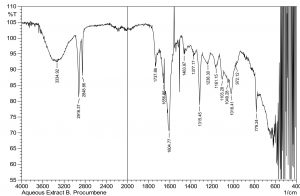
The current study was performed to evaluate the antimicrobial potential of B. purocumbens crude extract, to synthesize AgNPs from B. purocumbens extract and to determine their biological effects against pathogenic bacteria and fungi. Leave extracts were prepared in methanol, ethyl acetate, ethanol, chloroform, n-hexane, and water. Ash (19.38%), moisture (8.2%), and extractive values were also determined (Table 1). The FTIR of all extracts was performed using FTIR Model IR Pretige-21 (Shimadzu, Japan). Different peaks of functional groups were detected in each fraction (Fig. 2-6).
Table (1):
Ash, moisture and extractive value (%) of B. procumbenc.
| Ash (%) | Moisture (%) | Extractive value (%) | |||||
|---|---|---|---|---|---|---|---|
| MeOH | EtOH | EtOAc | CHCl3 | n-hexane | Aqueous | ||
| 19.38 | 8.206 | 13 | 12 | 8 | 7 | 5 | 18 |
Phytochemical analysis of B. purocumbens extract
The results of the phytochemical analyses, shown in Table 2, reveal that tannins were present in methanol, ethanol, ethyl acetate, chloroform extracts. Flavonoids were absent in the n-hexane extract, while present in all other extracts. Alkaloids and terpenoids were absent in all extracts. Saponins were absent in the n-hexane extract, while present in methanol, ethanol, ethyl acetate, chloroform, and water extracts. Steroids were present is less quantity in n-hexane extract, while strongly found in all the remaining extracts. Quinones was absent in n-hexane extract, while detected in chloroform, ethyl acetate, ethanol, methanol, and aqueous extracts. Phenols were present in much higher concentration in methanol and ethanol extracts, while moderately present in n-hexane and EtOAc extracts, and least detected in chloroform and water extracts.
Table (2):
Phytochemical analysis of B. procumbenc.
S. No. |
Test name |
MeOH |
EtOH |
CHCl3 |
n-hexane |
EtOAc |
H2O |
|---|---|---|---|---|---|---|---|
1 |
Tannins |
++ |
+++ |
++ |
+ |
++ |
++ |
2 |
Flavonoids |
++ |
++ |
++ |
– |
++ |
+ |
3 |
Alkaloids |
– |
– |
– |
– |
– |
– |
4 |
Terpiniods |
– |
– |
– |
– |
– |
– |
5 |
Saponins |
++ |
++ |
+ |
– |
++ |
+++ |
6 |
Steroids |
+++ |
+++ |
+++ |
++ |
++ |
– |
7 |
Quinones |
++ |
++ |
+ |
– |
+ |
+ |
8 |
Phenols |
+++ |
+++ |
+ |
++ |
++ |
+ |
+++: strongly, ++moderately, + weak
FTIR analysis of B. purocumbens extract
All fraction of crude extract of B. purocumbens were evaluated for the presence of functional groups using FTIR (Fig 2-7). The Fig. of each fraction was analyzed from its IR values and presented in a tabulated form (Table 5-10).
Table (3):
Zone of inhibition by B. procumbens crude extract.
| Bacterial Species | Zone of inhibition (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H2O | MeOH | EtOH | EtOAc | CHCl3 | n-hexane | AgNPs | Positive Control | Negative Control | |
| E. coli | 09±0.38 | 0±0 | 13±0.73 | 12±0.44 | 0±0 | 0±0 | 23±0.46 | 16±0.94 | – |
| K. pneumoniae | 17±0.85 | 11±0.75 | 14±0.19 | 13±0.84 | 10±0.66 | 0±0 | 22±0.28 | 11±0.26 | – |
| S. aureus | 11±0.02 | 0 | 14±0.33 | 14±0.95 | 0±0 | 0±0 | 22±0.18 | 15±1.03 | – |
| P.aeruginosa | 0±0 | 12±0.66 | 15±0.85 | 14±0.28 | 12±0.92 | 0±0 | 19±1.03 | 18±0.37 | – |
| C. braakii, | 13±0.38 | 12±1.03 | 16±0.86 | 14±1.06 | 11±0.02 | 09±0.55 | 20±0.44 | 13±0.68 | – |
| Providentia Spp. | 10±0.28 | 12±0.23 | 15±0.90 | 13±0.46 | 09±0.28 | 0±0 | 17±0.90 | 18±0.39 | – |
| S. typhi | 15±0.55 | 09±0.35 | 12±1.00 | 14±0.94 | 09±1.04 | 09±0.99 | 20±0.23 | 15±1.02 | – |
| S. para typhi | 0±0 | 10±0.69 | 15±0 | 13±0.28 | 0±0 | 0±0 | 23±0.84 | 14±0.33 | – |
| V. cholerae | 12±0.53 | 0±0 | 13±1.02 | 11±0.49 | 0±0 | 0±0 | 15±0.92 | 19±0.91 | – |
| P. vulgaris
|
09±0.18 | 08±0.39 | 14±0,29 | 12±0.84 | 0±0 | 07±0.18 | 15±0.49 | 13±0.25 | – |
DMSO was used as –ve while gentamicin was used as +ve control
Table (4):
Zones of inhibition of B. percumbence extracts.
Fungal Species |
H2O |
MeOH |
EtOH |
EtOAc |
CHCl3 |
n-hexane |
AgNPs |
Positive Control |
Negative Control |
|---|---|---|---|---|---|---|---|---|---|
A. oryzae |
13±0.23 |
21±0.65 |
17±0.24 |
15±0.84 |
13±0.84 |
00±00 |
22±0.38 |
14±0.48 |
– |
C. albicans |
10±0.39 |
17±0.22 |
20±0.84 |
18±0.37 |
16±0.58 |
16±0.65 |
20±0.74 |
17±0.65 |
– |
A. niger |
15±0.18 |
19±0.26 |
22±0.26 |
18±0.74 |
18±0.49 |
19±0.77 |
24±0.97 |
14±0.25 |
– |
A. alternata |
9±0.73 |
20±0.83 |
21±1.33 |
20±0.64 |
19±0.65 |
19±0.84 |
23±0.88 |
13±0.79 |
– |
A. flavus |
14±0.02 |
17±0.99 |
18±0.83 |
14±0.29 |
14±0.26 |
14±0.26 |
20±0.38 |
17±0.84 |
– |
DMSO was used as –ve while fluconazole was used as +ve control
Table (5):
FTIR analysis of MeOH fraction of B. percumberns.
IR values |
Bond |
Functional group |
|---|---|---|
3340 |
O-H Stretch |
Phenols & Alcohols |
2922 |
O-H Stretch |
Carboxylic acid |
1722 |
C=O Stretch |
Ketones |
1600 |
C-C=C Symmetric |
Alkenes |
1359 |
N=O Bend |
Nitro group |
1201 |
C-O Stretch |
Ethers |
1116 |
C-O Stretch |
Ethers |
1070 |
C-O Stretch |
Ethers |
1047 |
C-O Stretch |
Ethers |
1010 |
C-O Stretch |
Ethers |
Table (6):
FTIR analysis of EtOH fraction of B. percumberns.
Values |
Bond |
Functional group |
values |
Bond |
Functional |
|---|---|---|---|---|---|
3348 |
C-C=C |
Amines secondary |
1278 |
C-O Stretch |
Ethers |
2922 |
H-C-H |
Alkanes |
1180 |
C-O Stretch |
Esters |
2850 |
C-H Stretch |
Aldehydes |
1118 |
C-O Stretch |
Ethers |
1651 |
C=O Stretch |
Ketones |
1072 |
C-O Stretch |
Ethers |
1485 |
H-C-H |
Alkanes |
1045 |
C-O Stretch |
Esters |
1375 |
N=O Bend |
Nitro group |
1012 |
C-O Stretch |
Ethers |
1361 |
N=O Bend |
Nitro group |
|||
1319 |
N=O Bend |
Nitro group |
Table (7):
FTIR analysis of EtOAc fraction of B. percumberns.
Values |
Bond |
Functional group |
values |
Bond |
Functional |
|---|---|---|---|---|---|
3288 |
C-C=C |
Amines primary |
1373 |
N=O Bend |
Nitro group |
2918 |
H-C-H |
Alkanes |
1361 |
N=O Bend |
Nitro group |
2848 |
C-H Stretch |
Aldehydes |
1315 |
N=O Bend |
Nitro group |
1732 |
C=O Stretch |
Esters |
1244 |
C-O Stretch |
Ethers |
1645 |
C=O Stretch |
Ketones |
1217 |
C-O Stretch |
Esters |
1606 |
C=O Stretch |
Ketones |
1263 |
C-O Stretch |
Ethers |
1456 |
H-C-H |
Alkanes |
1029 |
C-O Stretch |
Esters |
1014 |
C-O Stretch |
Ethers |
Table (8):
FTIR analysis of EtOAc fraction of B. percumberns.
Values |
Bond |
Functional group |
values |
Bond |
Functional |
|---|---|---|---|---|---|
3346 |
C-C=C |
Amines secondary |
1242 |
C-O Stretch |
Esters |
2918 |
H-C-H |
Alkanes |
1161 |
C-O Stretch |
Ethers |
2848 |
C-H Stretch |
Aldehydes |
1033 |
C-O Stretch |
Esters |
1738 |
C=O Stretch |
Esters |
|||
1732 |
C=O Stretch |
Esters |
|||
1602 |
C=O Stretch |
Ketones |
|||
1558 |
N=O Stretch |
Nitro group |
|||
1539 |
N=O Stretch |
Nitro group |
|||
1456 |
H-C-H |
Alkanes |
|||
1373 |
N=O Bend |
Nitro group |
|||
1315 |
N=O Bend |
Nitro group |
|||
1269 |
C-O Stretch |
Ethers |
Table (9):
FTIR analysis of CHCl3 fraction of B. percumberns.
IR values |
Bond |
Functional group |
|---|---|---|
2918 |
O-H Stretch |
Carboxylic acid |
1602 |
C-C=C Symmetric |
Alkenes |
1375 |
N=O Bend |
Nitro group |
1315 |
N=O Bend |
Nitro group |
1249 |
C-O Stretch |
Ethers |
1159 |
C-O Stretch |
Esters |
1035 |
C-O Stretch |
Ethers |
Table (10):
FTIR analysis of an aqueous extract of B. percumberns
IR values |
Bond |
Functional group |
|---|---|---|
3340 |
O-H Stretch |
Phenols & Alcohols |
2922 |
O-H Stretch |
Carboxylic acid |
1722 |
C=O Stretch |
Ketones |
1600 |
C-C=C Symmetric |
Alkenes |
1359 |
N=O Bend |
Nitro group |
1201 |
C-O Stretch |
Ethers |
1116 |
C-O Stretch |
Ethers |
1070 |
C-O Stretch |
Ethers |
1047 |
C-O Stretch |
Ethers |
1010 |
C-O Stretch |
Ethers |
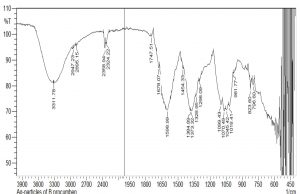
Characterization of AgNPs
FTIR of AgNPs
The FTIR analysis was carried out to identify AgNPs associated biomolecules. The results showed that different compounds were detected in the AgNPs sample, i.e., phenols, alcohols, carboxylic acid, ketones, alkenes, nitro group, and ethers. After comparing the spectra of AgNPs and aqueous extract, it has been confirmed that AgNPs are synthesized. The differences of IR spectra of AgNPs and aqueous extract were due to the compounds of aqueous extracts that have been oxidized by silver ions. Some of the peaks present in aqueous IR spectra were absent in AgNPs extracts. This means that AgNPs have reacted with these groups (Fig. 7-8). The FTIR analysis of AgNPS was interpreted using IR values and presented in Table 11.
Table (11):
FTIR analysis of AgNPs of B. percumberns.
IR values |
Bond |
Functional group |
|---|---|---|
3340 |
O-H Stretch |
Phenols & Alcohols |
2922 |
O-H Stretch |
Carboxylic acid |
1722 |
C=O Stretch |
Ketones |
1600 |
C-C=C Symmetric |
Alkenes |
1359 |
N=O Bend |
Nitro group |
1201 |
C-O Stretch |
Ethers |
1116 |
C-O Stretch |
Ethers |
1070 |
C-O Stretch |
Ethers |
1047 |
C-O Stretch |
Ethers |
1010 |
C-O Stretch |
Ethers |
pH and color
Synthesized AgNPs can also be confirmed physically by pH and color changes. AgNPs change their color from yellowish to dark brown, and pH changes from 6 to 5.5. The change in pH and color confirms the presence of AgNPs.
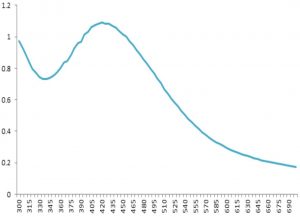
UV-Vis Spectroscopy of AgNPs
The UV-Vis spectroscopy is performed to characterize the AgNPs by the surface plasmon resonance (SPR) with a spectrum of 200 to 700 nm. The maximum peak absorbance with the high band was noted at 395 nm (Fig. 9).
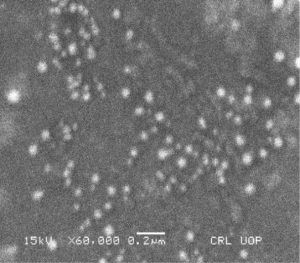
Scanning Electron Microscopy (SEM) of AgNPs
The SEM picture shows the shape and size of the AgNPs. The SEM images showed spherical shape AgNPs in scattered form focused on 0.5 µm of area, with diameter ranges from 20-80 nm (Fig. 10).
Antimicrobial activity
For antimicrobial activities, B. procumbens extracts obtained from different solvents, as well as synthesized AgNPs were screened against bacterial and fungal pathogens (Table 3 and 4). B. Percumbens plant extracts prepared using different solvents, including water, MeOH, CHCl3, EtOH, EtOAc, and n-hexane. MeOH and EtOH fractions resulted in significant activity against pathogenic bacteria and fungi. The extracts of the remaining fraction have no or very few activities. Furthermore, it was also clear that B. Percumbens synthesized AgNPs were more active than crude extracts (Tables 3 and 4). B. Percumbens synthesized AgNPs can be used to enhance the antimicrobial potential of plant constituents against a wide range of infections.
Medicinal plants have attracted significant attention to science communities due to the promising potential in life science (Antony and Sing., 2011). Pakistan is very rich in natural wealth because of it is excellent geographical condition, multiple ecological niches, and soil conditions. It is estimated that 400-600 species out of 5700 are present in Pakistan (Ahmad et al., 2007).
Nanotechnology continues to allow researchers and technologists to find new materials and essential benefits to people. However, it is also an essential part to consider the potential harmful impacts of nanoscale generated products on health as well as our environment (Hull and Bowman, 2018). Due to these facts, it is essential to determine how to decrease dangerous impacts interred by new materials. In the present study, green synthesis of AgNPs was adopted to reduce the negative impact on health and environment because this is the safest method of preparation of AgNPs. The procedure used by Ahmed et al. (2016) for the synthesis of AgNPs by the chemical method was followed. The methodology for the synthesis of AgNPs was the same, but instead of chemical synthesis, the green synthesis method was used in the present study.
The most valuable NPs for medical uses are AgNPs, which are well known for their high antimicrobial activity. AgNPs has a remarkable option for the development of bioactive and novel drugs due to their broad-spectrum activities. Silver ion is famous as a metal ion that shows antimicrobial, antifungal, and anti-algal properties. Mainly, it has been widely used as silver nitrate solution in water, which has sterilizing and also disinfecting activities (Kheybari, 2010). Hussain et al. (2012) revealed that roots containing oil of B. procumbens are composed of methyl ester derivatives of fatty acids. Eighteen types of different components were found and quantified as well. The most important and abundant was the methyl ester of linoleic acid (9%). In the present study, the phytochemical analysis revealed different types of phytochemicals, in which steroids are of great interest nowadays. B. procumbens was used as an entire plant for the process of phytochemical analysis, the plant contains eight important phytochemicals and having good antimicrobial activity. Furthermore, this ability was enhanced by synthesizing the AgNPs and transferring nanomaterials and nanoparticles through a cost-effective route.
Plants having different medically-imported bioactive components could be used to treat severe human pathogenic diseases. There are two types of categories of phytochemicals: i.e., the primary and secondary compounds. The primary compounds contain proteins, chlorophyll, sugar, and amino acids. Secondary compounds include alkaloids and terpenoids (Wadood et al., 2013). The objective of the present study was the same as both studies investigated the phytochemical profile. Important phytochemicals were identified, among them are terpenoids, flavonoids, reducing sugars, and alkaloids.
These compounds serve as a plant defense against microorganisms, insects, and herbivores. For example, phenolics are the simplest phytochemicals, which are effective against viruses (Wild, 1994), bacteria (Thomson, 1978), and fungi (Duke, 1985). Catechol and pyrogallol are both hydroxylated phenols, toxic to microorganisms. The mechanism of action is enzyme inhibition in different ways. Flavonoids are effective antimicrobial due to their ability to complex with extracellular and soluble proteins and to complex with bacterial cell walls (Tsuchiya et al. 1996). Furthermore, quinones cause inactivation of the (Kazmi et al. 1994), tannins could inactivate microbial adhesins, enzymes, cell envelope transport proteins (Nonaka et al., 1990) and alkaloids intercalate with DNA (Phillipson and O’Neil, 1987).
The clinical trial concerning the antimicrobial significance of AgNPs was carried out with the zone of inhibition produced by pathogenic organisms (gram-negative and gram-positive bacteria) and some disease-causing fungus. (Geoprincy, 2013). The present study aimed to evaluate the antimicrobial activity of B. procumbens. Among extracts used, methanol and ethanol showed significant activity against targeted pathogenic bacteria and fungi. The extract of the remaining fractions did not show or showed less activity. Interestingly, the prepared AgNPs from B. procumbens showed better results as compared to crude extracts.
The authors are grateful for the financial support provided by the Higher Education Commission of Pakistan for awarding a startup grant No (21:619/SRGP/R&D/HEC/2014. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for its funding this prolific research group no. (R.G.P.2.115/41).
ACKNOWLEDGMENTS
The authors are grateful for the financial support provided by the Higher Education Commission of Pakistan for awarding a startup grant No (21:619/SRGP/R&D/HEC/2014. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for its funding this prolific research group no. (R.G.P.2.115/41).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MR, SA, BKM, AR, MS, KU, SB, UM, YNM drafted the manuscript, compiled information from the literature, and designed the Fig. and tables. MR, SA, BKM, AR, MS, KU, SB, UM, YNM drafted the manuscript and gathered information from the literature. MR, SA, BKM, AR, MS, KU, SB, UM, YNM supervised, and reviewed the manuscript. MFR supervised and reviewed the manuscript.
FUNDING
This work was supported by the Deanship of Scientific Research at King Khalid University (Grant number R.G.P. 2/23/41/2019).
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Abo El-Maati MF, Mahgoub SA, Labib SM, Al-Gaby AMA, Ramadan MF. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur J Integr Med. 2016;8:494-504.
Crossref - Ahmed S, Ahmad M, Swami BL, et al. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci.2016;9(1):1-7.
Crossref - Ali KW, MI,Shinwari, S Khan. Screening of 196 medicinal plant species leaf litter for allelopathic potential. Pak J Bot. 2019;51(6).
Crossref - Bajguz A. Metabolism of brassinosteroids in plants. Plant Physiol Biochem. 2007;45(2):95-107.
Crossref - Choudhury R, Majumder M, Roy DN, Basumallick S, Misra TK. Phytotoxicity of Ag nanoparticles prepared by biogenic and chemical methods. Int Nano Lett. 2016;6(3):153-159.
Crossref - Chouhan, N. Silver Nanoparticles: Synthesis, Characterization, and Applications. In Silver Nanoparticles-Fabrication, Characterization, and Applications. IntechOpen. 2018.
Crossref - Cushnie TT, Cushnie B, Lamb AJ. Alkaloids: An overview of their antibacterial, antibiotic-enhancing, and antivirulence activities. Int J Antimicrob Agents. 2014;44(5):377-386.
Crossref - Duke JA. Handbook of medicinal herbs. CRC Press, Inc., Boca Raton, Fla. 1985.
- Geoprincy G, Srri BV, Poonguzhali U, et al.. ‘A review on green synthesis of silver nanoparticles,’ Asian J Pharm Clin Res. 2013;6(1):8-12.
- Hooda, Vinita, Nidhi Chauhan. A Novel Eco-friendly Approach for the Synthesis of Silver Nanoparticles Using Immobilized Glucose Oxidase. Nanoscience & Nanotechnology-Asia. 2018;8(1):55-66.
Crossref - Hull, M., & Bowman, D. (2018). Nanotechnology environmental health and safety: risks, regulation, and management. William Andrew.
- Hussain I, Talha M, Ullah R, et al.. ‘Fatty acid composition of Boerhaavia procumbens L. roots oil by gas chromatography mass spectrometry. J Med PlantsRes. 2012;6(14):2789-2792.
- Iravani S, Korbekandi H, Mirmohammadi SV, et al. ‘Synthesis of silver nanoparticles: chemical, physical and biological methods.Res Pharm Sci. 2014;9(6):385-406.
- Iwu MW, Duncan AR, Okunji CO. New antimicrobials of plant origin. Perspectives on new crops and new uses. ASHS Press, Alexandria, VA, 1999;457-462.
- Kazmi MH, A Malik S Hameed N Akhtar, S Noor Ali. 1994. An anthraquinone derivative from Cassia italica. Phytochemistry. 1999;36:761–763.
Crossref - Khalil A, Z Iqbal, A Adhikari. Chemical Composition, Phytochemical Analysis, and Antimicrobial Activity of Boerhavia Procumbens. Bangladesh Journal of Pharmacology. 2017;12(4):427-33.
Crossref - Khan AU, Akram M, Daniyal M, et al. Awareness and current knowledge of epilepsy. Metab Brain Dis. 2020;35(1):45-63.
Crossref - Khattak U, Ullah R, Khan SA, Jan SA, Rauf A, Ramadan MF. Synthesis, characteristics, and biological activities of silver nanoparticles from Euphorbia dracunculoides. Eurasia J Biosci. 2019;13(2):2249-2260.
- Mansoori GA, Soelaiman TF. Nanotechnology-an introduction for the standards community. Journal of ASTM International. 2005;2(6):1-22.
Crossref - Mohdaly AAA, Mahmoud AA, Roby MHH, Smetanska I, Ramadan MF Phenolic extract from propolis and bee pollen: composition, antioxidant, and antibacterial activities. Journal of Food Biochemistry. 2015;39:538-547.
Crossref - Morsy MK, Khalaf HH, Sharoba AM, El-Tanahi HH, Cutter CN. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. Journal of Food Science. 2014;79(4):M675-M684.
Crossref - Nonaka G-I, I Nishioka, M Nishizawa, et al. Anti-AIDS agents. 2. Inhibitory effects of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells. J. Nat. Prod. 1990;53:587-595.
Crossref - Phillipson JD, MJ O’Neill. New leads to the treatment of protozoal infections based on natural product molecules. Acta Pharm. Nord. 1987; 1:131-144.
- Pirtarighat S, Ghannadnia M, Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostructure Chem. 2019;9(1):1-9.
Crossref - Premnath D, Gomez, P. ‘Antifungal and Antibacterial Activities of Chemical Constituents from Heliotropium indicum Linn. Plant’.Drug Invention Today. 2012;4(11).
- Ramadan MF.Quercetin increases antioxidant activity of soy lecithin in a triolein model system. LWT-Food Science and Technology. 2008;41:581-587.
Crossref - Ramadan MF, Amer MMA, Awad A. Coriander (Coriandrum sativum L.) seed oil improves plasma lipid profile in rats fed diet containing cholesterol. Eur Food Res Technol. 2008;227:1173-1182.
Crossref - Rauf A, Bawazeer S, Uddin G, et al.. Muscle relaxant activities of pistagremic acid isolated from Pistacia integerrima. Z Naturforsch C J Biosci. 2018;73(11-12), 413-416.
Crossref - Shakya AK. Medicinal plants: future source of new drugs. International Journal of Herbal Medicine. 2016;4(4):59-64.
- Singh S, Khatoon S, Singh H, et al. ‘A report on pharmacognostical evaluation of four Adiantum species, Pteridophyta, for their authentication and quality control,’. Rev. Bras. Farmacogn. 2013;23(2):207-216.
Crossref - Sultana K, Bashir A, Abdur R, Linfang H, Ramadan MF. Synthesis, characterization and pharmacological activities of silver nanoparticles using Bistorta affinis and Malcolmia cabulica extracts. Bioinspired, Biomimetic, and Nanobiomaterials. 2019;9(1):7-15.
Crossref - Thomson WA, R. (ed.). Medicines from the Earth. McGraw-Hill Book Co., Maidenhead, United Kingdom. 1978.
- Tran QH, Le AT. Silver nanoparticles: synthesis, properties, toxicology, applications, and perspectives. Advances in Natural Sciences: J Nanosci Nanotechnol.2013;4(3):033001.
Crossref - Tsuchiya H, M Sato, T Miyazaki, et al. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50:27-34.
Crossref - Udapudi B, Naik PK, Savadatti ST, Sharma R, Balgi S. Synthesis and characterization of silver nanoparticles. IOSR J Pharm Biol Sci. 2012;2(3):10-14.
- Vazhacharickal PJ, Mathew JJ, Tomy C. The Honey Apple and its phytochemical analysis. ISBN-10: 3668470073, Grin Publishing. 2016.
- Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar, R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2(4):1-4.
Crossref - Wajid M, Wajid S, Aslam MS, Ahmad MA. An Update Review on Ethnomedicinal, Phytochemical and Pharmacological Profile of genus Boerhavia. Int J Complement Alt Med. 2017;6(3):00189.
Crossref - Wild, R. (ed.). The complete book of natural and medicinal cures. Rodale Press, Inc., Emmaus, Pa. 1994.
- World Health Organization. The world health report 2002: reducing risks, promoting healthy life. World Health Organization. 2002.
- Zhao Q, Dixon RA. Transcriptional networks for lignin biosynthesis: more complicated than we thought?. Trends in Plant Science. 2011;16(4):227-233.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.