Mastitis is a highly frequent chronic ailment with inflammation in the udder of the milking cows. The causative agents are mostly microbes. It is economically prominent contamination of lactating cows resulting in reduced milk production. The disease is diagnosed by chemical, physical and nutritional changes in the milk and pathological changes in the milk glands. Prevention measures for the disease can be taken by proper and timely sanitation of the cowshed through and time again disinfection of the teat, mechanized milking process, etc. The application of bactericidal drugs generates resistant varieties of microbes that cross the allopathic boundary. In this regard, an attempt is taken to focus the plant-based pharmacopoeia. Medicinal plants are traditionally used to cure various diseases as they are comparatively accessible to administer orally in different forms and can be along with fodder. Keeping the above facts in view, the present review deals with different types of mastitis, causative pathogens, detection and diagnosis, and effective plant-based treatment process available to date.
Bovine Mastitis, Causative Agents, Lactating Cows, Medicinal Plants
Dairy cows are considered the mother of human society. Fresh and hygienic milk is very much popular due to abundantly available nutraceutical.1 As all the macro and micronutrients, vitamins, minerals growth factors and some other nutritional factors are there in the milk for which it is the most balanced and natural diet obtained universally.2 Poor production of milk is always less than the requirement due to various factors. Some constraints are inappropriate management, mismatched genetics, nutritional deficiency, unhealthy reproductive condition, and frequently infected teat along with the udder, internally and externally. These factors contribute adversely to insufficient production of milk by lactating cows.3 It is one of the most frequent diseases of cattle and capable of causing serious damage to dairy herds worldwide.4 The symptoms of the disease are unusual increment in the number of somatic cells and deterioration in the quality of milk.5 High somatic cell count (SCC) in mastic cows is detected by the fluro-optoelectronic method.6 SCC approximately 200,000 cells/ml is the threshold value for mastitis.7 Mastitis also can transmit chronic diseases like tuberculosis brucellosis, leptospirosis, etc. through contaminated milk to consumers.8 The disease differs from other diseases of cattle as a plethora of mostly bacterial pathogens invades udder tissue. These stubborn pathogens enter the udder, navigate, infect and produce the toxin which inflammate the udder along with the teats which were more susceptible. The outcome of which is a drastic change in milk quality and quantity.9 The cost-effective benefits of the poor farmers were adversely affected due to contaminated and unsafe dairy products.9-11 The total economic estimation towards loss is approximately ₹ 7824 in one month per cow.10
Types of Mastitis
Depending on the infection pattern, mastitis is of different types; contagious and environmental with a broad spectrum of microbes. Besides the above types, it can be subclinical or clinical.12,13 Contagious mastitis is generated from inflected ones and contaminated to healthy cows by the time of milking through hands. It can also spread through improper sanitization of milking machines or by cloths used during milking but in environmental mastitis, the causal pathogens originate from cowsheds, unhealthy filthy water for preparation of udder prior to milking, flies, mud holes.13 Intramammary infections (IMIS) were diagnosed as SCM. However, it is difficult to identify SCM as initially it appears inside without any visible inflammation or redness in the udder or teat but an increase of somatic cells in the milk act as an indicator.14 The occurrence of SCM was ~32.48% and considerably lowers in clinical mastitis 9.4%. 15 SCM could not be noticed so easily but is always associated with loss of milk in the dairy herd.16 Traditional and advanced therapies are applicable for the control of mastitis. The treatments were through bacteriocins, nanoparticle based, vaccination, antibiotics, and phytotherapy. These therapies are not effective because multiple factors (host, pathogen, and environment) are responsible for the disease.17 To date for curing mastitis, the broad-spectrum antibiotics were used as the common therapy. However, the consequence of this antibiotic therapy is the drug resistance microbes. Substitutes for this type of therapy were explored by many researchers.18-20 Thus, herbal therapy for mastitis treatment is an accurate alternative to be replaced for synthetic drug.21
Causative Pathogens
Many mastitis pathogens are identified to dates, such as Staphylococcus aureus, Streptococcus agalactiae and other Streptococcus spp.22-26 Streptococcus dysgalctiae is a major causal microbe reported in the case of SCM in bovine herds followed by Clostridium perfrigens, Mycobacterium, Mycoplasma, Prototheca, Pasturella, Nocardia asteroids, Pseudomonas auriginosa, Staphylococcus aureus and yeasts.27 Actinomyces spp., Staphylococcus spp. and Streptococcus spp. are some pathogenic bacteria isolated from bovine mastitis.28 Staphylococcus aureus, Streptococcus agalactiae and Mycoplasma spp. are contagious bacteria causing contagious mastitis.13 Microorganisms that cause mastitis are categorised into three categories; 1. Contagious (Corynebacterium bovis, Staphylococcus aureus, Streptococcus agalactia, Mycoplasma sp.) 2. Environmental (Enterobacter aerogenes, Escherichia coli, Klebsiella pneumonia, Klebsiella oxytoca, Streptococcus uberis, Streptococcus bovis, Streptococcus dysgalactiae, Citrobacter sp. and Serratia sp.) and 3. Other (Coagulase-negative Staphylococci sp., Arcanobacterium pyogenes, Candida sp., Nocardia asteroids, Pseudomonas aeruginosa, Prototheca sp. and Serratia sp.29 Staphylococcus aureus is predominant in mastitis samples as reported by many workers.30-33 Escherichia spp. (22.16%), Klebsiella spp. (1.47%), Pasteurella spp. (2.45%), Pseudomonas spp. (0.45%) Staphylococcus aureus (20.19%), Streptococcus spp. (13.3%), Streptococcus agalactiae (12.8%) and Streptococcus dysgalactia (0.5%) are the most common pathogens isolated from mastitis milk sample.34 Staphylococcus, Aerococcus, Streptococcus, Enterobacter, Macrococcus, Corynebacterium, Acinetobacter, Psychrobacter, Ignavigranum, and Atopostipes are the bacteria species which are found to be causative pathogen for bovine mastitis. Staphylococcus spp. and Streptococcus spp. are maximum contaminants amongst all these.35 Escherichia coli, Klebsiella spp. and Staphylococcus aureus are isolated from the mastitis milk sample.36 Escherichia coli, Klebsiella oxytoca, Klebsiella pneumonia, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, and Staphylococcus aureus are isolated and identified from samples collected from mastitis cow.5 The most predominant bacterial pathogen responsible for the high prevalence in both subclinical and clinical mastitis was S. aureus followed by E. coli and S. agalactiae. The frequency of mastitis due to fungi and yeast was found to be very less as compared to the bacterial pathogens.37 Overuse of antibiotics and poor sanitation contributes to yeast mastitis.34
Detection and Diagnosis
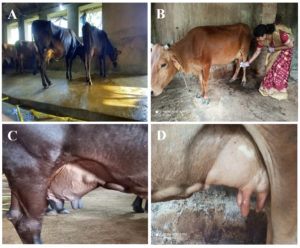
Mastitis is easy to detect and diagnose clinically, in which the type phenotypical observations are quite visible like red and swollen udder and fever in dairy herds (Figure). The texture of milk is different with flakes and clots than the normal type.35,39 It was also notified that during infection an uncountable group of white blood cells (leukocytes) invade the mammary gland.
Figure. Images showing cow affected with mastitis during the sample collection. A: Filthy cowshed; B: Sample collection; C: Infected udder; D: Swollen udder
Risk Factors
Some incidental factors for pathogens of mastitis are parity, stage and lactation, quantity production, hygienic nutrition and breeds.40-42 Several risk factors like body weight, age, milk yield, udder type, teat size, floor and bedding, season, parity and duration of lactation are associated strongly with the pathogens of mastitis.15 Other factors are inherent like susceptibility of mammary gland for intramammary infections (IMI), breed type, previous mastitis history and lactation stage are also countable for the prevalence of mastitis.43 Due to the pathogenic load in lactating animals milk stasis (hindrance to milking) also enhances the density of disease. Various factors concerned with this disease are discussed below.
Age
Older cows having more body weights are susceptible to mastitis.15 There is a correlation between the age of the lactating cow and mastitis due to the relaxed sphincter muscles of teat.44 Stress associated with old age makes the animal prone to a weak immune system. Hence the older cow’s infection rate is persistent and ultimate loss is more due to the incidence of mastitis.15
Breed
Cross breed jersey (70.41%) is more susceptible to mastitis in comparison to HF (16.33%) and local breeds (13.27%).15 In India, Jersey-Holstein cross breed cows are more prone (94.54%) to mastitis than indigenous breeds (31.25%).16 Maximum report of mastitis was observed in cross breed herd than local indigenous one as reported.45
Species
Buffaloes are less susceptible to mastitis than cows.46,47 Buffaloes are highly resistant as they have tightly closed teat orifices.
Milk Yield
Increased incidence of mastitis in high yielders than low yielders stated by many workers. This may be due to variable immune response of cow against infection.15,48,49 Cross breeds are high yielders and have heavy body weight than indigenous cows. So, exposure to more body surface area also invites environmental microbes to generate mastitis. The body area and weight of the hybrid bovines have more physiological stress. Due to the higher production of milk opening of teat sphincters for a continuous period invites the colonization of microbes for mastitis.15
Parity
The highest incidence of mastitis was seen in 5th parity followed by 4th, 3rd, 2nd and 6th with the lowest incidence in 8th one. The attributable reason is that IMI remains persistently up to 5th parity stage and gradually declines.50,51
Stage of Lactation
Onset of lactation also sometimes generates contagious disease in milking animals. First month of lactation and last months were more critical for mastitis than the in between period.15,52 Lactation also harbours microorganisms due to lack of sanitation or other factors.53 Accordingly, first month of lactation is prominent for mastitis and gradually diminishes towards the onset of dry periods i.e., the last months of milk formation. The frequency of mastitis is more in first two months of lactation and 2-3 weeks from the beginning of dry period.54 Sometimes cows at the lactating period of early maximum days are more prone to environmental contaminants as reported by.55,56 Generally, first day of lactation is more susceptible (62.7% for mastitis might be due to the hypersensitivity of mammary glands). It is also observed that gradually it declines towards the late stage (11.2%).52 One of the prime factors of mastitis during the early lactation period is that oxidative stress is more and could not be neutralised by antioxidative defence.43
Herd Size
There are significantly more (46.6%) mastitis reports with larger herd size than smaller one.15,48 The population of herds in the dairy industry is also a prominent factor for the maintenance of healthy lactating animals. Improper management of sanitation, hygiene, maintenance of the floor and bedding area are contributing significantly to the proliferation and transmission of mastitis microbiota.15
Season
Highest incidence of mastitis is observed in the rainy season (62.24%) followed by the summer (26.53%) and the winter (4.08%).15 It seems that breakout of mastitis is more in low temperature. This depicts that the bacterial growth is more suitable in low temperature climate.57,58
Floor Type of the Shed
Cowshed matters much for the origin of mastitis. Earthen floor of shed makes the dairy animals maximum (48.98%) susceptible than brick floor (38.78%) and concrete floor (12.24%). Maximum occurrence of the disease is reported in the earthen floors might be due to improper cleaning and dampening.15 Poorly designed facilities increase the incidence of environmental mastitis.41 Cowsheds are mostly in unhygienic condition due to obvious reasons, which plays a major role in harbouring environmental pathogens for mastitis. Due to the humid climate basement of cowshed mostly remains muddy or swampy favouring the growth of mastitis.43
Udder Type Teat Wise Prevalence
Prevalence of mastitis is highest in cows with cup shaped udder (48.98%), then bowl shaped udder (30.61%) and round shaped udder (20.41%).15 Cup or pendulous udder (depending on the depth) is closer to filthy ground in unhygienic condition in a suitable basement for mastitis.44 Occurrence of mastitis is comparatively less in fore quarter 42.85% than hind (57.14%). Accordingly, the right hind teat is more prone (40%) than the left hind (17%), right fore 19% and left fore 24%.15 Teat wise or udder wise contamination is more in hind part and the reason could be milk yield potential followed by contaminated hind legs and relaxed teat sphincters.59 Generally, in dairy cattle prevalence of mastitis in hind quarters were comparatively more due to the exposure of that part to dung and urine.60
Method of Milking
Occurrence of mastitis is more frequent in hand milking process than the mechanised ones.15 Mechanised milking process is always advantageous to obtain safe and healthy milk.61
Teat Injury
Prime pathway for entry of mastitis is the teat canal. Hence attention should and must be focused on the maintenance of a healthy and clean teat. Most important is to avoid teat injury, which is a risk factor for contamination of the intra- mammary glands.62
Use of Teat Disinfectant
Disinfectants are always advisable particularly for teat and udder which lowers a load of contaminants of mastitis proper sanitation is mandatory during the lactation period to control the spread of microbial infections and their colonial growth.63
Habitat
Incidence of the disease is more in rural habitats than that of urban. Urban areas were more facilitated as regards general awareness of the disease, proper veterinary services and immediate attention to the animals as and when required. In contrast, rural areas have slow pace lifestyle, sluggish and careless towards the dairy herds. Negligence in sanitation, hygiene and hospital service in rural areas make the destitute cows more susceptible to microbes for mastitis or any other disease.15
Dry Period
There is an increased risk of clinical mastitis with longer dry period >40 days.64 The frequency of infection increases during the two weeks prior to calving and two weeks following drying.65 However, the intramammary infection is 2 to 12 times higher during the dry period.66
Transition Period
A transition phase during parturition is more critical for any contagious disease in dairy cows. This phase is 4 weeks before the birth of calf and continues up to 4 weeks after.67 This is due to physiological stress associated with other factors like intensive growth of mammary glands, onset of overflowing synthesis and secretion of milk with higher energy requirement. To meet the energy, the need of oxygen is improved to a higher level.68 It is also experienced that during the peripartum duration alteration in defence mechanism is an inevitable change may be due to hormonal imbalance or any type of stress.69
Milking Interval
Due to irregular intervals for milking and the pre, post milking treatment of the teat and teat canals mostly the time interval in lactating cows are neglected seriously. As the time gap is more than 12 hours per day, which allows the bacteria to colonize at the teat ends.70
Blood Group
In Red Danish dairy cattle, the blood group is correlated with the emergence of mastitis during the lactation period. It was observed that bovines with the M blood group were more susceptible to bacterial infection as compared to the cattle herds lacking it.71
Control and Treatment
In spite of application of all the synthetic and strong antimicrobial agents, the microorganisms put their inherent quality of resistance by altering their genome structure.72 The outcome of this is drug resistance, which is sometimes considered a progressive evolutionary trend. This type of system generates resistant microbes which can survive, reproduce and stabilize themselves along with the microbiocidal drugs.73 The above facts forcibly paved the pathway in biological science to develop effectively the alternate branch of drug from the living world in which lower and higher groups of plants occupied the prime position in livestock health research.74 It was also observed that supplementation of vitamin D also reduces the bacterial load. Ancient literatures already established the fact that (Vanaspati), the plants were utilised in Ayurveda, Siddha and Unani for curing different ailments of living world.75 According to the ethnomedicinal branch of science most or all plants possess an array of secondary metabolites otherwise known as natural products like terpenes, phenolics and nitrogen containing metabolites, alkaloids glucosinates, cyanogenic glycosides, exhibit significant defensive activity for their pests and most of the microbes.76,77
Medicinal Plants used Against Mastitis
The pharmacopoeia database evidenced the ethnomedicinal property of plants against various contamination created by the pathogenic organism. The significant aspect of application of plant-based therapeutics is the minimal side effects.78,79 Several plants (whole or part) and plant extracts were traditionally applied for the treatment of mastitis in dairy cows. The most frequently reported plant species are Amomum subulatum, Allium sativum, Capsicum annuum, Centratherum anthelmisticum, Citrus limon, Citrullus colocynthis, Curcuma longa, Cuminum cyminum, Lepidium sativum, Nigella sativa, Peganum harmala, Rosa indica, Sesamum indicum, Triticum aestivum, and Zingiber officinale.21 Extracts of Artemisia absinthium, Baccharis dracunculifolia, Cymbopogon nardus, Senna macranthera, with different solvents exhibited antibacterial activity against Staphylococcus aureus strains isolated from mastitic cows.80 Methanolic extracts of Abtulion indicum, Brachiaria sp., Cenchrus ciliaris and Coccinia grandis showed conspicuous antimicrobial activities against bovine mastitis pathogens.81 Antibacterial activity and chemical profiling of Punica granatum was effective against pathogens isolated from cows with mastitis.82 In vitro bactericidal properties of some selected wild medicinal plants used to cure the most frequent disease of lactating bovine, the mastitis.83 Methanolic extracts of Abutilon indicum, Asteracantha longifolia, Brachiaria sp and Trichodesma indicum displayed antimicrobial activity against Staphylococcus aureus isolated from Bovine mastitis.77 Distilled water extracts of Aloe barbadensis, Annona squamosal, Azadirachta indica, Curcuma longa, Macrotyloma uniflorum, Phyllanthus niruri and Terminalia chebula were found to be effective in treatment of Bovine mastitis.36 Traditionally used ethnoveterinary herbs in northwest Pakistan like Oryza sativa, Triticum aestivum, Bunium persicum and Allium sativum were therapeutically active against most common microbial pathogen. Out of the above-mentioned plants, alkaloid of two (B. persicum and A. sativum) strongly inhibits the growth of mastitis causing bacteria.84 Mastitis could be treated with intramammary infusion of extract of Rheum officinale and Angelica dahurica.85 Extracts of Allium sativum, Zingiber ofcinale and Capsicum annuum were highly effective against multidrug resistant Staphylococcus aureus and Streptococcus pyogenes isolated from buffalo mastitic milk.86 Bactericidal property of Artemisia nialgirica was found to be effective against Bacillus subtilis, Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Salmonella typhi, Staphylococcus aureus and Yersinia enterocolitica.87 Plant extracts of Artemisia herba-alba and Jasonia montana were found efficacious to counter bacterial flora of clinical or sub clinical mastitis.34 Extracts of Combretum mole and Xanthium strumarium manifested good in vitro antibacterial effect against Streptococcus agalactiae and Staphylococcus aureus isolated from cows with mastitis.88 Leave extracts of Syzygium cumini, Millingtonia hortensis and Zizyphus mauritiana have a strong antimicrobial effect against Staphylococci (Coagulase-negative) collected from lactating cows having mastitis.89 Hexane extract of Artemisia nilagirica has a low MIC value against Bacillus subtilis, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella typhi, Shigella flaxneri and Yersinia enterocolitica. Artemisia nilagirica also proved to be an active agent for treating related microbial diseases.90 Acetone solvent extract of J. montana plant showed antibacterial activity against S. agalactiae, E. coli, S. aureus, Klebsiella spp and coagulase-negative Staphylococci.34 Crushed roots of Asparagus racemosus were with about 100gms of fruits of Trigonella foenum-graecum, Foeniculum vulgare, Terminalia chebula, Terminalia bellirica, Piper nigrum, Elettaria cardamomum, flower buds of Eugenia caryophyllu and 200gm of Alium cepa blended with water and further a small amount of jaggery was added to that, these formulations were administered orally to cure mastitis.91 Essential oil of Thymus serpyllum and T. vulgaris in different formulations were tested against the microbes of clinical and sub clinical mastitis, which can be a substitute for the common antibiotics in the near future.92 Acetone extracts of A. nilotica bark and Tetradenia riparia flowers showed the best activity against mastitis causing bacteria.93 Similarly, the alcoholic extracts of marigold, absinthe wormwood, essential oils of oregano, lavender, and rosemary could be effective against mastitis.6 Methanolic and ethanolic extracts of Laggera alata showed antibacterial activity against Streptococcus agalactiae and Staphylococcus aureus isolated from bovine mastitis.94 Methanolic extracts of Gymnema sylvestre, Holarrhenaanti dysenterica, Vernonia anthelmintica, Enicostemma littorale, Momordica charantia, Swertia chirata, Azadirachta indica and Caesalpinia bonducella showed more or less antibacterial activity against mastitis pathogens. Among these Azadirachta indica showed the most promising antibacterial properties.95 Bactericidal properties of extracts (aqueous and methanolic) of Tridax procumbens were applied against Staphylococcus aureus. It was noticed that the extracts were quite effective against the causal pathogens of mastitis in lactating animals.96 The therapeutic property of herbal raw materials as a treatment for mastitis can be used as an appropriate dose at the initial stage is a cost effective one. Above all to enhance the resistance of the body, contamination and inflammation of the teat and udder it is mandatory to follow the maintenance of aseptic environment around habitat of the lactating animal. Moreover, the efficacy of proper and balanced fodder helps a lot for the reduction of contamination.
The rising dairy market and its versatile products throughout the globe are very lucrative and significant. As the consumers are of all the age groups like infants to super senior citizens, so care must and should be taken for the deliverance of healthy dairy products. With the increasing trend of antibiotic resistance, the frequent occurrence of bovine mastitis thrust a stake and stress on the dairy industry, particularly with more milking breeds. Attempts should be taken to reduce the prevalence of microbial contamination at the udder and the associated glands or tissues. Besides antibiotics, alternative therapies in the form of herbal application or supplementation in fodder can help in the treatment of mastitis.
ACKNOWLEDGMENTS
The authors would like to thank Sri Sri Bayababa College, Maharaja Sriram Chandra Bhanja Deo University, Kendrapara Autonomous College, Utkal University and AIPH University for providing encouragement and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The data generated during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Etgen WM, Paul M. Reaves. Dairy cattle feeding and management. 6th ed. John Wiley & Sons. 1978.
- Chaplin LC, Lyster RLJ. Effect of temperature on the pH of skim milk. Journal of Dairy Research.1988;55(2):277-280.
Crossref - Mukasa-Mugerwa E. Review of reproductive performance in female Bas indicus (Zebu) cattle. ILCA monograph, No.6.ILCA, Addis Ababa. 1998.
- Ojo OE, Oyekunle MA, Ogunleye AO, Otesile EB. Escherichia coli, O157:H7 in food animals in part of South-Western Nigeria: Prevalence and in vitro antimicrobial susceptibility. Trop Vet. 2009;26(3):23-30.
- Abdi RD, Gillespie BE, Ivey S, Pighetti GM, Almeida RA, Dego OK. Antimicrobial Resistance of Major Bacterial Pathogens from Dairy Cows with High Somatic Cell Count and Clinical Mastitis. Animals. 2021;11(1):131.
Crossref - Pasca C, Marghitas LA, Dezmirean DS, et al. Efficacy of natural formulations in bovine mastitis pathology: alternative solution to antibiotic treatment. J Vet Res. 2020;64(4):523-529.
Crossref - Dohoo IR, Meek AH. Somatic cell counts in bovine milk. Can Vet J. 1982;23:119-125.
- Moges N, Asfaw Y, Belihu K, Tadasse A. Antimicrobial susceptibility of mastitis pathogen from smallholder dairy herds in and around Gondar, Ethiopia. Anim Vet Adv. 2011;10(12):1616-1622.
Crossref - Aghamohammadi M, Haine D, Kelton DF, et al. Herd-level mastitis-associated costs on Canadian dairy farms. Front Vet Sci. 2018;5:100.
Crossref - Das D, Panda SK, Jena B, Sahoo AK. Economic impact of subclinical and clinical mastitis in Odisha, India. Int. J Curr Microbiol App Sci. 2018;7(3):3651-3654.
Crossref - Izquierdo AC, Liera JEG, Cervantes RE, et al. Production of Milk and Bovine Mastitis. J Adv Dairy Res. 2017;5(2):174.
Crossref - Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12(1):270.
Crossref - Garcia A. Contagious vs. Environmental Mastitis. Extension Extra. 2004. http://openprairie.sdstate.edu/extension_extra/126
- Kumar GSN. Apannavar MM, Surnagi MD, Kotresh AM.Study on incidence and economics of clinical mastitis. Karnataka. J Agric Sci.2010;23(2):407-408.
- Tripathy RK, Rath PK, Panda SK, Mishra BP, Jena B, Karna DK. Studies on Prevalence and Epidemiological Risk Factors of Bovine Mastitis in and around Bhubaneswar, Odisha. Int J Live Res. 2018;8(9):151-157.
Crossref - Sharma N, Maiti SK. Prevalence and etiology of sub-clinical mastitis in cow. Indian J Vet Res. 2010;19(2):45-54.
- Gomes F, Henriques M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr Microbiol. 2016;72(4);377-382.
Crossref - Sharun K, Dhama K, Tiwari R, et al. Advances in therapeutic and managemental approaches of bovine mastitis: a comprehensive review. Vet Q.2021;41(1):107-136.
Crossref - Babra C, Tiwari JG, Pier G, et al. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol. 2013;58(6):469-474.
Crossref - Park YK, Fox LK, Hancock DD, McMahan W, Park YH. Prevalence and antibiotic resistance of mastitis pathogens isolated from dairy herds transitioning to organic management. J Vet Sci. 2012;13(1):103-105.
Crossref - Dilshad SMR, Rehman NU, Ahmad N, Iqbal A. Documentation of ethno veterinary practices for mastitis in dairy animals in Pakistan. Pak Vet J. 2009;30(3):167-171.
- Chahar A, Gahlot AK, Tanwar RK, Fakhruddin. Evaluation of different screening tests for diagnosis of sub clinical mastitis in cattle. Indian J Vet Med. 2008;28:91-93.
- Kader MA, Samad MA, Saha S, Taleb MA. Prevalence and ethology of sub clinical mastitis with antibiotic sensitivity to isolated organisms among milch cows in Bangladesh. Ind J Dairy Sci. 2002;55:218-223.
- Sharma N.Foot and mouth disease mastitis cascade in dairy cattle: A field study. Int J Zool Res. 2008;4(1):64-67.
Crossref - Sudhan NA, Singh R, Singh M, Soodan JS. Studies on prevalence, etiology and diagnosis of sub clinical mastitis among cross breed cows. Indian J Anim Res. 2005;39(2):127-130.
- Young ZJ, Fang X, Mei Y, et al. Isolation and identification of pathogens from mastitis cow and drug sensitivity test. China Anim: Husb. Vet Med. 2009;36:136-140.
- Kumar M, Goel P, Sharma A, Kumar A. Prevalence of sub clinical mastitis in cow at a Goshala. Proceeding of compendium of 27th ISVM International summit and convention at Chennai, February, Tamilnadu, India. 2009:4.
- Sharma N. Economically important production diseases of diary animals. Sarva Manav Vikash Samiti, Gurgaon, India. 2010.
- Sudhan NA, Sharma N. Mastitis. An important production disease of dairy animals 1st Ed. Sarva Manav Vikash Samiti, Gurgaon, India.2010.
- Abdel-Rady A, Sayed M. Epidemiological studies on subclinical mastitis in dairy cows in Assiut Governorate. Vet World. 2009;2(10):373-380.
Crossref - Rahman MM, Islam MR, Uddin MB, Aktaruzzaman M. Prevalence of subclinical mastitis in dairy cows reared in Sylhet District of Bangladesh. Int J BioRes. 2010;1(2):23-28.
- Hee-Jung K, Ik-Chun K, Jin-Hoe K, Won-Geun S, Du-Sik L. Identification and antimicrobial susceptibility of microorganism isolated from bovine mastitic milk. Korean J Vet Res. 2001;41(4):511-521.
- Sharma N. Alternative approach to control intramammary infection in dairy cows: A review. Asian J Anim Vet Adv. 2007;2(2):50-62.
Crossref - Zeedan GSG, Abdalhamed AM, Abdeen E, Ottai ME, Abdel-Shafy S. Evaluation of antibacterial effect of some Sinai medicinal plant extracts on bacteria isolated from bovine mastitis. Vet World. 2014;7(11):991-998.
Crossref - Sokolov S, Fursova K, Shulcheva I, et al. Comparative analysis of milk microbiomes and their association with Bovine mastitis in two farms in central Russia. Animals. 2021; 11(5):1401.
Crossref - Dinesh MD, George A, Vijayan A, et al. Prevention of Mastitis in Dairy Cattle’s at Wayanad District, Kerala, South India using “Herbalism”. Int J Appl Pure Sci Agric. 2016;2(2):253-260.
Crossref - Kirk JH, Bartlett PC. Bovine mycotic mastitis. The Compendium on continuing education for the practicing veterinarian. 1987;8(11):106-11.
- Ganguly S. Mastitis an Economically Important Disease in Milching Ruminants. Agri-BioVet Press (a unit of Prashant Book Agency), Daryaganj, New Delhi, India. 2018.
- Khan MZ, Khan A. Basic factors of mastitis in Dairy animals: A review. Pak Vet J. 2006;26(4):204-208.
- Smith KL, Hogan JS, Weiss WP. Dietary vitamin E and selenium affect mastitis and milk quality. J Anim Sci. 1997;75(6):1659-1665.
Crossref - Barkema HW, Schukken YH, Lam TJGM, Beiboer ML, Benedictus G, Brand A. Management practices associated with the incidence rate. J Dairy Sci. 1999;82(8):1643-1654.
Crossref - Zadoks RN, Allore HG, Barkema HW, Sampimon OC, Wellenberg GJ, Grohn YT. Cow and quarter level risk factors for Streptococcus uberis and Staphylococcus aureus mastitis. J Dairy Sci. 2001;84(12)2649-2663.
Crossref - Tezera M, Ali A. Prevalence and associated risk factors of Bovine mastitis in dairy cows in and around Assosa town, Benishangul-Gumuz Regional State, Western Ethiopia. Vet Med Sci. 2021;7(4):1280-1286.
Crossref - Qayyum, A, Khan JA, Hussain R, et al. Prevalence and association of possible risk factors with sub-clinical mastitis in Cholistani cattle. Pak J Zool. 2016;48(2):519-525.
- Saini SS, Sharma JK, Kwatra MS. Prevalence and etiology of subclinical mastitis among crossbred cows and buffaloes in Panjab”. Indian Journal of Dairy Science. 1994;47(2):103-106.
- Hussain M, Khalid N, Neem I. Subclinical mastitis in cows and buffaloes, identification and drug sensitivity of causative organism. Pak Vet J. 1984;4(3):161-164.
- Sharma N. Epidemiological investigation on subclinical mastitis in dairy animals Role of vitamin E and selenium supplementation on its control. M.VSc. Thesis IGKVV. Raipur, India. 2003.
- Sarba EJ, Tola GK. Cross-sectional study on bovine mastitis and its associated risk factors in Ambo district of West Shewa zone, Oromia, Ethiopia. Vet World. 2017;10(4):398-402.
Crossref - Awale MM, Dudhatra GB, Avinash K, et al. Bovine mastitis: a threat to economy. Sci Rep. 2012;1:295.
- Moroni P, Pisoni G, Antonini M, Villa R, Boettcher P, Carli S. Short communication: Antimicrobial drugs susceptibility of Staphylococcus aureus from subclinical mastitis in Italy. J Dairy Sci. 2006;89(8):2973-2976.
Crossref - Rabbani AF, MG, Samad MA. Host determinants based comparative prevalence of sub-clinical mastitis in lactating Holstein-Friesian cross cows and Red Chittagong cows in Bangladesh. Bangl J Vet Med. 2010;8(1):17-21.
Crossref - Fadelamula AAM, AI Dughaym GE, Mohamed MK, AI-Deiband AJ, AI-Zubaidy. Bovine mastitis: Epidemiological, Clinical and etiological study in a Saudi Arabian large dairy farm. Bulg J Vet Med. 2009;12(3):199-206.
- Sharma N, Singh NK, Singh OP, Pandey V, Verma PK. Oxidative stress and antioxidant status during transition period in dairy cow. Asian-Australas J Ani Sci. 2011;24(4):479-484.
Crossref - Sharma N, Rho GJ, Hong, YH, et al. Bovine mastitis: an Asian perspective. Asian J Anim Vet Adv. 2012;7(6):454-476.
Crossref - Wagter LC, Mallard BA, Wilkie BN, Leslie KE, Boettcher PJ, Dekkers JCM. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J Dairy Sci. 2000;83(3):488-498.
Crossref - Burvenich C, Merris V, Mehrzad J, Diez-Fraile A, Duchateau L. Severity of E. coli mastitis is mainly determined by cow factors. Vet Res. 2003;34(5): 521-564.
Crossref - Kurjogi MM, Kaliwal BB. Epidemiology of Bovine Mastitis in Cows of Dharwad District. Int Sch Res Notices. 2014;(1):1-9.
Crossref - Ranjan R, Gupta MK, Singh KK. Study of bovine mastitis in different climatic conditions in Jharkhand, India.Vet World. 2011;4(5):205-208.
Crossref - Tripura TK, Sarker SC, Roy SK, et al. Prevalence of subclinical mastitis in lactating cows and efficacy of intramammary infusion therapy. Bangl J Vet Med. 2014;12(1):55-61.
Crossref - Guha A, Guha R. Comparison of somatic cell count, California mastitis test, chloride test and rennet coagulation time with bacterial culture examination to detect subclinical mastitis in riverine buffalo (Bubalus bubalis). African Journal of Agriculture Research. 2012;7(41):5578-5584.
Crossref - Rakesh R, Sharma S, Swarup D. Prevalence of mastitis in machine and hand milked dairy cows. Indian Vet J. 2004;28:77-78.
- Hamann J, Burvenich C, Mayntz M, Osteras O, Halder W. Machine induced changes in the status of the bovine teat tissue with respect to new infection risk. IDF Bull. 1994;297:13-22.
- duPreez JH. Bovine mastitis therapy and why its fails. J S Afr Vet Assoc. 2000;71(3):201-208.
Crossref - Peeler EJ, Green MJ, Fitzpatrick JL, Morgan KL, Green LE. Risk factors associated with clinical mastitis in low somatic cell count British dairy herds. J Dairy Sci. 2000;83(11):2464-2472.
Crossref - Smith KL, Todhunter DA, Schoenberger PS. Environmental Mastitis: Cause, Prevalence, Prevention. J Dairy Sc. 1985;68(6):1531-1553.
Crossref - Vlieststra RJ. Managing new intramammary infections in the fresh cow. Proceeding of National Mastitis Council Regional Meeting. 2003:30-35.
- Sordillo LM, Boyle NO, Gandy JC, Corl M, Hamilton E. Shift in Thioredoxin reductase activity and oxidant status in mononuclear cells obtained from transition dairy cattle. J Dairy Sci. 2007;90(3):1186-1192.
Crossref - Gitto G, Reiter RJ, Karbownik M, et al. Causes of oxidative stress in the pre and perinatal period. Biol Neonate. 2002;81(3):146-157.
Crossref - Mallard BA, Dekkers JC, Ireland MJ, et al. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J Dairy Sci. 1998;81(2):585-595.
Crossref - Doherr MG, Roesch M, Schaeren W, Schallibaum M, Blum JW. Risk factors associated with subclinical mastitis in dairy cows on Swiss organic and conventional production system farms. Vet Med. 2007;52(11):487-495.
Crossref - Larsen B, Jensen NE, Madse P, Nielsen SM, Klastrup O, Madsen PS. Association of the M blood group system with bovine mastitis. Anim Blood Groups Biochem Genet.1985;16(3):165-173.
Crossref - Islam MA, Islam MZ, Islam MA, Rahman MS, Islam MT. Prevalence of subclinical mastitis in dairy cows in selected areas of Bangladesh. Bangladesh Vet Med. 2011;9(1):73-78.
Crossref - Suleiman TS, Karimuribo ED, Mdegela RH. Prevalence of bovine subclinical mastitis and antibiotic susceptibility patterns of major mastitis pathogens isolated in Unguja island of Zanzibar, Tanzania. Trop Anim Health Prod. 2018;50:259-266.
Crossref - Rios JL, Recio M. Medicinal plants and antimicrobial activity. Ethnopharmacol. 2005;100(1-2):80-84.
Crossref - Palaniswamy M, Pradeep BV, Sathya R, Angayarkanni J. In vitro anti-plasmodial activity of Trigonella foenum-graecum L. eCAM. 2010;7(4):441-445.
Crossref - Lewis K, Ausubel FM. Prospects of plant derived antibacterial. Nat Biotechnol. 2006;24(12):1504-1507.
Crossref - Mubarack HM, Doss A, Vijayasanthi M, Venkataswamy R. Antimicrobial drug susceptibility of Staphylococcus aureus from subclinical bovine mastitis in Coimbatore, Tamilnadu, South India. Vet World. 2012;5(6):352-355.
Crossref - Unakal CG, Kaliwal BB. Prevalence and antibiotic susceptibility of Staphylococcus aureus from bovine mastitis. Vet World. 2010;3(2):65-67.
- Lee SB, Cha KH, Kim SN, et al. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. J Microbiol. 2007;45(1):53-57.
- Diaz AN, Rossi CC, Mendonca VR, et al. Screening of medicinal plants for antibacterial activities on Staphylococcus aureus strains isolated from bovine mastitis. Brazilian Journal of Pharmacognosy.2010;20(5):724-728.
Crossref - Mubarack HM, Doss A, Dhanabalan R, Venkataswamy R. Activity of some selected medicinal plant extracts against bovine mastitis pathogens. J Anim Vet Adv. 2011;10(6):738-74121.
Crossref - Gopinath SM, Suneetha TB, Mruganka VD, Ananda S. Chemical profiling and antibacterial activity of Punica granatum L. against pathogens causing Bovine Mastitis. J Chem Pharm Res. 2011;3(5):514-518.
- Doss A, Muhamed H, Mubarack HM, Vijayasanthi M, Venkataswamy R. In vitro antibacterial activity of certain wild medicinal plants against bovine mastitis isolated contagious pathogens. Asian J Pharm Clin Res. 2012;5(2):90-93.
- Amber R, Adnan M, Tariq A, et al. Antibacterial activity of selected medicinal plants of northwest Pakistan traditionally used against Mastitis in livestock. Saudi J Biol Sci. 2018;25(1):154-161.
Crossref - Yang WT, Ke CY, Wu WT, Lee RP, Tseng YH. Effective treatment of Bovine mastitis with intramammary infusion of Angelica dahurica and Rheum officinale extracts. Evid Based Complement Alternat Med. 2019;2019():1-8.
Crossref - Naseer M, Kamboh A, Soho AB, Burriro R. In vitro antimicrobial efficacy of some plant extracts against multi-drug resistant Staphylococcus aureus and Streptococcus pyogenes isolated from buffalo mastitic milk. Buffalo Bulletin. 2021;40(1):31-44.
- Parameswari P, Devika R, Vijayaraghavan P. In vitro anti-inflammatory and antimicrobial potential of leaf extract from Artemisia nilagirica (Clarke.) Pamp. Saudi J Biol Sci. 2019;26(3):460-463.
Crossref - Kinde H, Regassa F, Asaye M, Wubie A. The in-vitro antibacterial effect of three selected plant extracts against Staphylococcus aureus and Streptococcus agalactiae isolated from Bovine Mastitis. J Veterinar Sci Technol. 2015;6(13).
Crossref - Boonkuso D, Tongbai W. Antimicrobal activity of ethnotraditional herb extracts against Coagulase-Negative Staphylococci (CNS) Isolated From dairy cows with mastitis in Lopburi province, Thailand. Ann Rom Soc Cell Biol. 2021;25(4):1800-1808.
- Ahameethunisa AR, Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complement Altern Med. 2010;10:6.
Crossref - Kumar R, Bharati KA. New claims in folk veterinary medicines from Uttar Pradesh, India. J Ethnopharmacol.2013;146(2):581-593.
Crossref - Kovacevic Z, Radinovic, M, Cabarkapa I, Kladar N, Bozin B. Natural Agents against Bovine Mastitis Pathogens. Antibiotics. 2021;10(2):205.
Crossref - Sserunkuma P, McGaw LJ, Nsahlai IV, Staden JV. Selected southern African medicinal plants with low cytotoxicity and good activity against bovine mastitis pathogens. S Afr J Bot. 2017;111:242-247.
Crossref - Amsalu A, Gelaye A, Fesseha H. In-Vitro Antibacterial Effects of Laggera Alata and Ehretia Cymosa against Staphylococcus aureus and Streptococcus aglactiae Isolated from Bovine Mastitis. Vet Med Animal Sci. 2020;3(1):1036.
- Patel R, Patel Y, Joshi C, Kunjadia A. Herbal plants: A potential agent to cure infectious mastitis in bovine animals. Int J Phytomedicine. 2013;5(3):362-366.
- Monika T, Sasikala P, Reddy MVB. A Study on In vitro Phytochemical Screening and Antibacterial Activity of Aqueous and Methanolic Leaf Extracts of Tridax procumbens against Bovine Mastitis Isolated Staphylococcus aureus. International Journal of Advanced Scientific and Technical Research. 2013;(3)4:2249-9954.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.