ISSN: 0973-7510
E-ISSN: 2581-690X
Due to poor hygiene and negligence of food safety standards, microbes find their way into the food supply chain by contaminating food ingredients and causing food-borne illnesses. Turmeric is not only a widely consumed spice but also a product that undergoes multiple stages of handling, from cultivation to packaging. These stages expose it to contamination risks, especially by non-pathogenic bacteria that may carry resistance genes. The study aims to bridge the gap in understanding the microbial burden in turmeric and the prevalence of AMR in its microbial communities. In this study, we investigated microbial burden in turmeric samples. Bacteria were isolated from loose and packaged turmeric samples and characterized for antimicrobial resistance, growth pattern, biochemical properties and later identified to assess potential risks to food safety. Out of 22 loose samples of turmeric, 82% had colony forming unit (CFU) >108, while most of the packed turmeric samples (98%) had CFU <108. From these samples, 13 distinct non-pathogenic bacteria associated with antimicrobial resistance (AMR) were isolated, out of which eleven were non-spore-forming bacteria. The bacterial isolates Stutzerimonas sp., Enterobacter sp., and Pantoea sp. exhibited resistant activity against clindamycin (macrolide), gentamycin (aminoglycoside), spectinomycin (aminocyclitol) and ampicillin (penicillin) with optimum growth at 45 °C and positive alkaline phosphatase (ALP) activity. This study clearly suggests the negligence of food safety standards may result in increased microbial burden, which directly poses a threat to food safety and shelf life of food commodities.
Bacterial Load, Turmeric, Food Safety, Shelf Life, Antimicrobial Resistance
Food ingredients such as spices are processed with various methods and have protocols to minimize contaminations, which may be threatening to food safety.1 These processes include harvesting, post-harvesting transportation, boiling, drying, grinding, packaging, storage, and shipping and these steps are critical in minimizing contamination.2,3 Bacterial contamination of spices is facilitated by poor sanitation practices of the processing environment and personnel, such as inappropriate drying and storage conditions, such as humidity and temperature. The bacterial presence raises questions about the quality of food and may be responsible for food spoilage and the shelf life of commodities.4-6 Further antimicrobial resistance (AMR) in bacteria makes this situation worse as far as food safety is concerned. When these antimicrobial resistant bacterial species enters in food commodities at any step during food supply chain, a more challenging situation develops.7-9
According to the World Health Organization, AMR occurs when bacteria, viruses, fungi, and parasites change over time and no longer respond to medicines, making infections harder to treat and increasing the risk of disease spread, severe illness, and death.10 Earlier report found that non-pathogenic bacterial species such as Lactobacilli, Pediococcus, Leuconostoc, Enterococcus, Bifidobacterium and others have gained AMR and these bacteria in the gut face stress favoring the rescue of adaptive resistance towards antimicrobials and promote gene transfer associated to AMR.11-13 Study has shown that the Lactobacillus plantarum M345 (transconjugant) can transfer AMR gene erm(B) to Enterococcus faecalis (recipient) through pLFE1 plasmid, a natural plasmid of transconjugant and suggest that gut environment is favourable for AMR gene transfer.12 However, non-pathogenic bacteria with AMR may operate as a source for the spread of the AMR gene in pathogenic bacteria, thereby generating risk to the safety of food commodities.14,15
The rhizome of Curcuma longa is used for making turmeric. Post-harvest, several steps are involved in turmeric processing, which includes scalding, drying, colouring and grinding. After boiling for 60-90 minutes, rhizomes are spread on mats or on the ground and dried in sunlight for 2 weeks.16 The boiling method can be a source of contamination by bacterial spores.17 Fouling enables resistant spores to germinate, and thrive thereby increasing mesophilic and thermophilic spore count in the turmeric after this step. Therefore, the goal of the current study was to evaluate and examine the microbial load and the frequency of genes associated with antimicrobial resistance in the microbial communities of turmeric. This research aims to offer a basic understanding of the microbiological risks related with turmeric, encompassing food contamination by microorganisms, possible hazards linked to it, and its evaluation in relation to the present AMR situation.
Bacterial isolation and identification from turmeric samples
Turmeric samples (n = 72) were procured from retail outlets from Lucknow as per Food Safety and Standards Authority of India (FSSAI) guidelines. Each turmeric sample whether loose or packed was purchased from retail shops in Lucknow city area. Packed samples of pack size 50 gm or more was purchased and 50 gm of loose samples were purchased. Samples were kept in plastic bags or vials and stored in dark at room temperature. Homogenized samples (1 gm) were mixed to sterile Phosphate Buffer Saline (PBS) and serially diluted in PBS and were spread on nutrient agar plates. Plates were kept at 37 ± 2 °C for incubation. The total CFU/gram of each sample was calculated. To check antibiotic resistance potential of isolated bacteria, 15 antibiotics belonging to different class and generation were used, namely amikacin (2.5 mg/ml), ampicillin (100 µg/ml), amoxicillin (50 µg/ml), ceftazidime (130 µg/ml), clindamycin, doxycycline, erythromycin (5 mg/ml), gentamicin (10 µg/ml), kanamycin (50 µg/ml), nalidixic acid (200 µg/ml), oxytetracycline (1 µg/ml), piperacillin (300 µg/ml), spectinomycin (100 µg/ml), tetracycline (15 µg/ml) and tylosin (0.5 µg/ml). All antibiotics were procured from HiMedia Laboratories Pvt. Ltd. Bacteria were inoculated in Luria Bertani medium in presence of antibiotic in microtiter plates. Plates were kept at 37 ± 2 °C and at 300 rpm. Absorbance was recorded at 600 nm after 24 hours. All antibiotics were used separately and in triplicates. A total thirteen phenotypically different bacteria with AMR were isolated from above samples using microplate assay, and further identified and characterized for antibacterial resistance gene.
Identification of bacterial isolates containing AMR by 16S rDNA sequencing
For identification of bacteria, their genomic DNA was isolated from bacterial culture grown for 14-16 hours at 37 ± 2 °C at 120 rpm, using MN NucleoSpin® Soil kit (Macherey Nagel, Germany). Manufacturer’s instructions were followed for isolation of genomic DNA. Isolated DNA was dissolved in 30 µL SE buffer and stored at -20 °C. 16S rDNA sequence was amplified using universal 16S rDNA primers (Forward 5’AGAGTTTGATCCTGGCTCAG3’ and Reverse 5’ACGGCTACCTTGTTACGACTT3’). A 50 µL PCR reaction was set up using following protocol of initial denaturation at 94 °C for 5 minutes followed by 35 cycles of 1 min denaturation at 94 °C, annealing at 56 °C for 30 sec and extension at 72 °C for 1 min. After 30 cycles a final extension of 5 min at 72 °C was done. The PCR product was then electrophoresed on 1% agarose gel. Bands were cut and amplified DNA was eluted (using MN PCR Cleanup kit) for DNA sequencing. After sequencing the resultant nucleotide sequences were aligned using nucleotide BLAST tool available at NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
qPCR analysis for antibiotic resistance genes
To confirm presence of antibiotic gene in the bacterial isolates quantitative PCR was performed. A 10 µL reaction mixture in triplicates containing 5 µL 2X master-mix (SyBr Green), 0.5 µL of each forward and reverse primer, 1 µL of total bacterial DNA as a template and 3 µL nuclease free water (PureGene, Genetix). qPCR conditions were: initial denaturation at 95 °C for 10 min followed by PCR stage of 40 cycles at 95 °C for 15 sec and 60 °C for 1 min. Fluorescence was recorded during annealing and extension stage at 60 °C. Primers used for detection of antibiotic resistance gene are listed in Supplementary Table 1. The multiple antibiotic resistance index of every bacterial isolate was determined by dividing the number of antibiotics, to which bacteria was found to be resistant (harboring resistant gene) with total number of antibiotics tested.18,19
Microbial growth at different temperature and pH
To study the impact of temperature on the growth, 13 isolated bacteria were inoculated in LB media separately in four sets, and incubated at 4 °C, 23 °C, 37 °C and 45 °C for growth analysis. Absorption at 600 nm was measured at a regular interval of one hour till 8 hours. Further, to study the effect of pH on the growth 3 set of each isolated bacteria were prepared in LB media at pH 4.5, 7.0 and 9.0. pH was adjusted using acetic acid and NaOH. For the growth analysis, OD at 600 nm was measured at a regular interval of 1 hour till 8 hours.
Alkaline phosphatase assay of bacterial isolates
ALP assay was performed using method developed by Brickman and Beckwith in 1975 method.20 Cells were harvested from bacterial culture grown at 37 °C for 16-18 hours (with constant shaking at 150 rpm) by centrifugation at 5000 rpm. Supernatant was discarded and pellet was washed and resuspended in 1 M TrisCl buffer (pH 8.0). OD was adjusted to 0.6 at 600 nm. Then to 1 ml of cell suspension, 0.1 ml pNPP (4%) is added and incubated at 37 °C in dark until a significant yellow color appears. To stop the reaction 0.1 ml of 1M K2HPO4 was added. Absorbance was recorded at 420 nm and 550 nm and units of phosphatase was calculated using formula given below.20
Activity = 1000 * [OD420 nm – 1.75 * OD550 nm / Time * OD600 nm] × Dilution Factor
Spices are widely used in Indian cuisine. Turmeric is one such spice that finds its use in almost every Indian household. Turmeric powder can become contaminated with bacteria at several points during the manufacturing, handling, and transportation processes. Major sources include water, which can include Salmonella and Escherichia coli, if untreated, and soil, which naturally contains bacteria like Clostridium and Bacillus. Unhygienic handling and processing equipment can potentially introduce bacteria like Staphylococcus and Listeria, causing contamination. Poor hygiene practices and human contact can also transfer pathogens such as E. coli. Bacterial contamination can also result from exposure to animals, insects, drying, grinding, and environmental toxins. Finally, the risk is further increased by contaminated packaging materials, cross-contamination, and improper storage conditions, compromising the safety and quality of turmeric powder. In the current study we have checked microbial burden and prevalence of AMR in turmeric samples.
Identification of bacterial isolates from turmeric samples
Bacterial isolates from turmeric were identified through phylogenetic analysis of 16S rDNA amplified PCR product (Supplementary Figure 1 and Supplementary Table 2). The predominant bacterial isolates were phylogenetically belonged to Brachybacterium sp. A2 (98.93%), Stutzerimonas sp. A3 (99.16%), Enterobacter sp. A4 (98.54%), Enterobacter sp. A9 (98.61%), Pantoea sp. B1 (96.67%), Pantoea sp. B2 (98.65%), Enterobacter sp. B3 (99.05%), Pantoea sp. B4 (85.53%), Enterobacter sp. B5 (97.4%), Pseudomonas sp. G21 (98.88%), Bacillus sp. G22 (98.54%), Bacillus sp. G23 (97.2%), Stutzerimonas sp. G24 (98%) (Supplementary Figure 2-14). Similar findings in turmeric and other spices were reported earlier that the prevalence of 12 different Bacillus species in 25 samples of turmeric with Bacillus cereus dominating 60%, followed by B. coagulans (44%) and B. polymyxa (32%). However, 8%-12% samples having other Bacillus species such as B. subtilis and B. licheniformis along with L. sphaericus.21 Several other researchers also demonstrated high level of mesophilic bacterial spore contamination ranging between 6.3 x 104 to 6.3 x 109 spores per gram22-24 and however, thermophilic spores were detected upto 1 x 106 spores per gram in turmeric.25 Previous studies have also revealed that AMR was present to different antibiotics in Enterobacter sp. and other isolates.26-31 The chemical composition of food ingredients, processing methods, environmental conditions, and even variation in handling can all be related to the broad variability in microbial profiles in different food products. In the current study, we focused on the prevalence of antimicrobial resistant bacteria in turmeric. The diversity in isolated bacteria can be attributed to handling conditions and several stages of processing of turmeric.
Antibiotic sensitivity assay and bacterial load in turmeric samples
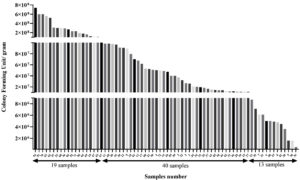
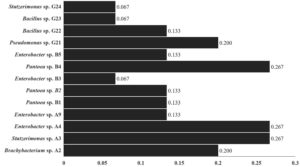
The bacterial burden was examined in term of CFU/gm and it was observed that the packed turmeric sample (n = 13) had low CFU/gm which was in the range of 5 x 105 – 8.7 x 106. While the majority of spice samples (n = 40) had the highest CFU ranged from 1.0 x 108 – 7.4 x 108 (in loose samples) (Figure 1). It was observed that loose sample had highest microbial load and about 82% loose sample had >1 x 108 CFU/gm. Contrary to that, in case of the packed sample only 2% had CFU >1 x 108. In a related investigation, a team of researchers assessed the levels of bacterial contamination in the spices namely, clove (zero), saffron (zero), ginger (3.9 x 104 CFU), pepper (1.5 x 104 CFU), turmeric (1.9 x 105 CFU) and cinnamon (1.99 x 102 CFU).32 The amount of Bacillus flora in spices was measured by Antai in 1988, and the results showed that it ranged from 1.8 x 104 to 1.1 x 108 per gram.33 In a different study, researchers enumerated the microbial load (up to 6 x 107 per gram) in 36 spices and correlated the results with the type, age, supplier, and production process or technique of the condiment.34 In another investigation, researcher assessed the total bacterial count and bacterial spore count in Indian turmeric sample up to 9.9 x 105 and 7.9 x 105, respectively.35 A recent study shows the presence of bacterial contamination in dairy and non-dairy food products with AMR and metal tolerance capable of forming biofilms.36 The microbial load in spices can be greatly influenced by several sources of contamination during cultivation, processing, and distribution.37 In our case, the microbial load of the packed samples was lower than that of the loose samples, indicating that appropriate storage and exposure to external conditions may have an impact on the microbial load.38 This high microbial population frequently contains bacteria that are resistant to antibiotics, a condition made worse by exposure to antibiotics during agricultural techniques and poor sanitation procedures.39-41 Turmeric may be contaminated by microorganisms from a variety of sources, including contaminated soil, water used during cultivation, as well as from inappropriate handling and storage practices, processing, and packing.42 Also, cross-contamination from workers’ personal hygiene and equipment might also contribute to contamination. Further, in order to check prevalence of antibiotic resistance in predominant bacterial isolates of turmeric samples, we firstly screened antibiotic susceptibility and then antibiotic resistant isolates were further examined for presence of antibiotic resistant gene. Our observation suggests that the all-bacterial isolates were showed resistant against gentamicin except Enterobacter sp. B3. Additionally, Stutzerimonas sp. A3, Enterobacter sp. A4 and Pantoea sp. B4 were found resistant against ampicillin, clindamycin, and spectinomycin, Brachybacterium sp. A2 was resistant against clindamycin and spectinomycin, Enterobacter sp. A9 was resistant against ampicillin while Enterobacter sp. B5 was found resistant against clindamycin. Enterobacter sp. B3 found resistant only against clindamycin. The percent inhibition was calculated as compared to control (Supplementary Table 3). A qPCR for resistance gene was done to corroborate the outcomes of antibiotic susceptibility test. Ampicillin, clindamycin, gentamicin and spectinomycin resistance genes were amplified in bacterial isolates by qPCR. The qPCR results were consistent with the results of antibiotic susceptibility assay (Table and Supplementary Table 3). The qPCR results confirm the presence of antibiotic resistance gene in conferring the antibiotic resistance in the isolates. The MAR index value reached up to 0.267 (Figure 2). Higher MAR index values indicates increased multiple antibiotic resistance against tested antibiotics.41 In recent studies, it was shown that the non-pathogens with AMR may operate as a source for the spread of the AMR genes in pathogenic bacteria, thereby generating risk to the safety of food commodities.14,15 These resistant bacteria in food commodities can easily spread to food supply chain and may contribute to AMR.11,12,43,44
Table:
Microbial growth observed by microplate assay against antibiotics. Bacteria were cultured in presence on different antibiotics listed above in microtiter plates. After incubation growth was observed and absorbance was recorded at 600 nm
Brachybacterium sp. A2 |
Stutzerimonas sp. A3 |
Enterobacter sp. A4 |
Enterobacter sp. A9 |
Pantoea sp. B1 |
Pantoea sp. B2 |
Enterobacter sp. B3 |
Pantoea sp. B4 |
Enterobacter sp. B5 |
Pseudomonas sp. G21 |
Bacillus sp. G22 |
Bacillus sp. G23 |
Stutzerimonas sp. G24 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Control |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
Amikacin |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Amoxicillin |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Ampicillin |
✖ |
✓ |
✓ |
✓ |
✓ |
✓ |
✖ |
✓ |
✖ |
✓ |
✖ |
✖ |
✖ |
Clindamycin |
✓ |
✓ |
✓ |
✖ |
✖ |
✖ |
✓ |
✓ |
✓ |
✓ |
✓ |
✖ |
✖ |
Ceftazidime |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Doxycycline |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Erythromycin |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Gentamicin |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✖ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
Kanamycin |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Nalidixic Acid |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Oxytetracycline |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Piperacillin |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Spectinomycin |
✓ |
✓ |
✓ |
✖ |
✖ |
✖ |
✖ |
✓ |
✖ |
✖ |
✖ |
✖ |
✖ |
Tetracycline |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Tylosine |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
✖ |
Figure 1. CFU/gram (total aerobic count) in seventy-two turmeric samples. Each turmeric sample was weighed 1 gm and mixed in sterile PBS and serially diluted. The dilutions were spread on nutrient agar plates and kept at 37 ± 2 °C. Colonies were counted and CFU per gram was calculated and graph was plotted using MS-Excel
Figure 2. Multiple Antibiotic Resistance (MAR) index values of isolated bacteria from Curcuma longa. The MAR index was calculated by formula MAR = x/y, where x = number of antibiotics to which bacteria is resistant and y = number of total antibiotics used
Growth at different temperature and pH
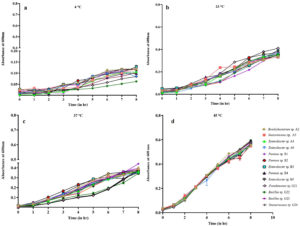
Bacteria isolated were cultured at different temperature and 45 °C was found to be most favorable temperature followed by 37 °C and 23 °C. The calculated generation time also supports most favorable temperature for bacterial isolates was 45 °C when compared to other temperature 37 °C and 23 °C (Supplementary Table 4). This is evident by decrease in OD with decrease in temperature. At 45 °C after 8 hours of incubation an average of 05-0.6 OD was observed followed by 0.3-0.4 OD at 37 °C and 23 °C and 0.1 OD at 4 °C (Figure 3) Similarly, neutral pH 7.0 (Figure 4) was found to be most suitable for bacterial growth followed by pH 9.0 as observed in Figure 4c. Little or no growth (insignificant) was observed at acidic pH of 4.5 (Figure 4a). Many reasons can be behind bacterial isolates from turmeric growing at 45 °C. Turmeric is grown in soil, which is a natural habitat for a variety of thermoduric bacteria, such as Bacillus and other bacterial species.45 The processing steps for turmeric, includes washing, drying, and grinding, often involve high temperatures. However, thermophilic bacteria can survive these conditions, if the processes employed, are not thorough enough to ensure complete sterilization.46 This may lead to the persistence of thermophilic bacteria in turmeric despite undergoing heat treatment processes designed to reduce microbial load. These thermophilic bacteria can thrive in stored turmeric if conditions favor their survival, such as in environments that experience fluctuating temperatures.47
Figure 3. Growth pattern of 13 isolated bacteria at temperatures (a) 4 °C, (b) 23 °C, (c) 37 °C and (d) 45 °C. Each bacterium at each temperature was inoculated in triplicates and its absorbance was measured. Mean of individual three absorbance readings was calculated for each bacterium at every hour to plot the curve. Absorbance was recorded at 1 hr interval at 600 nm using BioTek UV-Vis spectrophotometer
Figure 4. Growth pattern of 13 isolated bacteria at different pH (a) 4.5, (b) 7.0 and (c) 9.0. Each bacterium at each pH was inoculated in triplicates and its absorbance was measured. Mean of individual three absorbance readings was calculated for each bacterium at every hour to plot the curve. Absorbance was recorded at 1 hr interval at 600 nm using BioTek UV-Vis spectrophotometer
Alkaline phosphatase (ALP) activity of isolated bacteria
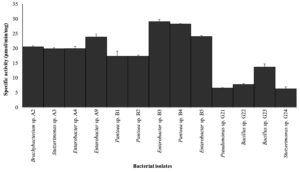
The ALP activity of isolated bacteria was evaluated, revealing a significant variance in enzymatic activity across different bacterial isolates. Notably, all bacterial isolates were tested positive for ALP with maximum activity reported in B3 (Enterobacter sp.) and B4 (Pantoea sp.) with specific activity of 29 and 28 µmol/min/mg respectively. Bacterial isolates Pseudomonas sp. and Bacillus sp. reported least specific activity below 10 µmol/min/mg. Rest bacterial isolates (8) exhibited specific activity between 10-20 μmol/min/mg (Figure 5). The high ALP activity in Enterobacter sp. and Pantoea sp. can be attributed to several factors, including their metabolic versatility and ability to adapt to diverse environmental conditions.48 These bacteria may possess unique genetic and regulatory mechanisms that enhance ALP production, which warrants further investigation into their genomic and proteomic profiles to identify specific genes and regulatory pathways involved in ALP synthesis and secretion.49,50
Figure 5. ALP activity of 13 isolated bacteria. Cells from overnight bacterial cultures were used after adjusting OD to 0.6 at 600 nm with tris buffer (pH 8.0). Cell suspensions were then incubated in dark for overnight at 37 °C after adding 0.1 ml of 4% pNPP to 1 ml cell suspension. Absorbance was recorded on Perkin Elmer LAMBDA 365 UV/Vis Spectrophotometer. Graph with error bars was generated using MS-Excel. The error bars denotes the standard error of mean of three observations
Food safety measures include implementing stringent hygiene protocols at all stages of turmeric production, including cultivation, post-harvest handling, processing, packaging, and storage. Our study’s results regarding the microbial burden and antimicrobial resistance (AMR) in turmeric make it clear that our food safety procedures are facing unprecedented challenges that necessitate futuristic interventions. It successfully highlights the role of loose turmeric samples as hotspots for microbial contamination, demonstrating the influence of poor handling and storage practices on microbial load and resistance profiles. However, the mechanism of AMR gene dissemination remains unresolved. It can be anticipated that advances in technology, processing methods and detection techniques combined with strict international food safety regulations will drastically lower food commodity contamination in the near future, which includes turmeric and other spices. Real-time detection of AMR genes and microbial contamination at different points in the food supply chain will probably be accomplished through the use of emerging technology and advanced monitoring systems. These developments will improve the precision and speed of microbiological detection while also facilitating the prompt resolution of contamination problems, protecting customers’ access to safer food products. A coordinated effort is needed to combat the spread of resistance genes throughout food ecosystems, as demonstrated by the increase in AMR in non-pathogenic bacteria. The development of novel antimicrobial drugs and the engineering of CRISPR-Cas systems to precisely remove AMR genes from bacterial populations are the next steps. Therefore, it will be essential to implement sustainable agricultural methods and uphold high standards of hygiene across the food processing chain to stop the introduction and subsequent spread of these resistant microbes. We can start to see a decrease in foodborne infections and create a more secure farm-to-fork continuum in the upcoming decades by finding the answers to many unanswered questions.
Additional file: Additional Table S1-S4 and Figure S1-S14.
ACKNOWLEDGMENTS
The authors are thankful to ICMR for providing Senior Research Fellowship. The Manuscript Communication Number is IU/R&D/2023-MCN0001952
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by ICMR India, through Senior Research Fellowship vide reference number 3/1/2/166/2019-Nut.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Klebukowska L, Zadernowska A, Chajecka-Wierzchowska W. Microbiological contamination of dried and lyophilized garlic as a potential source of food spoilage. J Food Sci Technol. 2015;52(3):1802-1807.
Crossref - Gallo M, Ferrara L, Calogero A, Montesano D, Naviglio D. Relationships between food and diseases: What to know to ensure food safety. Food Res Int. 2020;137:109414.
Crossref - Machado-Moreira B, Richards K, Brennan F, Abram F, Burgess CM. Microbial Contamination of Fresh Produce: What, Where, and How? Compr Rev Food Sci Food Saf. 2019;18(6):1727-1750.
Crossref - la Pena MM, Welti-Chanes J, Martin-Belloso O. Novel technologies to improve food safety and quality. Curr Opin Food Sci. 2019;30:1-7.
Crossref - Fusco V, Chieffi D, Fanelli F, et al. Microbial quality and safety of milk and milk products in the 21st century. Compr Rev Food Sci Food Saf. 2020;19(4):2013-2049.
Crossref - Letuka P, Nkhebenyane J, Thekisoe O. Street food handlers’ food safety knowledge, attitudes and self-reported practices and consumers’ perceptions about street food vending in Maseru, Lesotho. Br Food J. 2021;123(13):302-316.
Crossref - European Food Safety Authority, Aerts M, Battisti A, et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019;17(6):e05709.
Crossref - Nelson DW, Moore JE, Rao JR. Antimicrobial resistance (AMR): significance to food quality and safety. Food Qual Safe. 2019;3(1):15-22.
Crossref - Koutsoumanis K, Allende A, Alvarez-Ordonez A, et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA Panel on Biological Hazards. 2021;19(6):e06651.
Crossref - World Health Organisation (WHO). Antimicrobial resistance. 2023. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 21 November 2023.
- Kheadr E, Dabour N, Lay CL, Lacroix C, Fliss I. Antibiotic susceptibility profile of bifidobacteria as affected by oxgall, acid, and hydrogen peroxide stress. Antimicrob Agents Chemother. 2007;51(1):169-74.
Crossref - Feld L, Schjorring S, Hammer K, et al. Selective pressure affects transfer and establishment of a Lactobacillus plantarum resistance plasmid in the gastrointestinal environment. J Antimicrob Chemother. 2008;61(4):845-852.
Crossref - Ruiz L, Alvarez-Ordonez A. The role of the food chain in the spread of antimicrobial resistance (AMR). In: Boukherroub R, Szunerits S, Drider D, Eds. Functionalized Nanomaterials for the Management of Microbial Infection. Micro and Nano Technology. Elsevier. 2017:23-47.
Crossref - Gandra S, Alvarez-Uria G, Turner P, Joshi J, Limmathurotsakul D, van Doorn HR. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin Microbiol Rev. 2020;33(3).
Crossref - Chandler CIR. Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun. 2019;5(1):53.
Crossref - Jayashree E. Post harvest processing and scope for mechanization in spices. University of Agricultural Sciences Dharwad. 2011:178-187.
- Durand L, Planchon S, Guinebretiere MH, Andre S, Carlin F, Remize F. Contamination pathways of spore-forming bacteria in a vegetable cannery. Int J Food Microbiol. 2015;202:10-9.
Crossref - Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281.
Crossref - Costa MdC, Cruz AIC, Bispo ASdR, Ferreira MA, Costa JA, Evangelista-Barreto NS. Occurrence and antimicrobial resistance of bacteria in retail market spices. J Ciencia Rural. 2020;50(4).
Crossref - Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and j80 transducing phages. J Mol Bio. 1975;96(2):307-316.
Crossref - Seenappa M, Kempton AG. A note on the occurrence of Bacillus cereus and other species of Bacillus in Indian spices of export quality. J Appl Microbiol. 1981;50(2):225-228.
Crossref - Debs-Louka E, El Zouki J, Dabboussi F. Assessment of the microbiological quality and safety of common spices and herbs sold in Lebanon. J Food Nutr Disord. 2013;2(4):2.
Crossref - Witkowska AM, Hickey DK, Alonso-Gomez M, Wilkinson MG. The microbiological quality of commercial herb and spice preparations used in the formulation of a chicken supreme ready meal and microbial survival following a simulated industrial heating process. J Food Control. 2011;22(3-4):616-625.
Crossref - Kneifel W, Berger E. Microbiological Criteria of Random Samples of Spices and Herbs Retailed on the Austrian Market. J Food Prot. 1994;57(10):893-901.
Crossref - Oomes SJCM, van Zuijlen ACM, Hehenkamp JO, Witsenboer H, van der Vossen JMBM, Brul S. The characterisation of Bacillus spores occurring in the manufacturing of (low acid) canned products. Int J Food Microbiol. 2007;120(1-2):85-94.
Crossref - Subedi D, Vijay AK, Willcox M. Overview of mechanisms of antibiotic resistance in Pseudomonas aeruginosa: an ocular perspective. Clin Exp Optom. 2018;101(2):162-171.
Crossref - Jiang N, Hong B, Luo K, Li Y, Fu H, Wang J. Isolation of Bacillus subtilis and Bacillus pumilus with Anti-Vibrio parahaemolyticus Activity and Identification of the Anti-Vibrio parahaemolyticus Substance. Microorganisms. 2023;11(7):1667.
Crossref - Davin-Regli A, Pages JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392.
Crossref - Casale R, Boattini M, Bianco G, et al. Bloodstream Infections by Pantoea Species: Clinical and Microbiological Findings from a Retrospective Study, Italy, 2018-2023. Antibiotics. 2023;12(12):1723.
Crossref - Adamski P, Byczkowska-Rostkowska Z, Gajewska J, Zakrzewski AJ, Klebukowska L. Prevalence and Antibiotic Resistance of Bacillus sp. Isolated from Raw Milk. Microorganisms. 2023;11(4):1065.
Crossref - Papapetropoulou M, Iliopoulou J, Rodopoulou G, Detorakis J, Paniara O. Occurrence and antibiotic-resistance of Pseudomonas species isolated from drinking water in southern Greece. J Chemother. 1994;6(2):111-116.
Crossref - Yehia HM, Al-Masoud AH, Elkhadragy MF, Sonbol H, Al-Dagal MM. Analysis of spore-forming bacterial contaminants in herbs and spices and evaluation of their heat resistance. Food Sci Technol. 2022;42(2):19422.
Crossref - Antai SP. Study of the Bacillus flora of Nigerian spices. Int J Food Microbiol. 1988;6(3):259-261.
Crossref - Baxter R, Holzapfel WH. A microbial investigation of selected spices, herbs, and additives in South Africa. J Food Sci. 1982;47(2):570-574.
Crossref - Munasiri MA, Parte MN, Ghanekar AS, Sharma A, Padwal-desai SR, Nadkarni GB. Sterilization of ground prepacked Indian spices by gamma irradiation. Food Sci. 1987;52(3):823-824.
Crossref - Ejaz H, Junaid K, Yasmeen H, et al. Multiple Antimicrobial Resistance and Heavy Metal Tolerance of Biofilm-Producing Bacteria Isolated from Dairy and Non-Dairy Food Products. Foods. 2022;11(18).
Crossref - Hordofa TS, Tolossa TT. Cultivation and postharvest handling practices affecting yield and quality of major spices crops in Ethiopia: A review. Cogent Food Agric. 2020;6(1):1788896.
Crossref - Karam L, Salloum T, El Hage R, Hassan H, Hassan HF. How can packaging, source and food safety management system affect the microbiological quality of spices and dried herbs? The case of a developing country. Int J Food Microbiol. 2021;353:109295.
Crossref - Brown JC, Jiang X. Prevalence of antibiotic-resistant bacteria in herbal products. J Food Prot. 2008;71(7):1486-1490.
Crossref - Walusansa A, Asiimwe S, Nakavuma JL, et al. Antibiotic-resistance in medically important bacteria isolated from commercial herbal medicines in Africa from 2000 to 2021: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2022;11(1):11.
Crossref - Gyorgy E, Laslo E, Antal M, Andras CD. Antibiotic resistance pattern of the allochthonous bacteria isolated from commercially available spices. Food Sci Nutr. 2021;9(8):4550-4560.
Crossref - Banerjee M, Sarkar PK. Microbiological quality of some retail spices in India. Food Res Int. 2003;36(5):469-474.
Crossref - Hart WS, Heuzenroeder MW, Barton MD. A study of the transfer of tetracycline resistance genes between Escherichia coli in the intestinal tract of a mouse and a chicken model. J Vet Med Ser B. 2006;53(7):333-40.
Crossref - Egervarn M, Lindmark H, Olsson J, Roos S. Transferability of a tetracycline resistance gene from probiotic Lactobacillus reuteri to bacteria in the gastrointestinal tract of humans. Antonie Van Leeuwenhoek. 2010;97(2):189-200.
Crossref - McKee L. Microbial contamination of spices and herbs: a review. LWT – Food Sci Technol. 1995;28(1):1-11.
Crossref - Mohammad ZH, Arias-Rios EV, Ahmad F, Juneja VK. Microbial Contamination in the Food Processing Environment. In: Ahmad F, Mohammad ZH, Ibrahim SA, Zaidi S, eds. Microbial Biotechnology in the Food Industry: Advances, Challenges, and Potential Solutions. Springer Cham; 2024:15-43.
Crossref - Mathot AG, Postollec F, Leguerinel I. Bacterial spores in spices and dried herbs: The risks for processed food. Compr Rev Food Sci Food Saf. 2021;20(1):840-862.
Crossref - Ragot SA, Kertesz MA, Meszaros Eֹ, Frossard E, Bunemann EK. Soil phoD and phoX alkaline phosphatase gene diversity responds to multiple environmental factors. FEMS Microbiol Ecol. 2016;93(1):fiw212.
Crossref - Santos-Beneit F. The Pho regulon: a huge regulatory network in bacteria. Review. Front Microbiol. 2015;6:402.
Crossref - Wanner BL. Gene regulation by phosphate in enteric bacteria. J Cell Biochem. 1993;51(1):47-54.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.