ISSN: 0973-7510
E-ISSN: 2581-690X
Sickle cell disease (SCD) is a blood illness occurs due to point mutation in the β-globin gene and leads to serious health problems. The aim of the study was to assess probiotic Lactobacillus plantarum F4’s (L. plantarum F4) capacity to induce fetal hemoglobin (HbF) synthesis and anti-sickling effects in sickle erythrocytes isolated from individuals with sickle cell disease. Erythrocytes were isolated from 15 SCD patients and 5 controls blood samples. Erythrocytes were treated with L. plantarum F4 culture supernatant (CS) at concentrations of 30% and 60% (v/v) for up to 120 minutes, following lysozyme treatment to purify the supernatant. The Emmel test was conducted by microscopic observation after treating the samples with different doses and durations of L. plantarum F4 (CS). HPLC was utilized to assess the HbF levels in patients, while normal saline and hydroxyurea served as negative and positive controls. Statistical analyses, including ANOVA and Student’s t-test, were conducted to compare treatment effects. Results demonstrated that at a concentration of 30% (v/v) for 120 minutes, L. plantarum F4 (CS) significantly decreased sickling of SCD erythrocytes (p < 0.0001) and was equivalent to the common medication hydroxyurea. Additionally, compared to the negative control, L. plantarum F4 (CS) considerably raised the HbF% in the erythrocytes of SCD patients (p < 0.0001). For the first time, our study suggests that L. plantarum F4 significantly enhance HbF and anti-sickling activity in sickled erythrocytes, in vitro and can be further explored for in vivo animal model study and developing therapeutics for SCD.
Sickle Cell Disease (SCD), Sickled Erythrocytes, L. plantarum, Anti-sickling Activity, Fetal Hemoglobin (HbF), Hydroxyurea
The genetic hematological condition known as sickle cell disease (SCD) occurs due to mutations in the β-globin (HBB) gene, that codes for hemoglobin subunit. SCD affects about 5% of individuals globally, and India has the second-highest predicted number of SCD newborns.1,2 Sickle hemoglobin mutations alter the structure and function of red blood cells (RBCs), causing a range of symptoms and severity. The manifestations of SCD can be acute or persistent. In some patients, they result in recurrent hospitalizations due to painful, unpredictable vaso-occlusive crises (VOC). The polymerization of deoxygenated hemoglobin results into change of shape and rigidity, thus making the sickled RBCs more prone to hemolysis.
These rigid RBCs obstruct small blood arteries, leading to VOC, diminished blood and oxygen flow to tissues, thus resulting in organ damage. The hemolysis and VOC can be reduced by certain drugs such as hydroxyurea, crizanlizumab, L-glutamine, voxelotor, and prolonged transfusion therapy. Although yet experimental, stem cell transplantation has the potential to cure.3 VOC complication is among the major reason for hospitalization in SCD observed in children with SCD. In order to reduce early childhood mortality, antibiotic treatment is considered critical to prevent infections in individuals with functional asplenia in SCD.3 However, antibiotics used during the first five years of life may affects diversity and gut microbiota composition due to SCD mediated subclinical ischemia and antibiotics.4
Numerous investigations have indicated the function of our gut flora.5,6 It is involved in various systemic mechanisms that can have an impact on the SCD pathophysiology. Furthermore, the gut microbiota is essential for metabolism, the gut-brain axis, and both local and systemic immunity. In addition, it plays important role in preserving the integrity of intestinal epithelium.7-10 Disruptions in intestinal integrity, resulting in a “leaky gut,” can cause microbial poisons and germs to circulate throughout the body. This circulation can result in severe infections and restricted blood flow, which can lead to amputations.4,11 Although gut bacteria contribute to systemic inflammation and increase susceptibility to nosocomial infections, a risk faced by some SCD patients due to frequent hospitalizations,12-16 the role of specific microbes in influencing each compartment of the hematopoietic system has been considered in some way. Despite the fact that connections between the microbiota and hematopoietic system products are widely documented, a mechanistic knowledge of how the microbiome directly or indirectly affects hematopoiesis is limited.
We looked beyond the present conventional and traditional treatments for SCD since we were curious about other methods. This led us to probiotics, which are live bacteria that, when given in adequate amounts, help the health of host. The development of alternative therapeutic agents for the induction of HbF, VOC inhibition, and regulation of epigenetic-based gene expression and cell function has been prompted by the many uses of probiotics in production of probiotic-mediated short chain fatty acids (SCFAs) (butyrate, acetate, propionate, etc.) and human health. Therefore, we concentrated on Lactobacillus plantarum F4’s (L. plantarum F4), a gram-positive bacterium, in this investigation. It has previously been documented to have numerous health-improving benefits on humans and animals.17 L. plantarum has recently been linked to SCFA expression on LepR+ (leptin receptor-expressing) mesenchymal stromal cells, which controls hematopoiesis and erythropoiesis in the bone marrow.18 Previous research has also revealed that L. plantarum has a function in folate synthesis.19 Furthermore, folate is required for erythroblast proliferation during differentiation.20 Its ability to induce HbF and prevent sickling, however, has not yet been studied. Probiotics particularly strains like L. plantarum, have garnered attention for the potential to modulate systemic inflammation, influence gut microbiota composition, and produce bioactive metabolites such as SCFAs. These properties suggest that probiotics could play a role in enhancing HbF production, reducing systemic inflammation, and alleviating oxidative stress, thereby targeting key aspects of SCD pathology. This investigation was carried out to assess the effects of L. plantarum F4’s anti-sickling and HbF-inducing action on sickled erythrocytes obtained from SCD patients.
Culture of L. plantarum F4 and preparation of the supernatant
L. plantarum F4 lyophilized culture (KF496215; isolated from fish gastrointestinal system) was obtained from the Ingress Bioinnovations Pvt. Ltd. laboratory in Ahmedabad, India. The strain was cultivated for 24 hours at 37 °C in De Man, Rogosa, and Sharpe Broth (MRSB) medium with supplementation of 5% yeast extract. After two consecutive transfers, 10 mL volumes of MRS broth were mixed with 10% (v/v) of the activated culture, which was subsequently cultured for another 24 hrs at 37 °C. Cell population of the strain was determined using MRS agar medium (Himedia) through pour-plate technique with 36-hours incubation at 37 °C. Colonies (in the range of 25 to 250) were counted, and colony-forming units (CFU) were calculated.
The supernatant of bacterial cells was produced for L. plantarum F4 using the procedure described earlier.21 The bacterial supernatant was separated by centrifuging the L. plantarum F4 culture for 10 minutes at 4 °C at 6,000 × g. Extracellular extracts were extracted using cell-free medium. After adding 15 µL of 40 mg/ml lysozyme to the solution, it was incubated for three hours at 37 °C. Lysozyme was used to lyse the bacterial cells by hydrolyzing the peptidoglycan layer in the cell walls, thereby releasing intracellular components into the supernatant. This step ensured the extraction of potentially active metabolites and proteins relevant to the anti-sickling and HbF inducing effects. This suspension was centrifuged at 10,000 × g for 20 min at 4 °C. The separated supernatants were dialyzed with 0.22 µm membrane filter against a 0.02 M K3PO4 buffer (pH 6.2) that contained 10 mM EDTA. To get rid of any remaining medium components, this L. plantarum F4 (CS), also known as the dialysis product, was treated three times with sterile peptone solution. Thirty percent and sixty percent v/v were the final cell concentrations. This process retained the bioactive molecules within the L. plantarum F4 (CS) while eliminating smaller, unwanted substances, ensuring a purified preparation for downstream assays.22
Study participants and blood samples’ collection
This study included 15 patients with SCD and 5 healthy controls from South Gujarat region of India. Table represents demographic for patients and healthy controls. Medical staff at General Hospital, Vyara, India, made the SCD diagnosis. SCD patients were not treated for four weeks prior to blood collection, and they were not given any blood transfusions. 2 ml of blood were extracted from HbSS (homozygous SCD) individuals between the ages of 18 and 39 years.
Before starting treatment with the CS of L. plantarum F4 and the pharmacological control (hydroxyurea), the patients were categorized into severe and less severe groups based on their phenotypic characteristics and HbF levels. Patients exhibiting severe symptoms such as leg ulcers, lung or heart injury, acute chest syndrome, splenic sequestration, liver congestion and osteomyelitis were classified under ‘severe group’ The patients with pain crises, anemia, weariness, frequent bacterial infections, dactylitis, and arthritic symptoms were classified under ‘less severe group’.23 Additionally, patients with HbF concentrations below 10% were classified under “severe group”, whilst those with concentrations above 10% were classified under “less severe group”.24 All participants signed a written consent form. The ethical guidelines outlined in the 1964 Helsinki Declaration and its later revisions were adhered to in this investigation. K3EDTA vials were used to collect the blood samples, which were then kept at 4 °C until the subsequent tests (which were completed within 24 hours) were conducted.
Evaluation of L. plantarum F4 (CS)’s anti-sickling activity in vitro
L. plantarum F4 (CS) anti-sickling activity was assessed using the Emmel Test.26 To get the culture supernatant (CS), centrifugation of 1 mL fresh whole blood was carried out for 5 minutes at 3000 rpm after being cleaned three times with 1 ml sterile normal saline. Additionally, 100 µL of washed RBCs and 100 µL of the CS (at various concentrations: 30% v/v, 60% v/v, 90% v/v, 120% v/v) were combined, followed by incubation for three hrs at 37 °C while being shaken occasionally. Further, 0.2 ml of 2% sodium metabisulphite (Merck, Germany) was added and well stirred to further deoxygenate the system.
Until four readings were acquired, the samples were taken in triplicate at different times (i.e., 0 min, 30 min, 60 min, 90 min, and 120 min) and from different combinations. Following its placement on a glass slide, each sample was fixed with 95% methanol, allowed to air dry, and then stained with Giemsa dye. We included two kinds of controls in the test: 30 mg/ml & 60 mg/ml Hydroxyurea as experimental medication control and 0.9% normal saline as negative control. The slides were visualized using phase-contrast microscopy (Leica Microsystems, Bangalore, India). Using four distinct frames of view, the RBCs were counted throughout the slide. Sickled cells percentage was calculated using the following formula.27
Sickled cells percentage = [Number of sickled cells / Total number of cells] × 100
Measurement of the level of HbF after treatment with the (CS) of the L. plantarum F4 culture
HPLC system with Bio-Rad VARIANT™ II β-thalassemia Short Program, CA, USA (Variant Haemoglobin Testing System) was used for measuring HbF levels. One milliliter of blood was centrifuged for five minutes at 3000 rpm. After removing the plasma, the cells underwent two rounds of washing in regular saline. 50 µL of L. plantarum F4 (CS) was combined with 50 µL of cleansed erythrocytes. Each tube was filled with 0.2 mL of sodium metabisulphite following three hours of incubation at 37 °C with intermittent shaking. Following 2 hrs of culture at 37 °C, samples were collected at 30 minutes intervals.
Further, dilution of samples was carried out by adding 1 mL of deionized water in 5 µL of the reaction mixture (blood sample). After incubation of 30 minutes, the samples were processed using Variant Haemoglobin Testing System cartridge. The drug and negative controls were hydroxyurea (30% v/v, 60% v/v) and normal saline (0.9%), respectively. Human erythrocyte hemolysate that had been lyophilized and contained EDTA, tobramycin, and gentamycin served as HbF calibrator. The analysis employed elution buffers 1 and 2 (Na3PO4-buffer) and 1-2% HbF as standard control. The concentration of HbF, area percentage of other peaks, structural alterations windows and retention time were measured.
Statistical analyses
Each experiment was carried out twice, and results were expressed using the mean ± SEM (standard error of mean). Analysis of variance, or ANOVA, was performed using SPSS 17.0 software to compare anti-sickling and HbF inducing activity within and between groups. Differences between 30% v/v and 60% v/v concentrations of L. plantarum F4 (CS) and hydroxyurea at various time intervals were examined using the student t-test. A p-value of less than 0.05 was considered to be statistically significant.
L. plantarum F4 (CS)’s ability to prevent sickling in sickled erythrocytes
The anti-sickling activity of L. plantarum F4 (CS) was tested in 15 blood samples from SCD patients (Figure S1). All patients were found to have homozygous TT genotypes for the rs334 (A/T) SNP of the HBB gene, confirming the SCD disease at the genetic level (supplementary information).25 This study investigated the effect of different doses of L. plantarum F4 (CS) at different time periods on sickled RBCs. Table shows the demographic information for SCD patients.
Table:
Demographics of sickle cell disease (SCD) patients and healthy controls
| Sickle Cell Disease Patients (n = 15) | Healthy Controls (n = 5) | |
|---|---|---|
| Average Age (Mean ± SEM) | 27.65 ± 5.423 years | 24.83 ± 4.329 years |
| Sex: | ||
| Male | 7 (46.66%) | 3 (60%) |
| Female | 8 (53.33%) | 2 (40%) |
| SCD Onset Age (Mean ± SEM) | 3.447 ± 2.786 years | – |
| Disease Duration (Mean ± SEM) | 24.52 ± 3.742 years | – |
| Family History | 12 (80%) | – |
| Body pains/Back pains | 15 (100%) | – |
| Hydroxyurea Medication | 12 (80%) | – |
| Folic acid Medication | 3 (20%) | – |
| Genotyping of rs334 (A/T)25 | ||
| AA | 0 (0%) | 5 (100%) |
| AT | 0 (0%) | 0 (0%) |
| TT | 15 (100%) | 0 (0%) |
| Severe Group | 6 (40%) | – |
| Less Severe Group | 9 (60%) | – |
| Severity Features: | ||
| Acute Chest Syndrome | 2 (13.33%) | – |
| Leg Ulcers | 2 (13.33%) | – |
| Splenic Sequestration | 2 (13.33%) | – |
| Osteomyelitis | 0 (0%) | – |
| Lung & Heart Injury | 0 (0%) | – |
| Liver Congestion | 0 (0%) | – |
| Less Severity Features: | ||
| Fatigue | 3 (20%) | – |
| Anemia | 2 (13.33%) | – |
| Pain Crises | 4 (26.66%) | – |
| Dactylitis | 0 (0%) | – |
| Arthritic symptoms | 0 (0%) | – |
| Frequent Bacterial Infection | 0 (0%) | – |
| HbF level (Severe group): | ||
| HbF < 10% | 6 (40%) | – |
| (Mean HbF ± SEM) | (4.00 ± 1.257) | – |
| HbF level (Less severe group): | ||
| HbF > 10% | 9 (60%) | – |
| (Mean HbF ± SEM) | (13.20 ± 0.432) | – |
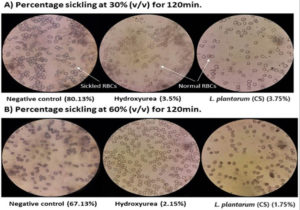
In addition to hydroxyurea (drug control), the RBCs of each patient were exposed to 30%, 60%, 90%, and 120% v/v concentrations of the L. plantarum F4 (CS) at five distinct time intervals: 0, 30, 60, 90, & 120 min. Normal saline was used as the negative control in the microscopic analysis to ascertain the proportion of sickling (Figure S1 A and B). We selected 30% (v/v) and 60% (v/v) for anti-sickling activity testing since they were the lowest concentrations of all the doses. At concentrations of 30% v/v and 60% v/v, the L. plantarum F4 (CS) demonstrated strong anti-sickling activity at all-time intervals in comparison to negative control (Figure 1A and 1B; Table S1). Consequently, 30% v/v and 60% v/v concentrations were chosen as appropriate for additional research.
Figure 1. Percentage sickling observed under a microscope after L. plantarum F4 (CS) and hydroxyurea treatment. (A) The sickling percentage at 30% (v/v) of L. plantarum F4 (CS) and hydroxyurea (Drug Control) for 120 minutes was observed under a 40X microscope, which revealed a decrease in sickled RBCs as compared to the negative control. When L. plantarum F4 (CS) was treated, the proportion of sickling was similar to that of hydroxyurea. (B) The percentage sickling at 60% (v/v) concentration of L. plantarum F4 (CS) and hydroxyurea (Drug Control) for 120 minutes was observed under a 40X microscope, which revealed a decrease in sickled RBCs in comparison to the negative control. When L. plantarum F4 (CS) was treated, the proportion of sickling was similar to that of hydroxyurea
Anti-sickling activity of L. plantarum F4 (CS) as compared to Hydroxyurea
Further, the anti-sickling efficacy of the L. plantarum F4 (CS) was compared to that of hydroxyurea (standard drug control). Previously, researchers28-30 used hydroxyurea as a conventional pharmacological control to assess anti-sickling potential of medicinal herbs. Sickling percentage seen after treating sickled erythrocytes with L. plantarum F4 (CS) and hydroxyurea was used to calculate anti-sickling activity.
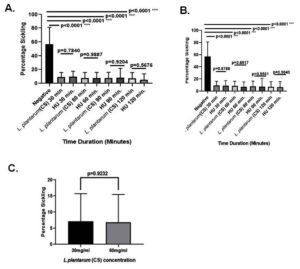
At 30% v/v dosage, the L. plantarum F4 (CS) significantly decreased the sickling percentage at all-time intervals (30, 60, 90, & 120 min; 9.066 ± 1.740%; 7.753 ± 7.703%; 7.717 ± 2.617% & 7.133 ± 2.212%, p < 0.0001) in comparison to negative control (Table S1). Nevertheless, for 30, 60, 90, & 120 min, there were no appreciable variations in the percentage of sickling between the L. plantarum F4 (CS) and hydroxyurea at 30% v/v concentration (9.066 ± 1.740% vs 9.803 ± 2.018%; p = 0.7840; 7.753 ± 7.703% vs 7.795 ± 2.100%; p = 0.9887; 7.717 ± 2.617% vs 8.134 ± 3.516%; p = 0.9204; & 7.133 ± 2.212% vs 5.344 ± 2.160%; p = 0.5676, respectively). The L. plantarum F4 (CS) demonstrated lowest sickling percentage in patients’ RBCs (7.133 ± 2.212%) at 30% v/v concentration for 120 minutes (Figure 2A).
Similarly, the L. plantarum F4 (CS) showed a substantial decrease in sickling percentage at all-time intervals (30, 60, 90, & 120 min; 5.892 ± 1.278%; 6.846 ± 2.344%; 8.053 ± 2.465%; 6.828 ± 2.224%; p < 0.0001) in comparison to negative control at a concentration of 60% v/v (Table S1). However, there were no discernible differences in the percentage of sickling between the L. plantarum F4 (CS) and hydroxyurea at the 60% v/v concentration during the 30, 60, 90, & 120 min. time intervals (5.892 ± 1.278% vs 8.910 ± 2.917%; p = 0.8788; 6.846 ± 2.344% vs 5.819 ± 1.436%; p = 0.6917; 8.053 ± 2.465% vs 6.4 ± 2.694%; p = 0.9561; & 6.828 ± 2.224 vs 4.741 ± 1.170; p = 0.9040, respectively). The 60% v/v L. plantarum F4 (CS) showed the lowest percentage of sickling (5.892 ± 1.278%) at 30 minutes (Figure 2B). Furthermore, the sickling percentage did not differ between 30% v/v & 60% v/v doses of the L. plantarum F4 (CS) (7.133 ± 2.212% vs 5.892 ± 1.278%; p = 0.9927) (Figure 2C).
Figure 2. L. plantarum’s (CS) ability to prevent sickling in SCD erythrocytes. (A) 30% (v/v) anti-sickling activity of (CS) in comparison to drug control: i) Sickling percentage in sickled RBCs did not significantly decrease with 30 minutes (p = 0.7840; Mean ± SEM = 9.066 ± 1.740 vs 9.803 ± 2.018, N = 15); ii) the percentage sickling in sickled RBCs did not significantly decrease with 60 minutes (p = 0.9887; Mean ± SEM = 7.753 ± 7.703 vs 7.95 ± 2.100, N = 15); iii) the percentage sickling in sickled RBCs did not significantly decrease with 90 minutes (p = 0.9204; Mean ± SEM = 7.717 ± 2.167 vs 8.134 ± 3.516, N = 15); iv) the percentage sickling in sickled RBCs did not significantly decrease with 120 minutes (p = 0.5676; Mean ± SEM = 7.133 ± 2.212 vs 5.344 ± 2.160, N = 15). (B) Drug control against (CS) for 60% (v/v) Anti-sickling Activity: i) Sickling percentage in sickled RBCs did not significantly decrease at 30 minutes (p = 0.8788; Mean ± SEM = 5.892 ± 1.278 vs 8.910 ± 2.917, N = 15); ii) Sickling percentage in sickled RBCs did not significantly decrease with 60 minutes (p = 0.6917; Mean ± SEM = 6.846 ± 2.344 vs 5.819 ± 1.436, N = 15); iii) the percentage sickling in sickled RBCs did not significantly decrease with 90 minutes (p = 0.9561; Mean ± SEM = 8.053 ± 2.465 vs 6.4 ± 2.694, N = 15); iv) the percentage sickling in sickled RBCs did not significantly decrease with 120 minutes (p = 0.9040; Mean ± SEM = 6.288 ± 2.224 vs 4.741 ± 1.170, N = 15). (C) Anti-sickling activity comparison at 120 minutes between 30% (v/v) and 60% (v/v): The percentage sickling decrease in sickled RBCs did not differ significantly (p = 0.9232; Mean ± SEM = 7.132 ± 2.212 vs. 6.288 ± 2.224, N = 15)
HbF induction by L. plantarum F4 (CS) in SCD erythrocytes
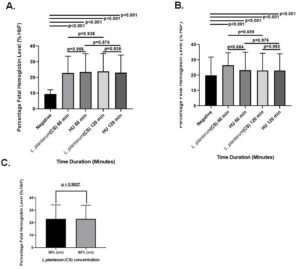
Blood samples of 15 patients with SCD and 5 controls were analyzed using HPLC to determine the effect of L. plantarum F4 (CS) on HbF concentration (Figure S2 A-B). In comparison to the negative control, the L. plantarum F4 (CS) had no influence on the levels of HbF in erythrocytes from healthy controls at concentrations of 30% v/v and 60% v/v. HbF concentrations in healthy people’s erythrocytes remained constant over time. Treatment with 30% v/v L. plantarum F4 (CS) and hydroxyurea markedly increased HbF levels at 60 and 120 min in comparison to negative control (Table S2; 22.98 ± 5.301% & 23.83 ± 5.771%; p < 0.0001). L. plantarum F4 (CS) (30% (v/v)) showed the biggest rise in HbF concentration at 120 minutes. However, the HbF concentration did not differ between L. plantarum F4 (CS) and hydroxyurea at 30% v/v for the given time intervals (22.98 ± 5.301% vs 23.43 ± 5.916%; p = 0.956 and 23.83 ± 5.771% vs 23.18 ± 5.591%; p = 0.938, respectively) (Figure 3A). Comparing the treatment with 60% v/v L. plantarum F4 (CS) and hydroxyurea to the negative control, HbF levels were significantly elevated at all the time points (26.40 ± 4.150% & 23.18 ± 5.591%; p < 0.0001; Table S2). Nevertheless, at the specified time periods, there was no difference in HbF levels between L. plantarum F4 (CS) and hydroxyurea at 60% v/v (26.40 ± 4.150% vs 23.35 ± 5.827%; p = 0.684 & 23.18 ± 5.591% vs 23.10 ± 5.474%; p = 0.992, respectively; Figure 3B). Furthermore, following 120 min, no change in HbF levels in erythrocytes was seen between the 30% v/v and 60% v/v L. plantarum F4 (CS) concentrations (23.18 ± 5.591% vs 23.10 ± 5.474%; p = 0.9927; Figure 3C).
Figure 3. Levels of HbF in SCD erythrocytes after exposure to L. plantarum F4 (CS). At 30% (v/v) and 60% (v/v) concentrations for all time periods, SCD erythrocytes produced more HbF in response to both (CS) and hydroxyurea than in the negative control (p < 0.01). (A) Drug control (30% (v/v)) versus L. plantarum (CS): i) There was no discernible rise in the percentage of HbF in sickled RBCs after 60 minutes (p = 0.956; Mean ± SEM = 22.98 ± 5.301 vs 23.43 ± 5.916, N = 15); ii) There was no discernible rise in the percentage of HbF in sickled RBCs after 120 minutes (p = 0.938; Mean ± SEM = 23.83 ± 5.771 vs 23.18 ± 5.591, N = 15). (B) L. plantarum F4 (CS) versus drug control (60% (v/v)): i) The percentage of HbF in sickled RBCs did not significantly increase after 60 minutes (p = 0.684; Mean ± SEM= 26.40 ± 4.150 vs 23.35 ± 5.827, N = 15); ii) the percentage of HbF in sickled RBCs did not significantly increase after 120 minutes (p = 0.992; Mean ± SEM= 23.18 ± 5.591 vs 23.10 ± 5.474, N = 15). (C) HbF production activity comparison at 120 minutes between 30% (v/v) and 60% (v/v): There was no discernible change in sickled RBCs’ HbF production (p = 0.9927; Mean ± SEM = 23.83 ± 5.771 vs 23.18 ± 5.591, N = 15)
L. plantarum F4 (CS)’s ability to prevent sickling in sickled erythrocytes from severe and less severe patients with SCD
Since we observed significant anti-sickling and HbF inducing activity at 30% v/v L. plantarum F4 (CS) for 120 min, this concentration and time were further investigated (Figure 2C and 3C). The patients were split into two groups: ‘severe group’ and ‘less severe group’, according to SCD severity (Table). The sickling percentage remained constant between negative controls of severe and less severe groups (Table S3 A; 65.32 ± 2.592% vs 59.34 ± 1.317%; p = 0.100). Similarly, when treated with hydroxyurea, the sickling percentage did not differ between the severe and less severe SCD groups (21.33 ± 3.751% vs 29.67 ± 3.851%; p = 0.146; Table S3 A). Additionally, there was no difference in sickling percentage between severe and less severe groups for L. plantarum F4 (CS) treatment (17.89 ± 3.890% vs 15.47 ± 3.174%; p = 0.635; Table S3A).
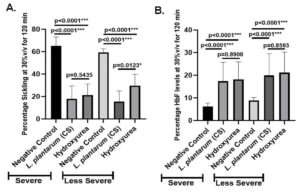
Additionally, hydroxyurea’s anti-sickling activity and negative control, L. plantarum F4 (CS), were examined in severe and less severe groups of SCD. Sickling percentage in the severe group varied substantially between hydroxyurea treatment and negative control (65.32 ± 2.592% vs 21.33 ± 3.751%; p < 0.0001) and between the negative control and L. plantarum F4 (CS) treatment (65.32 ± 2.592% vs 17.89 ± 3.890%; p < 0.0001) (Figure 4A). Sickling percentage in the less severe group also varied substantially between hydroxyurea treatment and negative control (59.34 ± 1.317 vs 29.67 ± 3.851; p < 0.0001) and between L. plantarum F4 (CS) treatment and negative control (59.34 ± 1.317% vs 15.47 ± 3.174%; p < 0.0001) (Figure 4A). Significant differences in percentage sickling between L. plantarum F4 (CS) and hydroxyurea treatment were also observed in the less severe SCD group (29.67 ± 3.851% vs 15.47 ± 3.174%; p = 0.0123). However, sickling percentage in severe group did not significantly differ between hydroxyurea and L. plantarum F4 (CS) treatments (17.89 ± 3.890% vs 21.33 ± 3.751%; p = 0.5435) (Figure 4A).
Figure 4. L. plantarum F4 (CS) has anti-sickling and HbF inducing activities in the RBCs of severe and less severe patients with SCD. (A) L. plantarum F4 (CS) anti-sickling activity in erythrocytes from severe and less severe groups at 30% v/v for 120 minutes: i) L. plantarum F4 (CS) vs Negative control in severe cases: L. plantarum F4 (CS) demonstrated a significant reduction in percentage sickling in comparison to negative control (p < 0.0001; Mean ± SEM = 65.32 ± 2.592 vs 59.34 ± 1.317, N = 6); (ii) Hydroxyurea vs Negative control in severe group: Hydroxyurea demonstrated a significant reduction in percentage sickling in comparison to negative control (p < 0.0001; Mean ± SEM = 65.32 ± 2.592 vs 21.33 ± 3.751, N = 6); (iii) L. plantarum F4 (CS) vs Hydroxyurea in severe group: The percentage of sickling in L. plantarum F4 (CS) and hydroxyurea did not differ significantly (p = 0.5435; Mean ± SEM = 17.89 ± 3.890 vs 21.33 ± 3.751, N = 6); (iv) In less severe cases, L. plantarum F4 (CS) showed a substantial reduction in sickling percentage in comparison to negative control (p < 0.0001; Mean ± SEM= 59.34 ± 1.317 vs 15.47 ± 3.174, N = 9); (v) In less severe cases, Negative control vs. Hydroxyurea: Hydroxyurea showed significant reduction in percentage sickling in comparison to negative control (p < 0.0001; Mean ± SEM = 59.34 ± 1.317 vs 29.67 ± 3.851, N = 9); (vi) L. plantarum F4 (CS) vs Hydroxyurea in Less Severe group: A significant difference in sickling percentage was observed between L. plantarum F4 (CS) and hydroxyurea (p = 0.0123; Mean ± SEM = 15.47 ± 3.174 vs 29.67 ± 3.851, N = 9) in comparison to negative control (p < 0.0001; Mean ± SEM = 59.34 ± 1.317 vs 29.67 ± 3.851, N = 9). (B) At 30% v/v for 120 minutes, the severe and less severe patients’ RBCs showed HbF induction by L. plantarum F4 (CS): (i) In severe group, negative control vs L. plantarum F4 (CS): In severe group, HbF% differed significantly between negative control and L. plantarum F4 (CS) (p < 0.0001; Mean ± SEM = 6.260 ± 0.647 vs 17.42 ± 3.713, N = 6); (ii) Negative control vs Hydroxyurea (p < 0.0001; Mean ± SEM = 6.260 ± 0.647 vs 18.14 ± 3.488, N = 6); (iii) In severe group, HbF% did not differ between L. plantarum F4 (CS) and Hydroxyurea (p = 0.8908; Mean ± SEM= 17.42 ± 3.713 vs 18.14 ± 3.488, N = 6); in less severe group, there was a difference between L. plantarum F4 (CS) and negative control. The HbF% of L. plantarum F4 (CS) and the negative control showed a significant difference (p < 0.0001; Mean ± SEM = 8.838 ± 0.60 vs 19.93 ± 4.797, N = 9); (v) In less severe group, HbF% differed significantly between hydroxyurea and negative control (p < 0.0001; Mean ± SEM = 8.838 ± 0.60 vs 21.15 ± 4.545, N = 9); (vi) In less severe group, there was a difference between hydroxyurea and L. plantarum F4 (CS). There was no discernible change in HbF% between hydroxyurea and L. plantarum F4 (CS) (p = 0.8593; Mean ± SEM = 19.93 ± 4.797 vs 21.15 ± 4.545, N = 9)
L. plantarum F4 (CS)’s ability to induce HbF in sickled erythrocytes from individuals with severe and less severe SCD
Additionally, erythrocytes from severe and less severe groups of patients were used for investigating the HbF inducing activity of L. plantarum F4 (CS). The optimal dose was 30% v/v, and the negative control, L. plantarum F4 (CS), and pharmaceutical control were all exposed for 120 minutes. HbF levels significantly differed between negative controls of severe and less severe patient groups (6.260 ± 0.647% vs 8.838 ± 0.60%; p = 0.019; Table S3 B). HbF levels, however, did not change between severe and less severe groups following hydroxyurea treatment (Table S3B; 18.14 ± 3.488% vs 21.15 ± 4.545%; p = 0.608). Likewise, HbF levels did not differ between severe and less severe groups for L. plantarum F4 (CS) treatment (17.42 ± 3.713% vs 19.93 ± 4.797%; p = 0.685; Table S3B).
Additionally, the HbF inducing activity of severe and less severe groups was examined using hydroxyurea and the negative control, L. plantarum F4 (CS). Significant differences in HbF% were seen in the severe SCD group between the negative control and L. plantarum F4 (CS) (6.260 ± 0.647% vs 17.42 ± 3.713%; p < 0.0001) and hydroxyurea (6.260 ± 0.647% vs 18.14 ± 3.488%, p < 0.0001; Figure 4B). Nevertheless, HbF% did not differ between hydroxyurea and L. plantarum F4 (CS) in severe group of SCD (17.42 ± 3.713% vs 18.14 ± 3.488%; p = 0.8908). Similarly, in the less severe SCD group, HbF% did not differ between hydroxyurea and negative control (p = 0.8593) (Figure 4B). Additionally, in the less severe SCD group, there was no difference in HbF% between L. plantarum F4 (CS) and hydroxyurea (19.93 ± 4.797% vs 21.15 ± 4.545%; p = 0.8593) (Figure 4B).
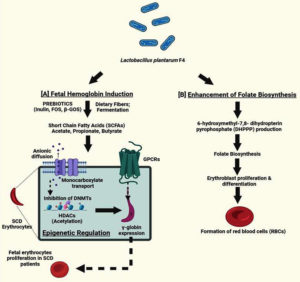
Sickle cell disease (SCD) is caused by a mutation in HBB gene, which results in aberrant hemoglobin and the characteristic sickle-shaped red blood cell.31 GBT440, pyridyl vanillin derivatives, and 5-hydroxymethylfurfural (5-HMF) have all been shown to be effective anti-sickling drugs in earlier studies.29,32,33 Anti-sickling properties have also been demonstrated by in vitro studies on anti-sickling compounds derived from plant sources include Boerhavia diffusa, Nigella sativa, Cyperus esculentus, Aloe vera, Cajanus cajan and Carica papaya.30,34-37 Many of the treatments or medications currently available for the treatment of SCD have been demonstrated to have a variety of negative effects on SCD patients when used for an extended period of time. Throughout all of these crises and painful episodes, the sole remedy for SCD was gene therapy and bone marrow transplant, but this has not been used due to lack of suitable donor and lack of knowledge.38 Therefore, we concentrated on investigating other potential alternative treatments for SCD. This has grabbed our focus towards gut microorganisms and probiotics that mediate short chain fatty acids (SCFAs) production. These SCFAs, act as an effective regulator of epigenetic mechanisms while also controlling VOC and inflammation via influencing several downstream molecules implicated in HbF signaling pathways.39 The folate molecule has a protein moiety that is linked to PABA (para-aminobenzoic acid). This protein moiety is produced from DHPPP (6-hydroxymethyl-7,8-dihydropterin pyrophosphate). Therefore, for folate de novo synthesis, both DHPPP and PABA are necessary (Figure 5). The shikimate pathway converts erythrose 4-phosphate and phosphoenolpyruvate into chorismate which is converted to 4-amino-4-deoxychorismate by aminodeoxychorismate synthase. Pyruvate is subsequently broken down by 4-amino-4-deoxychorismate lyase to produce PABA, which is used to produce folate (Figure 5). According to one study, L. plantarum possesses the folate biosynthesis cluster, which consists of all required genes (apart from alkaline phosphatase gene) for DHPPP synthesis and gene encoding dihydropteroate synthase. L. plantarum may therefore be considered an efficient folate producer if it is cultivated with PABA, as it is likely to synthesize DHPPP.19 Moreover, erythroblast proliferation during differentiation20 depends on folate (Figure 5).
Figure 5. The suggested mechanism by which sickle cell patients’ HbF is produced by L. plantarum F4. (A) L. plantarum produces SCFAs such as butyrate, acetate, propionate and valerate by consuming dietary fibers like inulin and fructooligosaccharides (FOSs). By activating different signaling pathways, these SCFAs may function as epigenetic modifiers in SCD patients. According to recent studies, SCFAs can directly enter epigenetic control through G-coupled protein cell receptors (GPCRs) or indirectly through anionic diffusion and monocarboxylate transport mechanisms. These SCFAs cause hyperacetylation of histone deacetylases (HDACs) and prevent DNA methylation in chromatin structures, which increases the generation of HbF. (B) One protein moiety, derived from DHPPP (6-hydroxymethyl-7,8-dihydropterin pyrophosphate), is attached to PABA (para-aminobenzoic acid) in the folate molecule. Therefore both PABA and DHPPP are required as precursors for de novo biosynthesis. Chorismate is the final product of the shikimate pathway, which is triggered by erythrose 4-phosphate and phosphoenolpyruvate. Aminodeoxychorismate synthase transforms chorismate into 4-amino-4-deoxychorismate. Subsequently, 4-amino-4-deoxychorismate lyase then breaks down pyruvate to produce pABA, which is then used in the production of folate. All of the genes involved in the production of DHPPP, except for alkaline phosphatase, including dihydropteroate synthase gene are part of cluster of folate synthesis that L. plantarum is capable of producing. As a result, L. plantarum is anticipated to generate DHPPP and might be regarded as a possible source of folate
L. plantarum has also been linked to SCFA expression on LepR+ (leptin receptor-expressing) MSCs (mesenchymal stromal cells), which affects haematopoiesis and erythropoiesis in bone marrow.18 Therefore, our hypothesis suggests that L. plantarum may have the potential for RBC proliferation, and thus the current study investigated the role of L. plantarum F4 (KF496215; isolated from the gastrointestinal tract of fish) in inducing anti-sickling activity and HbF in sickled RBCs).
For the first time we are looking into the potential role of probiotic L. plantarum F4 (CS) in preventing sickling and HbF induction in sickled erythrocytes in vitro. The anti-sickling activity of L. plantarum F4 (CS) at four different concentrations (30%, 60%, 90% and 120% (v/v)) was evaluated using 15 SCD blood samples. This was done in order to ascertain whether the (CS) of L. plantarum F4 has the potential to function as an anti-sickling agent. In this study, we acknowledge the limitation posted by the relatively small size, comprising 15 patients with sickle cell disease and 5 healthy controls. While this sample size provided initial insights into the anti-sicking and HbF inducing effects of L. plantarum F4 (CS), a larger cohort would strengthen the statistical power and generalizability of the findings. On SCD erythrocytes, anti-sickling action was detected at all concentrations of L. plantarum F4 (CS). We selected 30% (v/v) and 60% (v/v) for additional anti-sickling activity testing since these were the lowest concentrations across all doses. Interestingly, anti-sickling activity percentage was increased at 30% and 60% (v/v) concentrations of L. plantarum F4 (CS) in comparison to negative control (Table S1; Figure 2; p < 0.0001). However, anti-sickling activity for 30% (v/v) L. plantarum F4 (CS) did not differ significantly from hydroxyurea at 30, 60, 90 and 120 min (9.066 ± 1.740% vs 9.803 ± 2.018%; p = 0.7840; 7.753 ± 7.703% vs 7.795 ± 2.100%; p = 0.9887; 7.717 ± 2.617% vs 8.134 ± 3.516%; p = 0.9204; & 7.133 ± 2.212% vs 5.344 ± 2.160%; p = 0.5676, respectively), indicating that the anti-sickling activity of L. plantarum F4 (CS) is comparable to that of hydroxyurea (Figure 2A). L. plantarum F4 (CS) showed a significant difference at 60% (v/v) concentration (p < 0.0001) compared to negative control for all the time intervals (Figure 2B; Table S1). However, at 30, 60, 90 and 120 min, there was no discernible difference between L. plantarum F4 (CS) and hydroxyurea (5.892 ± 1.278% vs 8.910 ± 2.917%; p = 0.8788; 6.846 ± 2.344% vs 5.819 ± 1.436%; p = 0.6917; 8.053 ± 2.465% vs 6.4 ± 2.694%; p = 0.9561; & 6.828 ± 2.224% vs 4.741 ± 1.170%; p = 0.9040, respectively; Figure 2B). This suggests that at all investigated doses and time periods, the effects of L. plantarum F4 (CS) are equivalent to those of the pharmacological control, hydroxyurea. Furthermore, the percentage sickling did not differ at 120 min duration between 30% (v/v) and 60% (v/v) concentrations of L. plantarum F4 (CS) (7.132 ± 2.212% vs. 6.288 ± 2.224%; p = 0.9232) (Figure 2C), suggesting that 30% (v/v) concentration can be considered as the optimal dose because it is the lower dose as compared to 60% (v/v). Similarly, comparing HbF concentrations in sickled RBCs at 30% (v/v) and 60% (v/v) concentrations of L. plantarum F4 (CS) for 120 min did not reveal significant difference in HbF concentration (23.83 ± 5.771% vs 23.18 ± 5.591%; p = 0.9927) (Figure 3C), suggesting that the 30% (v/v) concentration can be considered as optimal dose because it is the lower dose as compared to 60% (v/v).
Notably, tiny molecular weight compounds generated from gut bacteria have been found to alter significant epigenetic variables directly or indirectly.39 Among these microbial metabolites, SCFAs have drawn a lot of attention due to their numerous health benefits. SCFAs such as lactate, butyrate, propionate, and succinate can activate and regulate G protein coupled receptors like GPR41 and GPR43. In this context, probiotic-induced epigenetic modifications may cause SCD patients to produce β-globin. Additionally, it has been demonstrated that SCFAs modify significant regulatory molecules by inhibiting histone deacetylases (HDACs) and exhibiting additional antimitogenic characteristics in relation to the increase in HbF.40 Gut microbes like Eubacterium and Butyrivibrio produce butyrate, a well-known class of epigenetic modifiers with many HDAC inhibitory mechanisms. These results suggest that HbF expression may be significantly influenced by probiotic-mediated epigenetic modifications (Figure 5). However, to fully assess the overall impact of these probiotics, more research is necessary.
Furthermore, investigations on gut microbiota in diverse murine and primate models have unambiguously proven the important function of SCFAs and other microbial products in driving embryonic erythropoiesis. The current research efforts aimed at understanding the treatment mechanisms for SCD have prompted researchers to place a greater emphasis on the regulatory component of HbF production. In the last two decades, numerous drugs have been proposed to revive HbF production via various routes. One such pathway includes SCFAs, specifically butyrate, which can stimulate HbF production.41 Many butyrate-related components found in promoter of β-globin, which is located away from the SSE and CCAAT box, are necessary for boosting HbF synthesis.42 One study claims that sodium butyrate alters microRNA expression to raise β-globin expression.43 Other SCFAs and their derivatives, including butyrate, have been shown to cause humans and baboons to produce HbF. Research has shown that oral treatment of propionate, phenylbutyrate, and valproic acid can increase HbF levels in baboons and transgenic mice.41 Additionally, it has been shown that SCFAs such as butyrate increase cGMP-signaling in K562 cells, which in turn increases NO (nitric oxide) and ROS. These factors then activate downstream transcription factors like p38-MAP kinase, which in turn inhibits HDAC and leads to the creation of HbF.41 However, the entire mechanism underlying butyrate-induced HDAC inhibition in SCD patients is still unknown. As a result, more research is needed to see if butyrate-producing gut bacteria can increase HbF production in SCD patients.
Hence, the present study sought to determine if the L. plantarum F4 (CS) has the capacity to boost the HbF concentration in SCD patients’ erythrocytes. Various reports in the literature imply that increasing HbF levels alleviates unpleasant crises such as VOC and acute chest syndrome. So, using HPLC, we determined the percentage of HbF in SCD patients’ blood samples after treatment with L. plantarum F4 (CS) through dose and time dependent manner. In sickled erythrocytes treated with both concentrations of L. plantarum F4 (CS), i.e., 30% and 60% (v/v), we found a substantial increase in HbF% (p < 0.0001) in comparison to the negative control (Table S2). Overall, the HPLC results showed that there is a percentage increase in the HbF concentration within the SCD erythrocytes at all the time intervals for both the concentrations of L. plantarum F4 (CS) and hydroxyurea. Although the HbF percentage between the L. plantarum F4 (CS) and hydroxyurea treated RBCs did not differ at 60 and 120 min. for both 30% and 60% (v/v) concentrations (22.98 ± 5.301% vs 23.43 ± 5.916%; p = 0.956 and 23.83 ± 5.771% vs 23.18 ± 5.591%; p = 0.938, 26.40 ± 4.150% vs 23.35 ± 5.827%; p = 0.684 & 23.18 ± 5.591% vs 23.10 ± 5.474%; p = 0.992; respectively, Figure 3 A, B), the HbF percentage in SCD blood samples increased by L. plantarum F4 (CS) was comparable to that of hydroxyurea (drug control) (Figure 3C). Inducing HbF and reducing the sickling of erythrocytes are pivotal strategies in the management of SCD. Elevated HbF levels reduce the polymerization of sickle hemoglobin (HbS), thereby decreasing the occurrence of sickling, hemolysis, and associated complications such as VOC and organ damage. HbF’s ability to inhibit HbS polymerization is particularly beneficial in alleviating the clinical severity of SCD and improving patient outcomes. Similarly, reducing erythrocyte sickling decreases microvascular occlusion, enhances blood flow, and mitigates chronic ischemic damage. Hence, exploring alternative approaches, such as probiotic derived therapies like L. plantarum F4 (CS), offers promise for improving the quality of life and long-term prognosis for SCD patients. Future experiments could explore the impact of shorter and longer incubation times to fully elucidate the kinetics of anti-sickling and HbF-induction activities, thus refining the therapeutic potential of L. plantarum F4 (CS). Additional studies are warranted to systemically evaluate the impact of incubation durations on anti-sickling and HbF-induction activities, enabling a comprehensive understanding of the temporal dynamics of L. plantarum F4 (CS) efficacy. To ensure the safe and effective clinical application of L. plantarum F4 (CS), future studies must rigorously address safety and efficacy concerns through comprehensive in vitro and in vivo investigations. Cell line studies using human erythroid progenitors and hematopoietic stem cells can assess cytotoxicity, metabolic stability, and the potential for off-target effects. These studies must include detailed toxicological assessments, including organ histopathology, immune modulation, and microbiota changes, to establish the safety profile. Additionally, pharmacokinetics and pharmacodynamics evaluations will be critical to determine optimal dosing regimens and efficacy endpoints. By addressing these preclinical considerations, future research can lay a robust foundation for the translational and clinical development of L. plantarum F4 as a novel therapeutic agent for SCD.
Future studies should focus on elucidating the mechanisms underlying the anti-sickling and HbF induction effects of L. plantarum F4 (CS). Identifying bioactive compounds using techniques such as liquid chromatography-mass spectrometry (LC-MS) and proteomics will help pinpoint the active metabolites or proteins responsible for these effects. Investigations into epigenetic modifications, including histone acetylation and DNA methylation of the β-globin promoter, can provide insights into regulatory pathways modulated by metabolites such as short-chain fatty acids (SCFAs). Additionally, studies on signaling pathways, including cAMP/PKA, NO/cGMP, and p38 MAPK, will help clarify the molecular processes involved. In vivo studies using transgenic SCD mouse models or clinical trials in humans will validate the therapeutic potential of L. plantarum F4. Exploring interactions with gut microbiota and assessing folate biosynthesis pathways could further explain the strains impact on erythropoiesis. Lastly, the stability and duration of the L. plantarum F4 (CS) effects, as well as its broader influence on hematopoietic and immune cells, warrant investigation to enhance its translational applicability for SCD treatment.
Overall, our results for the first time showed that L. plantarum F4 (CS) might have anti-sickling properties for sickled erythrocytes in vitro. It might also lead to the increased HbF in sickle cell erythrocytes. To validate the findings of this investigation, more research utilizing cell lines or animal models is necessary. Additionally, the bioactive substances and mechanisms underlying the L. plantarum F4 (CS)’s anti-sickling and HbF-inducing properties should also be elucidated, including epigenetic regulation and signaling pathways. Moreover, in vivo studies using transgenic SCD mouse models are essential to validate the efficacy and safety of L. plantarum F4 in a physiological context. Furthermore, assessing dosage optimization, long-term outcomes, and patient-reported benefits will be critical to translating these findings into effective therapeutic strategies for SCD.
Additional file: Additional Table S1-S3 and Figure S1-S2.
ACKNOWLEDGMENTS
The authors are thankful to all Sickle cell disease patients and control subjects for their participation in this study, and to Dr. Naitik Chaudhary, Chief District Medical Officer of Tapi, India for providing permission to collect patients’ blood samples. The authors are also grateful to Dr. Chirag V. Parikh, General Hospital, Vyara, India, who helped us in conduction of HPLC experiments, and to Mr. Akash Chaudhary for supporting in blood collection.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MD conceptualized the study, funding acquisition and supervised the study. PK, MD and NCL collected resources and visualization. SRS, FS, MD and ASP applied methodology. SRS, FS, MD and ASP performed analysis and data validation. SRS, FS, MD and ASP peformed Investigation. FS wrote the original draft. PK, MD and NCL wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was supported by Uka Tarsadia University-SSIP Cell, Gujarat, India, through grant number UTU/SSIP/2021-22/3445.
DATA AVAILABILITY
The data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional-Human Research Ethics Committee (IHREC), Maliba Pharmacy College, Uka Tarsadia University, Bardoli, Gujarat, India (Approval No.: MPC/IHEC/15/2022; Dated: 24-12-2022).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
Crossref - Sridevi P, Sharma Y, Balakrishna SL, Babu BV. Sickle cell disease treatment and management in India: a systematic review of interventional studies. Trans R Soc Trop Med Hyg. 2022;116(12):1101-1111.
Crossref - Amid A, Odame I. Improving outcomes in children with sickle cell disease: treatment considerations and strategies. Paediatr Drugs. 2014;16(4):255-266.
Crossref - Maximo C, Saad STO, Thome E, Queiroz AMM, Lobo C, Ballas SK. Amputations in Sickle Cell Disease: Case Series and Literature Review. Hemoglobin. 2016;40(3):150-155.
Crossref - Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69-75.
Crossref - Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar RD. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787-803.
Crossref - Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017;46(1):77-89.
Crossref - Ehrlich SD. The human gut microbiome impacts health and disease. C R Biol. 2016;339(7-8):319-323.
Crossref - Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med. (Encinitas) 2014:13(6):17-22.
- Wu GD. The Gut Microbiome, Its Metabolome, and Their Relationship to Health and Disease. Nestle Nutr Inst Workshop Ser. 2016;84:103-110.
Crossref - Gouveia C, Duarte M, Norte S, Alves P, Kjollerstrom P, Brito M, Tavares D. Osteoarticular infections in paediatric sickle cell disease: in the era of multidrugresistant bacteria. Br J Haematol. 2020;189(4):e147-e150.
Crossref - Bansil NH, Kim TY, Tieu L, Barcega B. Incidence of serious bacterial infections in febrile children with sickle cell disease. Clin Pediatr (Phila). 2013;52(7):661-666.
Crossref - Yanda ANA, Nansseu JR, Awa HDM, et al. Burden and spectrum of bacterial infections among sickle cell disease children living in Cameroon. BMC Infect Dis. 2017;17(1):211.
Crossref - Obaro SK, Tam PYI. Preventing Infections in Sickle Cell Disease: The Unfinished Business. Pediatr Blood Cancer. 2016;63(5):781-785.
Crossref - Odey F, Okomo U, Oyo-Ita A. Vaccines for preventing invasive salmonella infections in people with sickle cell disease. Cochrane Database Syst Rev. 2018;12(12):CD006975.
Crossref - Kondani DA, Gini-Ehungu JL, Bodi JM, Ekulu PM, Kunuanunua TS, Aloni MN. Prevalence of sickle cell disease in a pediatric population suffering from severe infections: a Congolese experience. Hemoglobin. 2014;38(4):225-229.
Crossref - Giraud E, Lelong B, Raimbault M. Influence of pH and initial lactate concentration on the growth of Lactobacillus plantarum. Appl Microbiol Biotechnol. 1991;36(1):96-99.
Crossref - Lee YS, Kim TY, Kim Y, et al. Microbiota-derived lactate promotes hematopoiesis and erythropoiesis by inducing stem cell factor production from leptin receptor+ niche cells. Exp Mol Med. 2021;53(9):1319-1331.
Crossref - Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3(1):118-134.
Crossref - Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105-131.
Crossref - Ningtiyas WD, Arief II, Budiman C, Utomo ARH. Inhibition of Human Cervical Cancer Hela Cell Line by Meat-Derived Lactic Acid Bacteria of L. plantarum IIA-1A5 and Lactobacillus acidophilus IIA-2B4. Pak J Biol Sci. 2021;24(12):1340-1349.
Crossref - Shekh SL, Boricha AA, Chavda JG, Vyas BRM. Probiotic potential of lyophilized Lactobacillus plantarum GP. Ann Microbiol. 2022;70:16.
Crossref - Ballas SK, Lieff S, Benjamin LJ, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85(1):6-13.
Crossref - Mpalampa L, Ndugwa CM, Ddungu H, Idro R. Foetal haemoglobin and disease severity in sickle cell anaemia patients in Kampala, Uganda. BMC Blood Disord. 2012;7(12):11.
Crossref - Waterfall CM and Cobb BD. Single tube genotyping of sickle cell anaemia using PCR-based SNP analysis. Nucleic Acids Res. 2001;29(23):E119.
Crossref - Mpiana PT, Lombe BK, Ombeni AM, et al. In vitro sickling inhibitory effects and antisickle erythrocytes hemolysis of Dicliptera colorata CB Clarke, Euphorbia hirta L and Sorghum bicolor (L.) Moench. Open J Blood Dis. 2013;3(1):43-48.
Crossref - Pauline N, Cabral BNP, Anatole PC, Jocelyne AMV, Bruno M, Jeanne NY. The in vitro antisickling and antioxidant effects of aqueous extracts Zanthoxyllum heitzii on sickle cell disorder. BMC Complement Altern Med. 2013;6(13):162.
Crossref - Nurain IO, Bewaji CO, Johnson JS, Davenport RD, Zhang Y. Potential of Three Ethnomedicinal Plants as Antisickling Agents. Mol Pharm. 2017;14(1):172-182.
Crossref - Lehrer-Graiwer J, Howard J, Hemmaway CJ, et al. GBT440, a potent anti-sickling hemoglobin modifier reduces hemolysis, improves anemia and nearly eliminates sickle cells in peripheral blood of patients with sickle cell disease. Blood. 2015;126(23):542.
Crossref - Shah F, Dwivedi M, Parikh CV. Promising Anti-sickling and Fetal Hemoglobin Inducing effects of Boerhavia diffusa Root Extract on Sickle Cell Erythrocytes. J Herb Med. 2020;24:100398.
Crossref - Shah F, Dwivedi M. Pathophysiology and recent therapeutic insights of sickle cell disease. Ann Hematol. 2020;99(5):925-935.
Crossref - Abdulmalik O, Safo MK, Chen Q, et al. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128(4):552-561.
Crossref - Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77(6): 1334-1341.
Crossref - Mojisola CC, Anthony EA, Alani DM. Antisickling properties of the fermented mixture of Carica papaya Linn and Sorghum bicolor (L.) Moench. Afr J Pharm Pharmacol. 2009;3(4):140-143.
- Anorue EC, Joshua PE. Evaluation of anti-sickling effects of two varieties of Cajanus cajan (L.) Huth on sickle cell beta thalassemia. J Ethnopharmacol. 2024; 331:118280.
- Nwaoguikpe RN, Braide W, Ezejiofor TIN. The effect of Aloe vera plant (aloe barbadensis) extracts on sickle cell blood (HbSS). Afr. J. Food Sci. Tech. 2010;1:58-63.
- Ibraheem NK, Ahmed JH, Hassan MK. The effect of fixed oil and water extracts of Nigella sativa on sickle cells: an in vitro study. Singapore Med J. 2010;51(3):230-234.
- Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 2017;376(5):429-439.
Crossref - Paul B, Barnes S, Demark-Wahnefried W, et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenet. 2015;7:112.
Crossref - Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111(6):2247-2252.
Crossref - Pace BS, Zein S. Understanding mechanisms of g-globin gene regulation to develop strategies for pharmacological fetal hemoglobin induction. Dev Dyn. 2006;235(7):1727-1737.
Crossref - Ikuta T, Kan YW, Swerdlow PS, Faller DV, Perrine SP. Alterations in protein-DNA interactions in the g-globin gene promoter in response to butyrate therapy. Blood. 1998;92(8):2924-2933.
Crossref - Tayebi B, Abrishami F, Alizadeh S, et al. Modulation of microRNAs expression in hematopoietic stem cells treated with sodium butyrate in inducing fetal hemoglobin expression. Artif Cells Nanomed Biotechnol. 2017;45(1):146-156.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.