ISSN: 0973-7510
E-ISSN: 2581-690X
Microsporidia, which are ubiquitous obligate intracellular parasites responsible for a variety of diseases and economic losses in farming, have different transmission strategies. While horizontal transmission relies on sufûcient parasite numbers released into environment, vertical transmission requires host reproduction to occur leading to a lower virulence, and also induces a sex ratio distortion. The second strategy has been reported in a broad range of hosts from protists to mammals, in which insects and amphipod crustaceans as the most common. The present study shows the first evidence of vertical transmission of a microsporidia in decapod crustaceans via an experimental description of the infection by Enterocytozoon hepatopenaei (EHP) in whiteleg shrimp Penaeus vannamei. The group of healthy shrimp was infected with EHP by feeding with infected-shrimp tissue and sharing habitat. The presence of EHP in the infected shrimps was detected by using Polymerase Chain Reaction (PCR) method with specific primers EHP-510F/EHP-510R and histopathological analysis. The Nauplius, Zoae 1 and Zoae 2 stages collected from the infected female broodstocks demonstrated that EHP can infect offspring from their parental shrimp, and interestingly EHP can be detected from Nauplius stage of shrimp by the use of PCR method.
Aquaculture, Diagnosis, Enterocytozoon hepatopenaei (EHP), PCR (Polymerase Chain Reaction), Pathogenesis, Shrimp
Microsporidia are ubiquitous obligate intracellular parasites responsible for a variety of diseases and economic losses due to their adverse effects1. With more than 1400 species identified worldwide, they have a broad impact on human and animal health, food security and other industries1,2. This is because that microsporidia have different transmission strategies including horizontal transmission (oral transmission of spores through contaminated food and water)2, and vertical transmission (from parents to oûspring)3,4. They can infect a wide variety of animals ranging from invertebrate to vertebrate hosts, including humans and arthropods (insects, crustaceans) of economic importance5.
In the shrimp industry, which is currently facing an increasingly complex and damaging epidemic due to the continuous development of farming areas and forms of farming to increase productivity6, microsporidia are serious pathogens associated with white faecal syndrome (WFS) and slow growth in shrimp in many of the shrimp growing countries in the world7,8. Microsporidia were found for the first time in tiger shrimp in Thailand in 19899. In 2001, it was reported the incidence of microsporidian infection associated with mortalities in Penaeus japonicus in Australia10. In 2004, an undesignated microsporidium was considered to associate with monodon slow growth syndrome (MSGS) in Thailand11. In 2009, Tourtip and co-authors identified for the first time Enterocytozoon hepatopenaei (EHP) as a new microsporidian using PCR based on the 18ssu rRNA of EHP12. More recently EHP has reported popularly in many shrimp growing countries of Asia such as Vietnam13, Thailand7, and India14. These studies have found that EHP enters shrimp hepatopancreas as the target organ and affects the host digestive and absorptive functioning resulting in poor growth and immunity.
There is no drug for the control of EHP infection in shrimp. Thus, a comprehensive understanding on the pathogenesis via different transmission strategies and an early diagnosis of EHP in shrimp are required for improved management practices and proper biosecurity in order to keep this parasite away from the aquaculture ecosystem. In this study, we show the first clues of the vertical transmission of Enterocytozoon hepatopenaei (EHP) in whiteleg shrimp Penaeus vannamei, thus it contributes to a comprehensive understanding on the pathogenesis of EHP as a serious pathogen related to slow growth in shrimp causing a heavy loss in the global shrimp industry. This is also probably the first evidence of the interaction between microsporidia and decapod crustaceans via the vertical transmission strategy, indicating the ecological impact of these parasites in aquatic ecosystems. Finally, a sensitive method of Polymerase Chain Reaction for the early detection of EHP from Nauplius stage is developed.
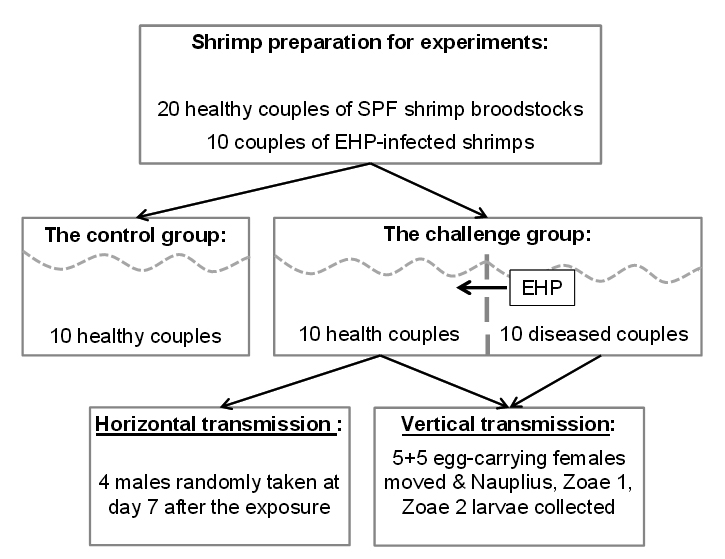
A summary of animal challenge experiment is presented in Fig. 3. The details are described below.
Experimental shrimp
Total 20 broodstock couples of healthy (SPF, specific pathogen-free) whiteleg shrimp P. vannamei (40 g per individual) were imported from Shrimp Improvement Systems Pte Ltd, Singapore and cultured as the guidance of the manufacturer. The shrimps were acclimatized in 2 weeks before experiments. Experimental shrimps were marked by elastomer/fin clipping method15.
EHP-infected shrimps were collected from shrimp ponds in Ninh Thuan province, Viet Nam and transported alive to the laboratory for further analysis.
Animal challenge experiment: Horizontal and vertical transmission trials
The animal challenge experiments were designed using cohabitation of healthy and diseased shrimps and feeding with EHP-infected shrimp hepatopancreatic tissue. In the challenge group, 10 healthy broodstock couples and 10 diseased shrimp couples were shared the same aquarium but separated by a plastic net to prevent physical contact. The control group included 10 healthy couples only.
The shrimp groups were cultured in cement tanks of 6 m3 which were supplied with filtered and chlorine-treated seawater. Environmental parameters were kept stably at salinity at 33 – 34 ppt, temperature at 28 – 29oC, pH 7.5 – 8.0 and continuously aerated during the experimental period of 30 days. Seawater was replaced 50% and waste was removed daily.
The control group was fed once a day with live feed (worms, oysters, squids at 25% body weight per day) until the end of the 30-day experiment. The challenge group was fed both live feed (at 15% BW) and EHP-infected shrimp tissue (at 10% BW).
In order to sample for horizontal transmission trial, samples of 4 males from the challenge group were randomly taken at day 7 after the exposure to detect the presence of EHP using histopathological analysis and PCR method. All remaining broodstock shrimps were harvested at the end of the experiment for EHP detection using PCR.
In order to sample for vertical transmission trial, the egg-carrying female broodstocks were moved to spawning tank (1 m3) containing clean seawater. Larvae of each female were collected and nursed separately for monitoring the transmission of EHP from mother to larvae. Larvae stages of Nauplius, Zoae 1 and Zoae 2 were collected every two days (50 larvae each time) to detect the presence of EHP using PCR. Their mothers were also collected to perform the same method.
For histopathological analysis, hepatopancreas tissues were immersion-fixed in Davidson’s solution and stained by standard Hematoxylin and Eosin staining method as described previously16. Histopathological images were then observed by microscopy BX41 (Olympus, Japan) with 100x magnification.
DNA extraction and PCR amplification
Before DNA extraction, hepatopancreatic tissue was homogenized in Lysis buffer (50 mM Tris–HCl, 100 mM EDTA, 50 mM NaCl, 2% SDS and 10 ìg ml-1 proteinase K). DNA extraction was performed using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
The PCRs were performed as described by Nguyen and coworkers16 with some modifications as follows. The primers for EHP-specific detection are EHP-510F (5’-GCC TGA GAG ATG GCT CCC ACG T-3’) and EHP-510R (5’-GCG TAC TAT CCC CAG AGC CCG A-3’)16. The thermal cycling conditions include an initial denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 90 s, and a ûnal extension at 72°C for 5 min.
Preliminary PCR screening to detect EHP prior to animal challenge experiment
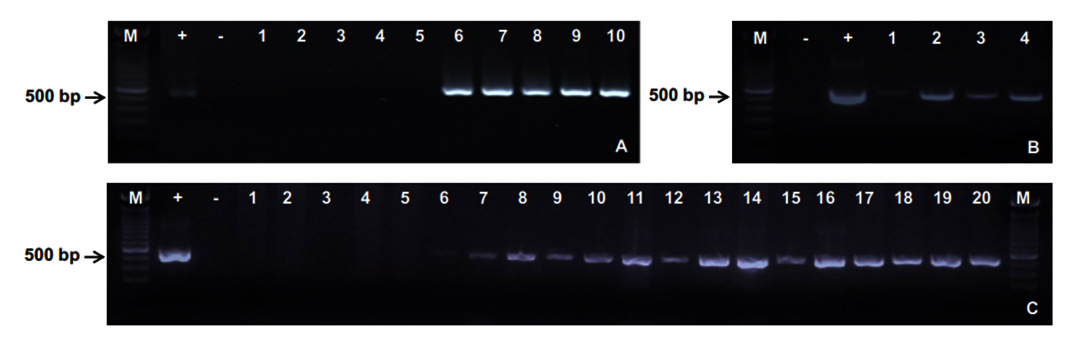
Before the animal experiment was carried out, hepatopancreas tissues were randomly taken from healthy shrimps to detect the presence of EHP by using PCR method. The amplification indicated negative results for EHP. In the same experiment condition, all of diseased shrimps collected from EHP-infected ponds in Ninh Thuan province, Vietnam, expressed positive results as demonstrated by 500 bp-sized PCR products appropriate to the used primer pair EHP-510F/ EHP-510R (Fig. 1A).
Four male shrimps after 7 days of exposure in horizontal transmission trial. Lanes 1 – 4: male shrimp broodstocks.
The offspring of the control and infected broodstocks in vertical transmission trial. Lanes 1 – 5: Zoae 2 (Z2) larvae from the control female broodstocks 1-5; Lanes 6 – 8: Nauplius (N), Zoae 1 (Z1) and Zoae 2 (Z2) larvae from the infected female broodstock 1; Lanes 9 – 11: N, Z1 and Z2 larvae from the infected female broodstock 2; Lanes 12 – 14: N, Z1 and Z2 larvae from the infected female broodstock 3; Lanes 15 – 17: N, Z1 and Z2 larvae from the infected female broodstock 4; Lanes 18 – 20: N, Z1 and Z2 larvae from the infected female broodstock 5.
(M: 100 bp Marker; +: Positive control; -: Negative control)
Horizontal transmission of EHP via cohabitation and feeding with EHP-infected shrimp tissue
Experimental shrimps showed gross signs after 7 days of co-habitation and feeding with EHP-infected tissues. Shrimps were less swimming, not sensitive to stresses, reduced or stopped eating compared to the control. However, no mortality was observed within the first 7 days. The PCR products of 4 samples in the challenge group exhibited bands with an expected size of 500 bp (Fig. 1B). This demonstrated that the healthy shrimps were susceptible and infected EHP from diseased shrimps via living environment and feed. At the end of experiment, all remaining shrimps in both control and challenge group were sampled to extract DNA and detect EHP by PCR. The control group showed 100% negative for EHP whereas 15/16 (93.7%) positive samples were found in the challenge group. Clearly, this study has confirmed a very high rate of horizontal transmission of EHP in whiteleg shrimp via sharing habitat and feed.
In this study, healthy whiteleg shrimps were infected EHP by feeding infected tissues and cohabitation with diseased shrimp. After 7 days of exposure, the shrimps were found positive with EHP as demonstrated by PCR. Similar results were also shown in the experiment of Tang and coworkers8 when they researched the horizontal transmission pathways of EHP by feeding shrimp with diseased tissues and cohabitation. In that case, healthy shrimps were infected after 14 days of feeding diseased shrimps and after 35 days of cohabitation together with diseased shrimps. In 2017, Salachan and co-authors described a successful transmission in a cohabitation model with natural EHP-infected shrimp in closed, perforated plastic containers placed in aquaria together with free-swimming, uninfected shrimp. After a period of 14 days all the free-swimming shrimps tested were positive to EHP in the hepatopancreas using PCR, histological and in situ hybridization analyses17. Comparing our results with their studies, our study showed the transmission time of EHP to healthy shrimp was one week earlier than in the feeding method and 4 weeks earlier than in the cohabitation procedure.
It could be explained that the combined infection method of feeding with infected tissue and cohabitation would increase the number of microsporidia exposed to shrimp and led to accelerate the process of infection. Normal shrimp could be infected to EHP by eating EHP-diseased shrimp and pathogen-infected organisms in ponds such as earthworms, crabs and crabs feces. On the other hand, EHP could also spread parasites on shrimp shells. For example, when shrimp molted, spores would stick to the soft shell, enter the liver and pancreas and then lead to be pathogenic to shrimp18. Molting shrimps have soft shells; therefore they are the most susceptible to EHP infection. After invading, the microsporidia multiplied to increase the number and released toxins causing necrosis. Furthermore, histological examination of EHP-infected P. vannamei collected from Vietnam showed basophilic inclusions within the cytoplasm of hepatopancreas tubule epithelial cells. These inclusions appeared to be at a plasmodium stage; mature, basophilic spores were also observed18.
Vertical transmission of EHP from parental shrimps to their offspring
Nauplius (N), Zoae 1 (Z1), and Zoae 2 (Z2) larvae of control and infected-female broodstocks (5 pairs per treatment) were collected to detect EHP using PCR with specific primers EHP-510F/ EHP-510R. The results showed that larvae of the infected group at all 3 stages expressed 500 bp-sized bands while those in the control group (Z2) were negative for EHP (Fig. 1C). Moreover, it has been confirmed that this PCR procedure was sensitive enough for the detection of EHP at Nauplius stage.
Vertical transmission of EHP from parental shrimps to their offspring was also confirmed by histopathological analysis. Abnormal cells (clustered nucleus) and many inclusions were observed within the cytoplasm of hepatopancreas tubule epithelial cells in tissue sections of shrimp exhibiting gross signs of the disease (Fig. 2). As expected, no EHP pathological characteristics were observed in the control shrimps.
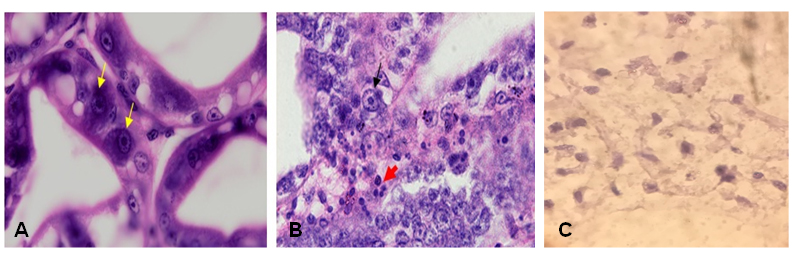 A: Hepatopancreas tubules with normal cells in control shrimp; B: Hepatopancreas cells with densed and clustered nucleus in in-fected shrimp (×40); C: Microsporidium spores in infected hepatopancreas (×100)
A: Hepatopancreas tubules with normal cells in control shrimp; B: Hepatopancreas cells with densed and clustered nucleus in in-fected shrimp (×40); C: Microsporidium spores in infected hepatopancreas (×100)
Fig 2. Manifestation of EHP infection within tissues and organs of whiteleg shrimp stained with hematoxylin and eosin
Although there are no specific signs and symptoms in shrimp due to EHP infection, it can be detected microscopically and by polymerase chain reaction (PCR) technique. Molecular assays like nested PCR7, LAMP assay19 and Real time PCR assay20 has been developed for detecting EHP. So far, shrimps Penaeus monodon, P. japonicas, and P. meruuiensis are also reported to be susceptible to EHP infection8. The present research has successfully used the PCR method for the detection of EHP in shrimp P. vannamei, and especially our procedure is highly sensitive to analyse the presence of this parasite from the Nauplius larvae stage.
Interestingly, this study is the first evidence of vertical transmission of a microsporidium in decapods (Order Decapoda) within the same Class Malacostraca, Subphylum Crustacea and Phylum Arthropoda with Order Amphipoda. Among arthropods, gammarids (Crustacea: Amphipoda) are a group in which vertically transmitted microsporidia infections are widespread4. A survey estimated that twothirds of gammarid species are infected by microsporidia4, most of them exhibiting vertical transmission21.
In addition, the infection of microsporidia in decapod crustaceans is associated with host sex ratio distortion, as revealed by correlative studies3,4,21. Most microsporidia are pathogens, inducing changes in their hosts at the level of reproduction, growth or behaviour, and some of these parasites must kill their host to fulûl their life-cycle22. The microsporidium EHP used in this study has been considered as a serious pathogen, which was reported to associate with retarded growth in whiteleg shrimp7,8.
In a previous study, Tang and coworkers18 reported that after stocking infected postlarvae into the shrimp ponds, shrimp grew at a normal rate during the first 25 days, and then shrimp health started to deteriorate; the infected shrimp exhibited a reduction in feed consumption (50–70%) and discolored hepatopancreas. We have also investigated the prevalence of EHP in P. vannamei in Vietnam and found that not only growing shrimp but also breeding shrimp were positive with this parasite by using PCR (our unpublished data). This has indicated that infected breeding shrimp and infected growing shrimp are sources of transmission to the healthy shrimp in the farm. The results of our present study showed that the Nauplius (N), Zoae 1 (Z1), and Zoae 2 (Z2) larvae of infected parental shrimp were positive with EHP by PCR. Most importantly, EHP was detected clearly in the Nauplius stage, when the larvae have not much time to contact external pathogens, thus Nauplius could only get infected by the transmission from parents rather than from environment.
In the challenge group, 10 healthy shrimp couples were cohabitated with 10 EHP-infected couples but separated by a plastic net to prevent physical contact.
Fig. 3. Diagram of animal challenge experiment
The vertical transmission strategy of microsporidia has been reported in a broad range of hosts from protists to mammals with a focus on several major groups of invertebrates (insects, crustaceans) and vertebrates (fish)23. Notably, almost half of the known genera of Microsporidia infect aquatic animals, which can be related to their important role for parasite persistence and dispersal in aquatic habitats. For example, during drought, Hamiltosporidium tvaerminnensis utilizes vertical transmission to survive diapause in Daphnia magna23. For aquatic crustaceans, microsporidia expressed their vertical transmission strategy mainly found on gammarids (Order Amphipoda) and is also associated with host sex ratio distortion4,21-23. This could be explained by the fact that microsporidia invaded the gonads (the mother’s ovaries), clung and persisted in the eggs, causing disease to the hatched larvae. Therefore, the vertical transmission of EHP occurred.
Horizontal and vertical transmission strategies are considered to influence the virulence of microsporidia. While horizontal transmission relies on sufûcient parasite numbers released into environment causing high parasite replication and virulence24, vertical transmission requires host reproduction to occur leading to a lower virulence24-26. In addition, the second strategy usually only occurs via female hosts25 related to reproductive manipulations and thus induce sex ratio distortion via male killing or feminisation26,27. In the present study, EHP from infected-female broodstocks was demonstrated to transfer to all 3 stages of offspring including Nauplius (N), Zoae 1 (Z1), and Zoae 2 (Z2) larvae. However, further evidences of its eûects on male killing or feminisation in naturally and artiûcially infected shrimps during and after vertical transmission should be confirmed and discussed.
ACKNOWLEDGMENTS
This work was funded by a research grant from Vietnamese Government via Ministry of Agriculture and Rural Development to HVK. Authors would like to thank Dr. Vy Hich Tran at Nha Trang University for the histological slide preparation and Dr. Khuong Van Dinh at Nha Trang University and Technical University of Denmark for his valuable comments to the manuscript. We also acknowledge Professor Simeon Keates at Faculty of Engineering and Science, University of Greenwich, UK for the research administration support to VDN.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Weiss, L.M. and Becnel, J.J. Microsporidia: Pathogens of Opportunity: First Edition. Wiley Blackwell, 2014. DOI: 10.1002/9781118395264.
- Stentiford, G., Becnel, J., Weiss, L., Keeling, P., Didier, E., et al. Microsporidia–Emergent Pathogens in the Global Food Chain. Trends Parasit., 2016; 32: 336-348.
- Dunn, A.M., Terry, R.S. and Smith, J.E. Transovarial transmission in the microsporidia. Adv. Parasitol., 2001; 48: 57–100.
- Terry, R.S., Smith, J.E., Sharpe, R.G., Rigaud, T., Littlewood, D.T.J., et al. Widespread vertical transmission and associated host sex–ratio distortion within the eukaryotic phylum Microspora. Proc. Royal. Soc. Lond. B: Biolog. Sci., 2004; 271: 1783–1789.
- Han, B., Polonais, V., Sugi, T., Yakubu, R., Takvorian, P.M., et al. The role of microsporidian polar tube protein 4 (PTP4) in host cell infection. PLoS Pathogens, 2017; 13: e1006341.
- Nguyen, V.D., Le, M.H. and Trang, S.T. Application of probiotics from marine microbes for sustainable marine aquaculture development. In: Marine microbiology: bioactive compounds and biotechnological applications (Kim SK, edi). Weinheim: Wiley VCH, 2013; pp 307-349.
- Tangprasittipap, A., Srisala, J., Chouwdee, S., Somboon, M., Chuchird, N., et al. The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus (Litopenaeus) vannamei. BMC Vet. Res., 2013; 9: 139.
- Tang, K.F.J., Han, J.E., Aranguren, L.F., White-Noble, B., Schmidt, M.M., et al. Dense populations of the microsporidian Enterocytozoon hepatopenaein (EHP) in feces of Penaeus vannamei exhibiting white feces syndrome and pathways of their transmission to healthy shrimp. J. Invertebr. Pathol., 2016; 140: 1–7.
- Anderson, I.G., Shariff, M. and Nash, G. A hepatopancreatic microsporidian in pond-reared tiger shrimp, Penaeus monodon, from Malaysia. J. Invertebr. Pathol., 1989; 53: 278– 280.
- Hudson, D.A., Hudson, N.B. and Pyecroft, S.B. Mortalities of Penaeus japonicus prawns associated with microsporidian infection. Aust. Vet. J., 2001; 79: 504–505.
- Chayaburakul, K., Nash, G., Pratanpipat, P., Sriurairatana, S. and Withyachumnarnkul, B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis. Aquat. Organ., 2004; 60: 89-96.
- Tourtip, S., Wongtripop, S., Stentiford, G.D., Bateman, K.S., Sriurairatana, S., et al. Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine structure and phylogenetic relationships. J. Invertebr. Pathol., 2009; 102: 21–29.
- Ha, N.T., Ha, D.T., Thuy, N.T. and Lien, V.T.K. Enterocytozoon hepatopenaei parasitizing on tiger shrimp (Penaeus monodon) infected by white feces culture in Vietnam has been detected. Agriculture and rural development: science and technology (translation from Vietnamese), 2010; 12: 45–50. (in Vietnamese with English abstract).
- Rajendran, K.V., Shivam, S., Praveena, P.E., Rajan, J.J.S, Kumar, T.S., et al. Emergence of Enterocytozoon hepatopenaei (EHP) in farmed Penaeus (Litopenaeus) vannamei in India. Aquaculture, 2016; 454: 272–280
- Guy, C.S., Blankenship, H.L. and Nielsen, L.A. Tagging and marking. In: Fisheries Techniques (Murphy BR and Willis DW, edi). Bethesda: American Fisheries Society, 1996; pp 353–379.
- Nguyen, V.D., Pham. T.T., Nguyen, T.H.T., Nguyen, T.T.X. and Lone, H. Screening of marine bacteria with bacteriocin-like activities and probiotic potential for ornate spiny lobster (Panulirus ornatus) juvenile. Fish Shellfish Immunol., 2014; 40: 49-60.
- Salachan, P.V., Jaroenlak, P., Thitamadee, S., Itsathitphaisarn, O. and Sritunyalucksana, K. Laboratory cohabitation challenge model for shrimp hepatopancreatic microsporidiosis (HPM) caused by Enterocytozoon hepatopenaei (EHP). BMC Vet. Res., 2017; 13: 9.
- Tang, K.F., Pantoja, C.R., Redman, R.M., Han, J.E., Tran, L.H. and Lightner, D.V. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei (EHP), a microsporidian parasite infecting penaeid shrimp. J. Invertebr. Pathol., 2015; 130: 37-41.
- Suebsing, R., Prombun, P., Srisala, J. and Kiatpathomchai, W. Loopmediated isothermal amplification combined with colorimetric nanogold for detection of the microsporidian Enterocytozoon hepatopenaei in penaeid shrimp. J. Appl. Microbiol., 2013; 114: 1254-1263.
- Liu, Z., Zhang, Q.L., Wan, X.Y. and Huang, J. Development of real-time PCR assay for detection of microsporidian Enterocytozoon hepatopenaei and detection in shrimp samples under different growth rates. Prog. Fish. Sci., 2015; 37: 119-126. (in Chinese with English abstract).
- Haine, E.R., Boucansaud, K. and Rigaud, T. Conflict between parasites with different transmission strategies infecting an amphipod host. Proc. R. Soc. B, 2005; 272: 2505–2510.
- Haine, E.R. Symbiont-mediated protection. Proc. R. Soc. B, 2008; 275: 353-61.
- Stentiford, G.D., Feist, S.W., Stone, D.M., Bateman, K.S. and Dunn, A.M. Microsporidia: diverse, dynamic, and emergent pathogens in aquatic systems. Trends Parasitol., 2013; 29: 567–578.
- Ebert, D. and Herre, E.A. The evolution of parasitic disease. Parasitol. Today, 1996; 12: 96–101.
- Bandi, C., Dunn, A.M., Hurst, G.D. and Rigaud, T. Inherited microorganisms, sex-speciûc virulence and reproductive parasitism. Trends Parasitol., 2001; 17: 88–94.
- Dunn, A.M. and Smith, J.E. Microsporidian life cycles and diversity: the relationship between virulence and transmission. Microbes Infect., 2001; 3: 381–388.
- Hurst, L.D. The incidences and evolution of cytoplasmic male killers. Proc. R. Soc. Lond. B: Biol. Sci., 1991; 244: 91–99.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.