ISSN: 0973-7510
E-ISSN: 2581-690X
Thailand is one of the major agricultural production countries in the world. Therefore, large amounts of agricultural waste are generated as by-products of the agroindustry. The wastes are usually discarded or burnt, resulting in environmental pollution. The main goal of this research was to evaluate the use of agricultural waste for mushroom cultivation. A total of 15 mushroom isolates were recovered from mother spawns and basidiocarps. They were screened for their cellulolytic enzyme activity on Czapek agar using carboxymethyl cellulose (CMC) as the sole carbon source. Two isolates of the oyster mushrooms, Pleurotus pulmonarius PP6 and Pleurotus ostreatus PO3, produced the best enzyme activities. To observe the mycelial growth on agricultural waste, the two oyster mushroom candidates were cultured in jars containing five different types of agricultural waste: corn husk, rice straw, coconut meal, coconut husk and sugarcane bagasse, and the jars were incubated at 25°C for six weeks. The results show that both isolates grew best on coconut meal, producing very densely packed mycelia. Meanwhile, corn husk and rice straw were also good sources for oyster mushroom cultivation. This study shows that these three substrates have the potential to be utilized in mushroom cultivation on a commercial scale.
Agricultural Waste, Coconut, Corn, Oyster Mushroom, Sugarcane
Edible mushrooms have been cultivated and used as a source of food for centuries because they can be grown on a wide range of substrates and are highly nutritious. Pleurotus, the members of which are commonly known as oyster mushrooms, is a genus of gilled mushrooms that includes numerous widely consumed mushrooms. Indeed, the genus includes some of the most common and often cultivated edible mushrooms.1 Pleurotus mushrooms are white rot fungi so they can produce various lignocellulolytic enzymes. Therefore, by-products from agricultural crops containing cellulose, hemicellulose, and lignin can be commonly used as substrates.2
In Thailand, agriculture plays an important role and forms the backbone of the Thai economy. A wide range of crops are grown, and they are consumed domestically or exported internationally. Each year, huge amounts of fruits and vegetables are harvested, and subsequently large quantities of agricultural waste are generated.3 After the crops are harvested, the waste is usually burnt causing soil depletion and critical air pollution. There is an urgent need to solve this problem.4,5 Previous researchers have given various instances of the use of agricultural waste as substrate for mushroom cultivation.6
Sawdust, a by-product from logging and timber mills, is widely used for mushroom cultivation because it is suitable as a growth medium for mushrooms and is reasonably cheap and cost effective. However, the use of sawdust has become difficult due to increasing concerns about deforestation.7 Such concerns have driven demand for sawdust, which has in turn led to increasing production costs for mushroom growers. Therefore, there is the challenge to find other sustainable sources of substrates. In this study, five types of agricultural waste that are abundant in Thailand, namely corn husk, rice straw, coconut meal, coconut husk and sugarcane bagasse, were evaluated for their utility in the cultivation of oyster mushrooms. The main objectives of this study were (1) to isolate the oyster mushrooms and screen for their ability to produce cellulolytic enzymes, and (2) to evaluate agricultural waste for mushroom cultivation.
Collection and isolation of Oyster mushrooms
Two types of oyster mushrooms, mother spawns and basidiocarps, were collected in this study (Figure 1). The samples were collected from commercial farms and local markets in the central parts of Thailand. The details of isolation codes, locations and numbers of samples are shown in Table 1. Isolation of a pure culture from a mother spawn was done by transferring a seed with fungal mycelium onto potato dextrose agar (PDA). For the fresh basidiocarp, a specimen was dissected using a flame sterilized blade, and pieces of mycelium (1×1 cm2) were cut from inside the cap and stalk of the basidiocarp. They were transferred onto PDA. The PDA plates were incubated at 25°C for seven days. After the pure cultures of oyster mushrooms were recovered, they were maintained on PDA slants and kept at 4°C.
Figure 1. Samples of oyster mushrooms collected from commercial farms in Thailand. (A) A mother spawn growing on sorghum seeds, and (B) Basidiocarps growing out from a substrate
Study of cellulolytic enzymes
Fifteen isolates of Pleurotus mushrooms were cultured on PDA for 7 days at 25°C. The screening method was modified from a study by Sazci et al.8 Agar discs were cut from the PDA using a Cork borer no. 1 (4 mm in diameter) and transferred to the center of CMC agar plates (Czapek agar supplemented with carboxymethyl cellulose as a carbon source). The plates of CMC agar were incubated at 25°C for 11 days. The negative control was CMC agar without inoculation. Afterwards, the plates were dyed by flooding with 20 ml of 1% Congo red solution (1 mg/ml) for 15 min. Then the plates were decolorized with 0.85% normal saline solution. The diameters of the mycelium circles on each plate were measured. There were five replicates (five plates) for each treatment. The data is presented as mean value ± standard error.
Cultivation of mushrooms in jars
Five types of agricultural waste were investigated for use as carbon sources for the cultivation of oyster mushrooms (Figure 2). They were collected from local farms and markets free of charge because they were seen as by-products and waste. After they had been allowed to dry in the sun during the day for 12 hours, they were chopped and ground into fiber and fine powder. Twenty grams of each substrate was added into 500 ml jars and 1.2 g of potato dextrose broth medium and 50 ml of distilled water were added into every jar. The jars were autoclaved at 121°C for 1 hour. After cooling down to room temperature, the oyster mushroom cultures were inoculated into the jars. This experiment was done in three replicates (three jars) for each substrate. The mushroom cultures were incubated at 25°C for six weeks. They were observed weekly, and the photographs of the jars were taken at the final week of incubation.
Isolation of mushrooms
Five species of oyster mushrooms were collected, and they were P. pulmonarius, P. ostreatus, P. cornucopiae, P. djamor and P. cystidiosus. They were collected from four provinces in central parts of Thailand. There were six isolates each for P. pulmonarius and P. ostreatus, while there was one isolate for each of the other three species. Isolation codes for each isolate are shown in Table 1. The pure cultures of oyster mushrooms were isolated from five and 10 samples of mother spawns and basidiocarps, respectively.
Table (1):
Pleurotus mushrooms collected in this study, their isolation code, locations, and types of samples
Mushroom |
Code |
Province |
Farms’ Name |
Type |
|---|---|---|---|---|
P. pulmonarius |
PP1 |
Nonthaburi |
Katom Hed |
Mother spawn |
P. pulmonarius |
PP2 |
Nonthaburi |
Pothong |
Mother spawn |
P. pulmonarius |
PP3 |
Bangkok |
Baan Hed |
Basidiocarp |
P. pulmonarius |
PP4 |
Kanchanaburi |
Aunty Chin |
Mother spawn |
P. pulmonarius |
PP5 |
Nakornprathom |
Kasetsart University |
Basidiocarp |
P. pulmonarius |
PP6 |
Kanchanaburi |
Takratum |
Basidiocarp |
P. ostreatus |
PO1 |
Nonthaburi |
Pothong |
Mother spawn |
P. ostreatus |
PO2 |
Kanchanaburi |
Aunty Chin |
Mother spawn |
P. ostreatus |
PO3 |
Bangkok |
Krisana |
Basidiocarp |
P. ostreatus |
PO4 |
Kanchanaburi |
Aunty Chin |
Mother spawn |
P. ostreatus |
PO5 |
Kanchanaburi |
Aunty Chin |
Mother spawn |
P. ostreatus |
PO6 |
Nakornprathom |
Kasetsart University |
Basidiocarp |
P. cornucopiae |
PCN1 |
Nonthaburi |
Pothong |
Mother spawn |
P. djamor |
PD1 |
Nonthaburi |
Pothong |
Mother spawn |
P. cystidiosus |
PC1 |
Kanchanaburi |
Aunty Chin |
Mother spawn |
Net (Isolates) |
15 |
Screening of cellulase enzyme
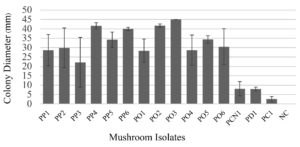
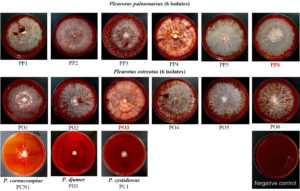
These 15 mushroom isolates were studied for their cellulolytic enzyme production activity. Most of them were able to utilize cellulose on CMC agar (Figures 3 and 4). They were categorized into three groups: (1) Isolates with low or without activity (2) Isolates with moderate activity, and (3) Isolates with high activity.
Figure 3. Colony diameters of Pleurotus mushrooms on CMC agar incubated at 25°C for 11 days. The colony diameters are expressed as means with standard deviation (mean±SD). Note: NC stands for negative control
Group (1) There were three isolates (PCN1, PD1 and PC1), of which one isolate (PD1) did not grow on CMC agar, showing that it was unable to utilize cellulose as a substrate. The other two isolates (PCN1 and PC1) produced small colonies that ranged from 2.58 – 8.10 mm in diameter.
Group (2) There were eight isolates (PP1, PP2, PP3, PP5, PO1, PO4, PO5, PO6) that grew well on CMC agar. They produced colonies ranging from 22.17-34.40 mm in diameter. However, they produced loosely flat mycelia on the medium.
Group (3) There were four isolates (PP4, PP6, PO2 and PO3) which grew very well on CMC agar. They produced large colonies of >40.00 mm in diameter. Two isolates, P. pulmonarius PP6 and P. ostreatus PO3, were selected for further study because they produced clearly visible white circles with dense mycelia as shown in Figure 4.
Figure 4. A screening of cellulase activity by Pleurotus mushrooms on CMC agar incubated at 25°C for 11 days
Growth of Pleurotus mushrooms on agricultural waste
Fungal mycelial development was categorized into 4 levels (Table 2). These were: (–) = no mycelial growth; (+) = mycelial growth on the surface; (++) = mycelial growth on the surface and inside substrate; (+++) = mycelial growth on the surface and inside substrate with a loose mass of mycelia; and (++++) = mycelial growth on and inside substrate with a dense mass of mycelia. Figures 5-7 show mycelial development in the jar experiment. The top, middle and bottom rows show the side view, top view, and close-up top view of the jars, respectively, for each substrate.
Table (2):
The development of fungal mycelia of Pleurotus mushrooms on five types of agricultural waste
| Treatment | Substrates | ||||
|---|---|---|---|---|---|
| Corn husk | Rice straw | Coconut meal | Coconut husk | Sugarcane bagasse | |
| Control | – | – | – | – | – |
| P. palmonarius PP6 | ++ | +++ | ++++ | +++ | + |
| P. ostreatus PO3 | +++ | +++ | ++++ | +++ | +++ |
Figure 5. Control treatments of five types of agricultural waste. The jars containing these substrates were incubated at 25°C for 6 weeks
Figure 6. Mycelial development of P. pulmonarius PP6 on five types of agricultural waste. The jars containing these substrates were incubated at 25°C for 6 weeks
Figure 7. Mycelial development of P. ostreatus PO3 on five types of agricultural waste. The jars containing these substrates were incubated at 25°C for 6 weeks
In the control treatments, in which no mushroom inoculation took place, there was no physical or appearance changes of substrates. All of them were still intact after they were incubated at 25°C for 6 weeks (Figure 5). They were used to compare with those that were inoculated with the two mushrooms to assess the mycelial development on the surface and in the interior layers of the substrates.
Pleurotus pulmonarius PP6
Isolate PP6 grew best on coconut meal, producing white mycelia both on the surface of and inside the substrate. When it was cultivated on rice straw, it produced dense white mycelia on the surface of the substrate as well as on the walls of the jar, and it produced a moderate amount of mycelia inside the rice straw. Growing on corn husk and coconut husk, Isolate PP6 grew moderately on the surface of these two substrates. However, it hardly grew on the surface of sugarcane bagasse, and only a tiny amount of mycelia was observed on the substrate.
Pleurotus ostreatus PO3
Isolate PO3 also grew rapidly on coconut meal, producing white and densely compact and sticky mycelia on and inside this substrate. The mycelia also grew and reached the top of the jars. Similarly, it followed the same pattern by growing well on the surface of rice straw and produced dense mycelia on the walls of the jars. When this mushroom was cultivated on the other three substrates (corn husk, coconut husk and sugarcane bagasse), it also produced dense mycelia on each substrate as well as on the walls of the jars.
The screening of cellulase-producing microbes using CMC as a carbon source and Congo red as dye indicator is widely used because the results generated by this plate method are clearly observable and easily interpreted.9 The technique is frequently used as an initial screening method for detection of cellulases due to it being cost-efficient, simple, and convenient.10 In this study, cellulases were chosen as key enzymes for the primary screening because they were essential enzymes for breaking down cellulose (a polysaccharide consisting of a linear chain of thousands of glucose units) into monosaccharides and oligosaccharides. This breakdown is considered as a crucial step from an industrial and economic perspective because it makes a major constituent of agricultural waste available for further application. Previous studies by Velazquea-Cedeno et al.11 and da Luz et al.12 demonstrated that Pleurotus ostreatus and Pleurotus pulmonarius were good sources of numerous enzymes including cellulases. In this study, our results also indicated their high biotechnological applicability.
Production of fungal mycelial biomass is one the most important criteria for mushroom cultivation because it can be used as an indicator of how rapidly mushrooms can penetrate and colonize substrates. Therefore, the oyster mushrooms were cultivated in jars to observe both aerial and vegetative mycelia. Girmay et al.13 reported that when P. ostreatus was cultivated on four substrates (cotton seed, paper waste, wheat straw and sawdust), it took from 14-19 days to colonize the substrates completely. If oyster mushrooms can colonize into cultivation materials within a short period of time, this is likely to indicate a shorter spawn running step. This means the fruiting bodies of the mushrooms can be formed and harvested sooner. It also shortens the production cycle and enables more profit for farmers.
Most of the research on mushroom production has been focused on coconut coir (sometimes called coconut fiber or coconut husk) because it is cheap and abundant.14 In this present study, coconut meal, which is a by-product generated from coconut milk production, was focused on. We wanted to expand upon the body of knowledge to do with waste from coconut production. In our study, among the five kinds of substrates, coconut meal was considered the best candidate substrate for mycelium production of Pleurotus mushrooms. Carbon: Nitrogen ratio (C/N ratio) plays an important role for mushroom cultivation. The C/N ratio for coconut meal is 25.51.15 While the C/N ratios for other 4 substrates including sugarcane bagasse, rice straw, corn husk and coconut coir are 65.88, 43.94, 15 and 10.77, respectively.15,16 The optimal C/N ratio for Pleurotus eryngii is between 20-25.17 Coconut meal also contains various soluble sugars, such as glucose, mannose, galactose and arabinose,18 dozens of amino acids, and an abundance of minerals and vitamins.19 These nutrients are a good substrate for mushrooms because they can act as precursors for fungal metabolism. This suggested that coconut meal can be used as a supplementary material to sawdust; therefore, it can enhance the mycelial growth of oyster mushrooms.
Corn husk is also an interesting alternative for mushroom cultivation. Adjapong et al.7 evaluated three parts of corn residue (husk, cob and stalk) as growth media for the P. ostreatus. When the three of them were compared with sawdust, a common substrate used for commercial mushroom production, corn husk gave higher yield of weight of fruiting bodies per crop. Similarly, Mohd Rashid et al.20, who studied white oyster mushrooms (Pleurotus florida), also reported that corn husk could be used as a substitute for sawdust because when P. florida was cultivated with two substrates, corn husk gave the higher yield of all major indicators (the total number of fruiting bodies, fresh weight, and dry weight). Rice straw and coconut husk are also promising substitutes for sawdust because they are widely studied and considered as low-cost substrates for oyster mushroom cultivation.5,21,22
Various studies suggested that sugarcane bagasse is a good source for the cultivation of P. ostreastus.23,24 Their studies showed that biomass from sugarcane either used alone, or in combination with other substrates, such as rubber tree sawdust and wheat straw, can increase the yield of oyster mushrooms. In this study, P. ostreatus PO3 also grew well on sugarcane bagasse; however, P. pulmonarius PP6 hardly grew on the same substrate. Although they are members of the same genus Pleurotus, they probably differ at species level in their ability to utilize different substrates. In our study, we found that P. ostreatus grew well on sugarcane bagasse, but P. pulmonarius did not follow the same pattern. Thus, it cannot be assumed that mushrooms in the same genus can utilize the same substrate. This emphasizes the importance of evaluating substrates using different species of mushrooms.
Cultivation of oyster mushroom (P. ostreatus) by using combinations of two substrates offer a good alternative for better yield and cost effectiveness. De et al.25 showed that combining rice straw and sugarcane bagasse improved yield of oyster mushroom. The mushroom grew faster and produced more weight when it was cultivated on a combined substrate compared to when it was cultivated on rice straw alone. Likewise, Elkanah et al.26 mixed palm oil waste (shaft and bunch) with sawdust to cultivate oyster mushroom, the combined substrates gave higher yield of mushroom production. Our results show the coconut meal is the best candidate for oyster mushroom cultivation in a lab condition. Further studies on combining coconut meal with other substrates and optimal cultivation conditions are needed.
For the successful cultivation of mushrooms, three factors must be carefully considered: (1) the reliability of the mushroom strain, (2) the suitability of the substrate, and (3) the optimality of conditions. In this study, we provided useful knowledge related to the first two factors. We identified two promising isolates of oyster mushrooms, and we identified a suitable substrate, coconut meal.
Among fifteen isolates of oyster mushrooms, the two candidates (P. pulmonarius PP6 and P. ostreatus PO3) that had the potential to use lignocellulosic materials, were chosen. When they were cultivated on different substrates, both produce dense and thick mycelia on coconut meal. However, P. pulmonarius PP6 grew rapidly on most substrates except for sugarcane bagasse. Of the five substrates, coconut meal proved to be the most promising source for the cultivation of oyster mushrooms.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by the King Mongkut’s Institute of Technology Ladkrabang (KMITL) Research Fund through grant number 2567-02-05-004.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Wan Ishak WR, Wan Ahmad WAN, Bakar N. Does oyster mushroom (Pleurotus sajorcaju) powder addition improve nutrient composition, sensory acceptability, and glycaemic index (GI) of flatbread (Tortilla)? Kuwait Journal of Science. 2021;48(2).
Crossref - Kumla J, Suwannarach N, Sujarit K, et al. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules. 2020;25(12):2811.
Crossref - Fukuda S. Agricultural and municipal waste management in Thailand. 2021:303-324.
Crossref - Arunrat N, Pumijumnong N, Sereenonchai S. Air-pollutant emissions from agricultural burning in Mae Chaem Basin, Chiang Mai Province, Thailand. Atmosphere. 2018;9(4):145.
Crossref - Mihai RA, Melo Heras EJ, Florescu LI, Catana RD. The edible gray oyster fungi Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm a potent waste consumer, a biofriendly species with antioxidant activity depending on the growth substrate. J Fungi. 2022;8(3):274.
Crossref - Lestari P, Elfrida N, Suryani A, Suryadi Y. Study on the production of bacterial cellulose from Acetobacter xylinum using agro-waste. Jordan J Biol Sci. 2014;7(1):75-80.
Crossref - Adjapong AO, Ansah KD, Angfaarabung F, Sintim HO. Maize residue as a viable substrate for farm scale cultivation of oyster Mushroom (Pleurotus ostreatus). Advances in Agriculture. 2015;213251.
Crossref - Sazci A, Erenler K, Radford A. Detection of cellulolytic fungi by using Congo red as an indicator: a comparative study with the dinitrosalicyclic acid reagent method. J Appl Bacteriol. 1986;61(6):559-562.
Crossref - Fen L, Xuwei Z, Nanyi L, et al. Screening of lignocellulose-degrading superior mushroom strains and determination of their CMCase and laccase activity. Sci World J. 2014;763108.
Crossref - Jo WS, Park HN, Cho DH, Yoo YB, Park SC. Optimal media conditions for the detection of extracellular cellulase activity in Ganoderma neo-japonicum. Mycobiology. 2011;39(2):129-132.
Crossref - Velazquez-Cedeno MA, Mata G, Savoie J-M. Waste-reducing cultivation of Pleurotus ostreatus and Pleurotus pulmonarius on coffee pulp: changes in the production of some lignocellulolytic enzymes. World J Microbiol Biotechnol. 2002;18(3):201-207.
Crossref - da Luz JM, Nunes MD, Paes SA, Torres DP, de Cassia Soares da Silva M, Kasuya MC. Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz J Microbiol. 2012;43(4):1508-1515.
Crossref - Girmay Z, Gorems W, Birhanu G, Zewdie S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Express. 2016;6(1):87.
Crossref - Ali B, Hawreen A, Ben Kahla N, Talha Amir M, Azab M, Raza A. A critical review on the utilization of coir (coconut fiber) in cementitious materials. Construction and Building Materials. 2022;351:128957.
Crossref - Fauzan F, Fadhil M, Irfan I, Yunita D, Erika C, Lahmer R. Study of C/N ratio of organic materials and its application in the production of natural fertilizer (bokashi). IOP Conference Series: Earth and Environmental Science. 2022;951:012105.
Crossref - Xu N, Bhadha JH, Rabbany A, et al. Crop nutrition and yield response of bagasse application on sugarcane grown on a mineral soil. Agronomy. 2021;11(8):1526.
Crossref - Zhou Y, Li Z, Xu C, et al. Evaluation of corn stalk as a substrate to cultivate king oyster mushroom (Pleurotus eryngii). Horticulturae. 2023;9(3):319.
Crossref - Khuwijitjaru P, Watsanit K, Adachi S. Carbohydrate content and composition of product from subcritical water treatment of coconut meal. J Indust Eng Chem. 2012;18(1):225-229.
Crossref - Sundu B, Hatta U, Mozin S, et al. Coconut meal as a feed ingredient and source of prebiotic for poultry. IOP Conf Ser: Earth Environ Sci. 2020;492(1):012126.
Crossref - Rakib MRM, Lee AM, Tan SY. Corn husk as lignocellulosic agricultural waste for the cultivation of Pleurotus florida mushroom. Bioresources. 2020;15(4):7980-7991.
Crossref - Zhang R, Li X, Fadel JG. Oyster mushroom cultivation with rice and wheat straw. Bioresour Technol. 2002;82(3):277-284.
Crossref - Yang W, Guo F, Wan Z. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J Biol Sci. 2013;20(4):333-338.
Crossref - Aguilar-Rivera N, De Jesus-Merales J. Edible mushroom Pleurotus ostreatus production on cellulosic biomass of sugar cane. Sugar Tech. 2010;12(2):176-178.
Crossref - Zakil FA, Hassan KHM, Sueb MSM, Isha R. Growth and yield of Pleurotus ostreatus using sugarcane bagasse as an alternative substrate in Malaysia. IOP Conf Ser: Mater Sci Eng. 2020;736(2):022021.
Crossref - De A, Mridha D, Roychowdhury T, Bandyopadhyay B, Panja AS. Substrate level optimization for better yield of oyster mushroom (Pleurotus ostreatus) production, using different ratio of rice straw and sugarcane bagasse. World J Microbiol Biotechnol. 2023;39(10):270.
Crossref - Elkanah FA, Oke MA, Adebayo EA. Substrate composition effect on the nutritional quality of Pleurotus ostreatus (MK751847) fruiting body. Heliyon. 2022;8(11):e11841.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.