ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed to investigate the way the treatment of pulmonary tuberculosis in the province of Thi-qar. Which included the expense of the number of patients infected with the disease in thoracic diseases center in the province and the manner of dealing with and the effectiveness of the three-year therapy (2013,2014,2015). It was reached a situation improved between the years where we note a decrease in the number of casualties, which numbered 255 patients in 2013 and 174 patients in 2014 Om 167 patients in 2015. Maybe it was due to the patient’s commitment to DOTS adopted by the Iraqi Ministry of Health and the World Health Organization system. Or increasing the health awareness of the community and its attention to personal hygiene.
Treatment, Tuberculosis, Thi-Qar
TB (Tuberculosis) is a major global health problem. It causes illness among millions of people each year and ranks as the second leading cause of death from an infectious disease worldwide, next to HIV (human immunodeficiency virus) infection. According to the WHO (World Health Organization) 2012 report, almost 9 million new cases and 1.4 million TB deaths occur worldwide by the year 2011 During 1993-2003, incidence of tuberculosis (TB) in the United States decreased 44% and is now occurring at a historic low level (14,874 cases in 2003). The Advisory Council for the Elimination of Tuberculosis (ACET). Multidrug-resistant TB (MDR-TB) remains a threat to the global tuberculosis (TB) control effort1. In the United Kingdom (UK), the annual number of culture confirmed cases of MDR-TB increased from 28 to 58 between 2000 and 20092 and there were a total of eight extensively drug-resistant (XDR) cases reported (data unpublished). The prolonged treatment associated with MDR-TB and the often severe adverse effects of second-line antibiotics increases the challenges to achieve treatment completion. The rise in the number of MDR-TB cases has important implications for clinical management, social support and financing of TB control programs3. Internationally, in resource rich settings, initial empirical treatment of MDR-TB patients should be based on past drug resistance results for patients with a previous TB episode, drug resistance profiles of an identified source case, or the levels of background drug resistance in the patient’s country of origin4,5. This should be followed by individually adapted drug regimens once drug susceptibility results become available4.In 2008, the World Health Organization (WHO) recom-mended that the MDR-TB treatment regimen should ideally consist of a combination of ethambutol and pyrazinamide, an injectable agent (e.g. aminoglyco-sides), a fluoroquinolone and if necessary, a bacterio-static drug should be added to give a total of at least four drugs to which resistance has not been demon- strated. Antibiotics with unknown efficacy should only be used when better options are exhausted4. Recently published WHO guidelines recommend the inclusion of the bacteriostatics ethionomide or pro- thionamide and either cycloserine or ñ-aminosalicylic acid in the regimen5. The treatment should last at least 20 months in total5 and be supervised by directly observed therapy (DOT)4. the terms latent TB infection (LTBI), TB, TB disease, and infectious TB disease are used. LTBI is used to designate a condition in which an individual is infected with Mycobacterium tuberculosis but does not currently have active disease. Such patients are at risk for progressing to tuberculosis disease. Treatment of LTBI (previously called preventive therapy or chemoprophylaxis) is indicated for those at increased risk for progression as described in the text. Persons with LTBI are asymptomatic and have a negative chest radiograph. TB, TB disease, and infectious TB indicate that the disease caused by M. tuberculosis is clinically active; patients with TB are generally symptomatic for disease. Positive culture results for M. tuberculosis complex are an indication of TB disease. Infectious TB refers to TB disease of the lungs or larynx; persons with infectious TB have the potential to transmit M. tuberculosis to other persons[6. Because transmission of TB may not be immediately recognized, common-source outbreaks can occur. Such outbreaks represent a challenge to public health efforts to control TB. Strategies for control of outbreaks include rapid identification, isolation, and treatment of infectious TB patients, evaluation of exposed persons for subclinical or latent disease, and preventive therapy for persons at high risk for infection. Such public health strategies have been complicated by the emergence of drug resistance, and some existing strategies may need to be modified7. aims of the treatment to cure TB patients, prevent death, prevent relapse, decrease transmission to others, prevent drug resistance.
Time and Place
Current study was a cohort study, conducted in AL-Nasiriyha during the period between 2013-2015. The patients who followed in present study were attending center of respiratory disease of AL-Nassiriyha province either as Direct attendants whom seek treatment.
Definition of Cases
The following are the WHO standard definitions of cases with modification8, 9.
A tuberculosis case is defined as a patient in whom tuberculosis has been bacteriologically confirmed or diagnosed by a clinician [10].
Smear positive patient: Patient with at least two initial sputum smear examinations positive for AFB (acid fast bacilli), or one sputum smear examination positive for AFB plus radiographic abnormalities consistent with active pulmonary tuberculosis as determined by a clinician. Follow up was restricted to smear positive cases. The cases are classified according to the presence or absence of history of taking anti TB drugs:
(1) New patient: a patient who had never taken treatment for TB or who had taken anti-TB drugs for less than four weeks.
(2) Retreatment patient: A patient who had taken anti-TB drug for one month or more at any time in the past, and is one of the following:
(a) Treatment Failure: A patient who, while on treatment remained or became again smear-positive five months or later after commencing treatment.
(b) Relapse: A patient who has been declared cured of any form of TB in the past by a physician, after one full course of chemotherapy, then became sputum smear positive.
(c) Defaulter: A patient who interrupts treatment for two months or more, and returns to the health service with smear positive sputum.
Treatment Outcomes
Treatment outcomes are classified into six groups which are:
(1) Cured: Initially smear positive patient who completed treatment and had negative sputum smear results, on at least two occasions of treatment.
(2) Completed Treatment: Sputum smear positive patient who completed treatment with negative sputum smear results at the end of the initial phase, but with no or only one negative sputum smear result in the continuation phase and none at the end of treatment.
(3) Died: Patient who died during treatment, regardless of cause.
(4) Failure: Smear positive patients who remained or became again smear positive five months or later after commencing treatment.
(5) Defaulter: Patient who, at any one time after registration, had not taken drugs for two months or more.
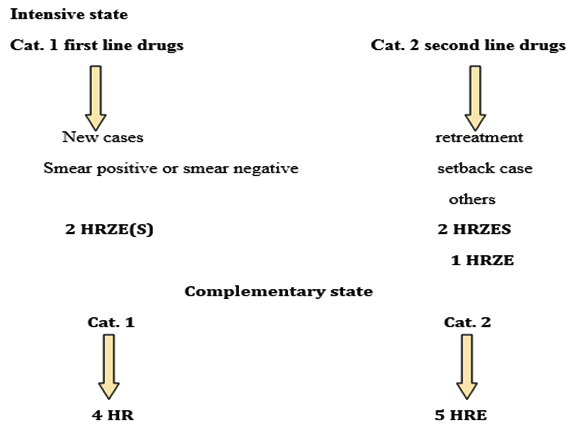
Scheme: Course of TB treatment in AL-Nasiriya
Table (1):
Anti-TB drugs currently used in AL-Nassiriyha.
| Drugs | Effect | Comments |
|---|---|---|
| Isoniazid(INH) | Bacteriocidal | INH, RIF, PZA, and EMB form the core of initial treatment regi-men |
| Rifampicin(RIF) | Bacteriocidal | |
| Ethambutol (EMB) | Bacteriostatic | |
| Pyrazinamide(PZA) | Bacteriocidal | |
| Streptomycin(SM) | Bacteriocidal | • SM was formerly considered to be a first-line drug and in some instances, is still used in initial treatment. • Increasing prevalence of resistance to SM in many parts of the world has decreased its overall usefulness |
Table (2):
Treatment Outcomes in center of respiratory disease of AL-Nassiriyha.
| Year | Sex | Cured | Completed treatment |
Died | Failure | Defaulter | Total Number |
|---|---|---|---|---|---|---|---|
| 2013
n=(205) |
male | 120 | 25 | 3 | 4 | 3 | 155 |
| female | 42 | 6 | 0 | 2 | 0 | 50 | |
| 2014
n=(174) |
male | 94 | 26 | 2 | 11 | 8 | 151 |
| female | 10 | 5 | 0 | 5 | 3 | 23 | |
| 2015
n=(167) |
male | 98 | 11 | 0 | 15 | 6 | 130 |
| female | 18 | 8 | 0 | 6 | 5 | 37 |
Table (3):
Comparison of performance indicators with the WHO targets.
Year |
Treatment Success Rate* |
WHO Global Target |
|---|---|---|
2013 |
73% |
85% |
2014 |
77% |
85% |
2015 |
80% |
85% |
*Success rate = (Number of new smear positive TB cases that cured or completed treatment/total n of new smear positive TB cases registered for treatment) ×100% (WHO 1996)
Table (4):
TB treatment phases.
Phase |
Purpose |
Treatment |
|---|---|---|
Initial phase |
• Kills most of the tubercle bacilli during the first 8 weeks of treatment, but some bacilli can survive longer • Prevents the emergence of drug resistance • Determines the ultimate outcome of the regimen |
Initial 2-month treatment regimen • Includes four drugs in the treatment (usually INH, RIF, PZA, and EMB) • Each of the drugs plays an important role for short-course regimens with high cure rates • Multiple drugs are needed to prevent the development of drug-resistant TB disease |
Continuation phase |
• Kills remaining tubercle bacilli (after initial phase) • If treatment is not continued long enough, the surviving bacilli may cause TB disease in the pa-tient at a later time |
An addition of either 4 or 7 months of treatment • 4 months is used for majority of patients • 7 months is recommended only for persons » Who have drug-susceptible cavitary or extensive pulmonary TB disease and whose sputum cul-ture obtained at the time of completion of 2 months of treatment is positive » Whose initial phase of treatment did not include PZA » Who are treated with once-weekly INH and RPT and whose sputum culture at the time of completion of the initial phase is positive |
Treatment completion |
Defines the number of doses ingested within a specified time frame Duration depends on • Drugs used • Drug susceptibility test results of the isolate • Patient’s response to therapy |
Most patients with previously untreated pulmonary TB disease can be treated with either • 6-month regimen (preferred) containing INH, RIF, and initially PZA or • 9-month regimen containing INH and RIF |
Table (5):
Dosage Recommendations for the Treatment of TB in Adults.
| Dose in mg/kg (maximum dosage in parenthe-ses) | |||||
|---|---|---|---|---|---|
| Drug | Adults | Daily | 1 time/ week |
2 times/ week |
3 times/ week |
| INH | – | 5 mg/kg (300 mg) |
15 mg/kg (900 mg) |
15 mg/kg (900 mg) |
15 mg/kg (900 mg) |
| RIF | – | 10 mg/kg (600 mg) |
= | 10 mg/kg (600 mg) |
10 mg/kg (600 mg) |
|
PZA |
40- 55 kg | 18.2- 25 mg/kg (1000 mg) |
= | 36.4- 50 mg/kg (2000 mg) |
27.3- 37.5 mg/kg (1500 mg) |
| 56- 75 kg | 20- 26.8 mg/kg (1500 mg) |
= | 40- 53.6 mg/kg (3000 mg) |
33.3- 44.6 (2500 mg) |
|
| 76- 90 kg | 22.2- 26.3 mg/kg (2000 mg) |
= | 44.4- 52.6 mg/kg (4000 mg) |
33.3- 39.5 mg/kg (3000 mg) |
|
|
EMB |
40- 55 kg | 14.5- 20 mg/kg (800 mg) |
= | 36.4- 50 mg/kg (2000 mg) |
21.8- 30 mg/kg (1200 mg) |
| 56- 75 kg | 16- 21.4 mg/kg (1200 mg) |
= | 37.3- 50 mg/kg (2800 mg) |
26.7- 35.7 mg/kg (2000 mg) |
|
| 76- 90 kg | 17.8- 21.1 mg/kg (1600 mg) |
= | 44.4- 52.6 mg/kg (4000 mg) |
26.7- 31.6 mg/kg (2400 mg) |
|
Tuberculosis is a transmissible, airborne-illness and remains a major global public health hazard11. TB mostly affects subjects in developing countries and it has been reported that more than 85% of TB cases have been noticed in the developing countries. Along with the new cases, a number of re-treatment cases were also being noticed12, 13. Little was known to evaluate the outcomes of this program in Nassiriyha Province. The evaluation approach set by WHO depends on treatment outcomes rate and treatment success rates. These criteria give a valid tool to evaluate performance of the program and can compare the setting in our Province with WHO targets. In the table 3 of the present study revealed that the highest recorded patients in the year of 2013 which amount to 205 patients and the number is became less in year of 2014 which amount to 174 patients and in the year of 2015 the number of patient is became of 167 patents .This goes back to that the people known to the seriousness of the TB . Although male infection in present study was predominant, female ratio could not be representing the actual situation because many women do not attend official health care centers due to fear from social stigma with fear from probable consequence of it like divorce, the present finding is in concurrence with other reports14, 15 that proposed this ratio could be attributable to biological characteristics and socioeconomic and cultural barriers to access healthcare. The case detection rate means the proportion of estimated new smear positive cases which are detected (diagnosed and notified to WHO) by DOTS programs divided by the number of cases estimated for that year, it provides an indication of how effective national tuberculosis programs are in finding people with tuberculosis and diagnosing the disease. The present data was lower than the goal of global STOP TB strategy. Moreover, the detection rate of new TB cases nationwide that was detected and notified to WHO by Iraqi’s Ministry of health was 59% and 67% in 2012 and 2013, respectively16. Concerning the success rate of treatment, it was lower than that of countrywide tuberculosis control program and WHO target. However, it was high than that reported in some Arabs countries like Lebanon (71%), Arab Emirates (76%), Bahrain (44%), and Saudi Arabia (64%)17. The lower success rates reflect poor program performance. The explanation might be due to defects in trained health personnel who was supposed to be responsible for ensuring of taking medicine by patients, or defects in regular drug supply.
It was reached a situation improved between the years where we note a decrease in the number of casualties, which numbered 205 patients in 2013 and 174 patients in 2014 Om 167 patients in 2015. Maybe it was due to the patient’s commitment to DOTS adopted by the Iraqi Ministry of Health and the World Health Organization system. Or increasing the health awareness of the community and its attention to personal hygiene.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- CDC. Tuberculosis elimination revisited: obstacles, opportunities, and a renewed commitment—Advisory Council for the Elimination of Tuberculosis (ACET). MMWR 1999;48(No. RR-9):1—13.
- World Health Organization (WHO). Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva: WHO; 2010. Available from: http://whqlibdoc.who.int/ publications/2010/9789241599191_eng.pdf
- Anderson L, Moore J, Kruijshaar M, Pedrazzoli D, Bradshaw L, Crofts J, et al. Tuberculosis in the UK: report on tuberculosis surveillance in the UK 2010. London: Health Protection Agency; 2010. Available from: http://www.hpa.org.uk/webc/ HPAwebFile/HPAweb_C/1287143594275
- White VL, Moore-Gillon J. Resource implications of patients with multidrug resistant tuberculosis. Thorax. 2000; 55(11):9623. http://dx.doi.org/10.1136/thorax.55.11.962. PMid:11050268. PMCid:PMC1745633.
- World Health Organization (WHO). Guidelines for the programmatic management of drug-resistant tuberculosis. Emergency update 2008. Geneva: WHO; 2008. Available from: http://whqlibdoc.who.int/publications/2008/9789241547581_ eng.pdf
- American Thoracic Society, CDC. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000; 161(4 Pt 2):S221—47.
- CDC. Nosocomial transmission of multidrug-resistant TB to health- care workers and HIV-infected patients in an urban hospital — Florida. MMWR 1990; 39:718-22.
- World Health Organization, International Union against Tuberculosis and Lung Disease, Royal Netherlands Tuberculosis Association. 2001. “Revised International Definitions in Tuberculosis Control.” Int. J. Tuberc. Lung Dis. 5(3): 213-5.
- World Health Organization. 1999. Global Tuberculosis Control. World health organization report,13.
- World Health Organization. 1996. Managing Tuberculosis at District Level A7: Quarterly report. EMRO, Egypt, 27-9.
- Soomro, M. H., Qadeer, E., Khan, M. A., and Morkve, O. “Treatment Supporters and Their Impact on Treatment Outcomes in Routine Tuberculosis Program Conditions in Rawalpindi District, Pakistan.” Tanaffos, 2012; 11(3): 15-22.
- World Health Organization. 2010. Global Tuberculosis Control. WHO report. Geneva, Switzerland. (WHO/HTM/TB/2010).
- Parry, C., and Davies, P. D. “The Resurgence of Tuberculosis.” Journal of Applied Bacteriology, 1996; 81 (25s): 23S-6S.
- Rao, S. “Tuberculosis and Patient Gender: An Analysis and Its Implications in Tuberculosis Control.” Lung India, 2009; 26 (2): 46-7.
- Borgdorff, M. W., Nagalderke, N. J., Dye, C., and Nunn, P. “Gender and Tuberculosis: A Comparison of Prevalence Surveys with Notification Data to Explore Gender Differences in Case Detection.” Int. J. Tuberc. Lung Dis. 2000; 4(2): 123-32.
- The World Bank. “Data: Tuberculosis Case Detection Rate (%, All Forms).” The World Bank. Org 2015.
- The World Bank. 2015. “Data: Tuberculosis Treatment Success Rate (% of New Cases).” The World Bank. Org. Accessed, 2015.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


