ISSN: 0973-7510
E-ISSN: 2581-690X
Antimicrobial resistance is a serious public health concern across the world. Gram-negative resistance has propagated over the globe via various methods, the most challenging of which include extended-spectrum β-lactamases, carbapenemases, and AmpC enzymes. Gram-negative bacterial infections are difficult to treat in critically extremely sick persons. Resistance to different antibiotic treatments nearly always lowers the probability of proper empirical coverage, sometimes resulting in severe outcomes. Multidrug resistance can be combated with varying degrees of success using a combination of older drugs with high toxicity levels and novel therapeutics. The current therapies for multidrug-resistant Gram-negative bacteria are discussed in this review, which includes innovative medications, older pharmaceuticals, creative combinations of the two, and therapeutic targets.
MDR, ICU, Extended-spectrum β-lactamases, β-lactam/β-lactamase Inhibitors, AmpC Enzymes, Carbapenemases
Resistance to antibiotics is a serious and complex phenomenon caused by a complex combination of direct and indirect impacts, such as antimicrobial misuse in humans and farm animals, spillover impacts, such as pollution and poor sanitation, and inherent bacteria features. Some researchers have identified previous antibiotic consumption, underlying illnesses, and invasive methods as the most common risk factors for resistance.1,2 Furthermore, the lifestyle factors that influence resistance distribution differ depending on where you live. Antibiotic resistance is more likely to spread in underdeveloped nations due to poor hygiene and a lack of clean water, as per WHO, but research in the United States (US) suggests that around one in every five infections conferring resistance is caused by contaminated food or livestock.3 Antibiotic overuse and abuse, including poor contamination management practices, have been identified as factors in Europe’s evolution of antimicrobial resistance. Antimicrobial resistance is a major problem to human health around the world.4 Resistance is more likely to spread in this area due to variables such as quickly developing and densely populated cities, rising income, and the resulting increase in large-scale procedures.5 However, thorough information on the relative contributions of numerous elements to the overall worldwide problem of MDR diseases has yet to be completely clarified.6 As a result, based on various epidemiological and socioeconomic scenarios, it is necessary to address the diverse aspects of MDR diseases both globally and locally.6 Mechanisms restricting penetration of drugs into or enhancing drug elimination from bacteria, mutation-selection of therapeutic targets, and enzymatic inactivation of medicines are some of the ways pathogens evolve antimicrobial resistance.7 Antimicrobial resistance, for whatever cause, has already impaired our ability to treat infections, posing a danger to human health successfully. Carbapenem-resistant Gram-negative bacteria, like carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Enterobacterales, and Acinetobacter baumannii extensively drug-resistant (XDR), are a global threat.8 This study looks at the current and future effects of MDR Negative bacteria infections, current and potential treatment choices, and other aspects of the active treatment of MDR Negative bacteria infections in hospitalized patients.
As the basis for the present narrative review, a literature search was performed in the MEDLINE / PubMed database using various combinations of pertinent keywords (e.g., “ICU,” “Gramnegative,” “therapy,” “management,” “novel antibiotics,” “novel drugs,” “Pseudomonas,” “Acinetobacter,” “Klebsiella,” “MDR”). Subsequently, retrieved papers were discussed and further iterative searches were conducted. Ultimately, three main narrative chapters were organized as follows: (i) MDR Gram-negative infections’ current and prospective treatment; (ii) Approaches to treating MDR Gram-negative infections; (iii) Future therapeutic possibilities for MDR-gram-negative bacteria.
MDR Gram-negative Infections’ Current and Prospective Treatment
Global and national organizations such as the European Centers for Disease Control and Prevention (ECDPC), the American Society for Infectious Diseases (IDSA), the World Health Organization (WHO), and the US Centers for Disease Control and Prevention (CDC), along with all MDRs, are the threats posed by gram-negative bacteria 9. Three carbapenem-resistant A. baumannii, Enterobacterales, and P. aeruginosa are Gram-negative bacteria on the WHO’s priority concern resistant pathogens list for 2016–17.10 Carbapenem-resistant enterobacterial infections accounted for 6.6 percent of the 140,000 maximum severe healthcare-associated enterobacterial infections in the United States each year. In comparison, multidrug-resistant Acinetobacter infections accounted for 63 percent of 12,000 infections and 13 percent of 51,000 Pseudomonas infections, according to a 2013 CDC report.11 Even though the frequency of carbapenem-resistant infections has remained steady, MDR continues to be recognized as a serious global concern, according to the 2019 study. Pseudomonas aeruginosa infection has the highest MDR infection rate in Europe, with carbapenem resistance reaching 63% in some countries in Southeastern Europe in 2017.11,12
The frequency of carbapenem-resistant enterobacteria became extremely low (2.8 percent) in a 2016 study of 177 studies carried out in several Southeast Asian nations, with the occurrence of carbapenem-resistant P. aeruginosa and A. baumannii.2 The prevalence of all three resistant strains increased by 73.0% and 29.8%, respectively. According to the China Antimicrobial Surveillance Network, resistance to carbapenems become discovered in 10% of Enterobacterales traces and 71.4% of Acinetobacter spp., and 20-30% of P. aeruginosa traces were diagnosed in 2017.13 Gram-negative MDR has the highest mortality rate in critically sick patients, and MDR is linked to MDR infection. Carbapenem resistance is rising in neutropenic patients, particularly Pseudomonas species, with deaths from carbapenem-resistant bloodstream infections (BSI) ranging from 33.3 percent to 71.4 percent in neutropenic patients. Inappropriate empirical antibiotic medication was provided in 46.2 percent of instances of MDR Gram- terrible contamination in hematopoietic stem cell recipients.13
Approaches to Treating MDR Gram-negative Infections
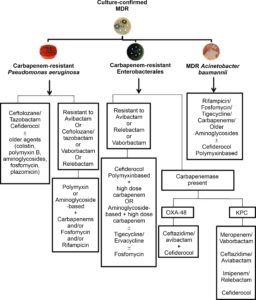
MDR Gram-negative infection poses the greatest risk to the critically sick, who frequently have numerous comorbidities. There are two methods for organizing and managing suggestions. Rather than specific MDR Gram-negative bacteria, most MDR Gram-negative infection treatment guidelines focus on general clinical and epidemiological conditions. In addition, meaningful suggestions must account for local resistance patterns and the possibility of fast changes in recommendations. However, suggestions grouped by MDR agent, antibacterial agent, or disease can be found in published studies and reviews. Bassetti et al. developed an MDR pathogen-based therapeutic approach for severely unwell patients in the intensive care unit. The first recommendations based on preclinical and clinical studies and taking into account local resistance patterns are: For carbapenem-resistant Enterobacterales, meropenem/vaborbactam, ceftazidime/avibactam, aztreonam/avibactam, or cefiderocol, imipenem/relebactam, ceftolozane/tazobactam for treatment of carbapenem-resistant P. aeruginosa; and A. baumannii with cefiderocol. Peri et al. published a new study with recommendations for treating A. baumannii, P. aeruginosa and carbapenem-resistant Enterobacterales (CRE)(Figure).14 In 2018, Hoki et al., Although the data were not organized by indication, did provide recommendations for the use of antibiotics. On the other hand, the 2020 IDSA rules provide precise recommendations for several forms of MDR illness (Table).15,16
Table:
The Infectious Diseases Society of America (IDSA)has suggested antibiotic therapy options15,16.
| Source of infection |
β-lactamase-producing Enterobacterales | Carbapenem-resistant Enterobacterales | P. aeruginosa with difficult-to-treat resistance | |||
|---|---|---|---|---|---|---|
| Preferred treatment | Alternative treatment | Preferred treatment | Alternative treatment | Preferred treatment | Alternative treatment | |
| Pyelonephritis or cUTI | Ertapenem, meropenem, imipenem/cilastatin, ciprofloxacin, levofloxacin, or trimethoprim/ sulfamethoxazole | Ceftazidime/avibactam, meropenem/vaborbactam, imipenem/cilastatin/ relebactam, and cefiderocol. Meropenemb (extended-infusion): only if ertapenem resistant, meropenem susceptible, AND carbapenemase testing results are either not available or negative. | Once-daily aminoglycosides. | Ceftolozane/tazobactam, ceftazidime/avibactam, imipenem/cilastatin/relebactam, and cefiderocol. | Once-daily aminoglycoside. | |
| Infections outside the urinary tract | Meropenem, imipenem/ cilastatin, ertapenem Oral step-down therapy to ciprofloxacin, levofloxacin, or trimethoprim/sulfamethoxazole can be considered | Ceftazidime/avibactam, meropenem/ vaborbactam, and imipenem/ cilastatin/relebactam. | Cefiderocol. Tigecycline, eravacycline (intra-abdominal infections). | Ceftolozane/tazobactam, ceftazidime/avibactam, or imipenem/cilastatin/ relebactam. | Cediferocol Aminoglycoside monotherapy: limited to uncomplicated BSI with complete source control. | |
| Cystitis | Nitrofurantoin, trimethoprim/ sulfamethoxazole. | Amoxicillin/clavulanate, single-dose aminoglycosides, fosfomycin (E. coli only). | Ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole, nitrofurantoin, or a single-dose of an aminoglycoside. Meropenem (standard infusion): only if ertapenem resistant, meropenem susceptible, AND carbapenemase testing results are either not available or negative | Ceftazidime/avibactam, meropenemvaborbactam, imipenem/cilastatin/ relebactam, and cefiderocol. Colistin (only when no alternative options are available). | Ceftolozane/tazobactam, ceftazidime/avibactam, imipenem/relebactam, cefiderocol, or a single-dose of an aminoglycoside | Colistin |
Indeed, issues with better prices and a shortage of comparative records with earlier antibiotics prevent widespread use because comparative research has not been performed or has become similar research.17 Furthermore, antibiotic treatment must be delivered as soon as feasible in critically ill patients to be successful, and doing antibiotic susceptibility tests can cause delays.16 As a result, specific empirical treatment guidelines based on the kind of infection have been developed. Here are some of the riskiest MDR clinical scenarios: However, it is important to remember that regional patterns of epidemic resistance must be considered.
The following paragraphs summarize the main properties of currently available drugs to treat the extreme form of MDR gram-negative infection in sick patients.
Eravacycline
Eravacycline (TP434) is synthesized by fluorocycline chemically identical to tigecycline and has just been approved by the European Medicines Agency (EMA) and FDA to treat cIAI.18 Eravacycline was developed to circumvent several of the tetracycline resistance mechanisms. Most bacteria with tetracycline efflux channels, ribosome protection, and beta-lactamase are resistant to it.19 Except for Burkholderia cenopacia and P. aeruginosa, erabacycline is effective against Gram-negative, Gram-positive and anaerobic bacteria. It is also highly effective against CRAB and is more effective than any other drug studied in one in vitro study.20 Eravacycline has a high bioavailability (over 90%) after oral administration, good metabolic stability, and minimal risk of drug side effects. Eravacycline has been tested in three scientific studies at four levels for cIAI and cUTI, with mixed outcomes: good overall performance in cIAI but poor overall performance in cUTI. The IGNITE study compared intravenous erabacycline 1.0 mg/kg to ertapenem 1 g every 12 hours in a controlled, twofold, quasi-trial.21 Errabacycline had an 86.8% cure rate in the population, while ertapenem had an 87.6% cure rate, suggesting that errabacycline was not inferior to ertapenem. IGNITE 3 (NCT019783938) and (NCT03032510) studies compared erabacycline with ertapenem and levofloxacin for the diagnosis of cUTI.22 Erabacycline was not less effective in any of the studies, raising concerns about its potential penetration into the lower urinary tract. Another phase 3 randomized trial (IGNITE 4) compared erabacycline and meropenem to treat non-infectious IAI and showed that erabacycline demonstrated 88.9% and 100% microbiological responses against enterobacterial and acinetobacterial infections, respectively reported to be seen. In our view, erabacycline can be a crucial remedy alternative for sufferers with cIAI due to MDR gram-terrible micro organism inclusive of CRAB.23 In addition, excessive oral bioavailability (>90%) permits conversion from injectables to oral dosage forms.
Fosfomycin
Fosfomycin is a phosphoenolpyruvate analog that prevents the peptidoglycan precursor UDP N-acetylmuramic acid from forming.24 When there were few or no active options, its intravenous formulation was used to treat MDR-gram-negative bacteria, sometimes combined with other medicines. In a quick RCT of ninety-four CRAB-inflamed sufferers handled with colistin plus fosfomycin compared to colistin alone, the mixture institution had a better microbiological reaction rate but no significant change in life expectancy.25 All-motive mortality inside 28 days in a small pattern of forty-eight sufferers with multidrug-resistant Gram-terrible bacterial infections handled with fosfomycin (generally in mixture with tigecycline or colistin) at a dose of 8 g every eight hours for 14 days turned into 37.5% people.26 Because of the lack of bigger trials and the apparent risk of quick resistance selection, we believe it is still recommended to use fosfomycin only in specific instances.24
Polymyxins
Polymyxins are antimicrobial detergents that attack gram-negative bacteria’s outer membrane. Polymyxin B and Colistin (polymyxin E) can be used in humans.8,27 Over the beyond few years, it has been regularly used to deal with infections resulting from gram-terrible micro organisms that motivate MDR and has been one of the few (and from time to time only) dependable options for CRE CRPA and CRAB.28 They are one of the first-line remedy alternatives for CRAB infection (more effective drugs await). However, because of the potential polymyxin-related hazards of nephrotoxicity or inadequate concentrations, new agents should be used whenever feasible for CRE and CRPA illnesses (especially in the lung).29
Furthermore, certain countries have documented signs of rising resistance (e.g., Italy and Greece). Consequently, the dosage and indications for polymyxin should be tailored to maximize efficacy while minimizing the development of polymyxin resistance. As a result, a recent international consensus declaration has been adopted to guide the correct use of polymyxin in all situations where polymyxin is still needed (e.g. CRAB infection, CRPA resistance to CRE, and novel BL/BLI).29
Aminoglycosides
Bactericidal aminoglycosides block the bacterial S30 ribosomal subunit in a concentration-dependent manner. They have been utilized a lot in recent years to treat carbapenem-resistant GNB, especially when it comes to polymyxin resistance.30 However, as with polymyxins, two problems prevent the powerful use of common aminoglycosides (e.g., amikacin, gentamicin, and tobramycin) to deal with infections as a result of gram-negative microorganism MDR: (i) can cause toxicity for the kidneys and reduce drug levels in the lungs; and (ii) increased resistance rate. On the other hand, plazomicin, a new aminoglycoside analog of sisomicin keeps balance towards many aminoglycoside-enhancing enzymes, on the other hand, has been shown to have a low rate of resistance in vitro.31 Although some NDM1-generated CREs have been reported to have apparent resistance due to co-expression of plasmomycin-inactivated methyltransferase, they are in vitro activity appears to be superior to CRE over CRPA CRAB. The FDA approved plasmomycin as a treatment for complex urinary tract infection (cUTI) primarily based on the outcomes of a segment three EPIC study that plasmomycin is superior to meropenem. A small randomized trial showed that patients with severe CRE infection who received plasmomycin had a lower mortality rate than patients who received colistin plus tigecycline or meropenem (BSI). Plazomycin has recently been submitted to the European Medicines Agency (EMA) to treat UTIs and other serious infections.32
Tigecycline
Because Pseudomonas aeruginosa is inherently resistant to tigecycline, and glycylcycline antibiotic that interacts with the subunit (30S) of the bacterial ribosome, CRE with CRAB is often more effective than CRE and CRPA.33 Tigecycline has been used with different tablets to treat severe CRE and CRAB infections, based on excellent in vitro effects and studies.34 It is well worth citing that, in step with an FDA caution primarily based on data obtained from controlled medical trials, tigecycline ought to be used with care withinside the remedy of ventilator-related pneumonia (VAP).35 It could purpose extra mortality than different regimens. Greater doses of tigecycline should be used to fulfill PK/PD targets while no different selections are available, and tigecycline is used to deal with pneumonia.36
Carbapenems
Although it may seem contradictory, carbapenem has been used with other antibiotics to treat carbapenem-resistant MDRgram-negative bacteria (before the advent of the new BL/BLI).37,38 This method was preferred because of its synergistic potential and aptitude to obtain appropriate carbapenem concentrations against some resistant bacteria at a slightly elevated carbapenem MIC. Large observational studies suggest that this method is ultimately more suitable for chronic CRE infection by KPC-producing strains. In contrast, a recent randomized trial showed no difference in survival with meropenem plus colistin compared to colistin alone in treating severe CRAB infection.27 In this context, in our opinion, the significant mortality observed in the study highlights the need for innovative anti-CRAB drugs. There are only a few observations of carbapenem CRPA combinations so that no firm conclusions can be drawn.25
Piperacillin/tazobactam
The piperacillin/tazobactam antibiotic is powerful in opposition to many Gram-negative bacteria, specifically Pseudomonas spp. It is one of the few antibiotics which could kill Pseudomonas spp.39 In ceftriaxone-resistant patients with E. coli or K. pneumoniae, the piperacillin/tazobactam combination did not show an increase in thirty-day mortality compared to meropenem.40 In the ZEUS study, piperacillin/tazobactam was relatively least active in patients with UTI than fosfomycin. Though, it became mentioned that the former’s dose in that test might also be inadequate.41 In many seriously unwell sufferers, the usual dosage of piperacillin/tazobactam (4.5 mg 3 instances a day) is inadequate to reap powerful bactericidal concentrations, and dose modifications can be indicated in sufferers with slight or intense renal impairment.
Ceftolozane/Tazobactam
Ceftolosan/tazobactam is likely the latest commercially available BL/BLI with the highest in vitro activity against CRPA; however, it is ineffective against CRE.42 The FDA and EMA have authorized ceftolozane/tazobactam to treat cUTI and cIAI based on the ASPECT-cUTI and ASPECT-cIAI studies. Currently, the most enticing use of ceftolosan/tazobactam is to treat CRPA infections and side effects. Indeed, an increase in post-advertising and marketing observational data on the use of ceftolosan/tazobactam for CRPA infection supports this and highlights the lack of a more aggressive labeling option.43 Following the announcement of quasi-meropenem in the recent ASPECTNP trial (NCT02070757), ceftolosan/tazobactam may be licensed to HP and VAP in the future.44 Finally, the use of ceftolosan/tazobactam has been proposed as a carbapenem-free alternative for contaminations triggered through extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriales, which may be beneficial in some cases until further clinical and economic evidence.
Cefoperazone/sulbactam
Current data provided by representatives of the SENTRY antimicrobial monitoring program indicate that Ceoperazone/sulbactam is one of the most active drugs in vitro with in vitro activity against 91.6% of Enterobacteriaceae.45 Susceptibility rates vary by area, with Western Europe having the highest rate at 94.4 percent and Eastern Europe having the lowest at 82.0 percent. When cefoperazone/sulbactam was compared with tigecycline in ICT, tigecycline caused by carbapenem-resistant Acinetobacter baumannii had a substantially greater 28-day death rate.46 There were no statistically important variations in the success rate (70.6 percent versus 73.9 percent, odds ratio 0.847, P = 0.761), sepsis-related death, or fourteen-day mortality in Enterobacterales-generating ESBL-induced ICT patients. Cefoperazone/sulbactam and treatment with carbapenem.47 Cefoperazone/sulbactam was not shown to be inferior to cefepime in the treatment of HP or medical-associated pneumonia, and a similar proportion of patients recovered after the study.48 Cefoperazone/sulbactam has been permitted in several European countries (Czech Republic, Bulgaria, Lithuania, Poland, Slovakia, and Italy) but not in the United States.48
Meropenem/Vaborbactam
Meropenem/vaborbactam is a new BL/BLI with strong and specific action against CREs producing class A carbapenemase (eg KPC).49 After FDA approval, the European Medicines Agency (EMA) has authorized meropenem/vabobactam to treat cIAI, cUTI, VAP, hospital-acquired pneumonia (HAP), and aerobic Gram-negative infections in adult patients treatment choices.49 Meropenem/vabobactam was used instead of piperacillin/tazobactam in a dual, twofold TANGOI trial for acute urinary tract infections, including acute pyelonephritis.50 In an open-label TANGOII research, meropenem/vaborbactam was contrasted to the best medicine available for CRE infection, which was prematurely due to the meropenem/vaborbactam.51 In most instances, patients who develop bacteremia, with scientific treatment costs of 65.6 percent in the meropenem/vaborbactam group (21/32), and 33.3 percent in the comparator group (5/15).52 In light of this, meropenem/vaborbactam is a unique and highly successful treatment for KPC-producing CRE. It should be used with care, as with ceftazidime/avibactam, since resistance may develop (although possibly less frequently).53 Consequently, we must carefully consider the unique properties and activity spectra of each of these two novel compounds for future CRE treatment algorithms to exploit the efficacy of CRE treatment in all situations while maintaining the efficacy of both drugs over time.
Ceftazidime/Avibactam
Ceftazidime/avibactam is a newly approved BL-BLI grouping effective against carbapenemases, and some CRPA isolates produce CRE classes A (e.g., KPC) and D (e.g., OXA).42 The European Medicines Agency and FDA have authorized ceftazidime/avibactam for cUTI, HAP, complex intra-abdominal infections (cIAI), and VAP.54 The European Medicines Agency (EMA) also authorized ceftazidime/avibactam for GNB infections in individuals with limited treatment options.55 Although randomized clinical trials demonstrated the efficacy of ceftazidime-avibactam against ceftazidime-resistant isolates but not CRE, favorable observational studies support its activity against the latter. For example, 104 sufferers with KPC-generating K. pneumoniae BSI who obtained ceftazidime-avibactam had a decreased 30-day mortality charge than a matched organization of 104 sufferers who obtained different medications (36.5 vs. 55.7 percent, respectively, p 0.005).56 As a result, ceftazidime/avibactam is a substantial, effective, and broadly to be had remedy alternative for CRE; nevertheless, its use must be optimized following antimicrobial stewardship standards.57 There have already been reviews of ceftazidime/avibactam resistance because of blaKPC mutations.
Future Therapeutic Possibilities for MDR-gram-negative Bacteria
For treating MDR- gram-negative bacteria infections, rifampin has shown a variable in vitro synergy when used with other drugs. In a randomized controlled trial, 210 patients with A. baumannii infection were randomly assigned to colistin or colistin plus rifampin to determine whether the addition of rifampin improved their microbiological response.58 However, the two arms had equal death rates. Aminomethylcycline Omadacycline is FDA-approved for treating community-acquired streptococcus pneumoniae and chronic skin-related diseases.59 Although efficacy has been demonstrated in vitro against some MDR gram-negative bacteria, therapeutic post-advertising and marketing evidence is desirable to evaluate whether this medicine will be included in future MDR gram-negative infection treatment algorithms.60
In this part, we will talk about novel medicines that are in the last stages of development against MDR Gram-negative infections. Examples of future clinical evidence that may guide antibiotic selection for treating Gram-negative MDR infection in critically sick patients.
Murepavadin
Murepavadine (POL7080) is a peptide-protein derivative that targets antibiotics and belongs to the outer membrane protein family (OMPTA).61 It works by targeting the lipopolysaccharide transport protein D (LptD), implicated in lipopolysaccharide production in Pseudomonas aeruginosa’s outer membrane.62 So, Murepavadin is particularly effective against P. aeruginosa, but has little effect on the normal intestinal flora or selection of resistance of other bacteria. It is effective against P. aeruginosa, particularly colistin-resistant, carbapenemase-producing, pan-resistant isolates, drug-resistant, and other Pseudomonas species in vitro, and not against quasi Gram-negative pathogens or Enterobacteriaceae.63 The medicine has broad tissue spreading, progressive and dose-proportional pharmacokinetics, and an elimination half-life of 2-5 hours. Murepavadin was given to 25 individuals with proven P. aeruginosa VAP in an open phase 2 study (NCT02096328). Clinical treatment was obtained in 91% of patients with established P. aeruginosa VAPs (nine had MDR or a highly drug-resistant infection), and the 28-day all-cause death rate was just 8%, significantly below the projected mortality rate of 20-40%.64 There were no signs of development of resistance to murepavadine during the study period. In the PRISMUDR study (NCT03582007), murepavadine and one antibiotic were compared with two antibiotics in the VAP, and in the PRIMSMDR trial (NCT03582007), murepavadine and one antibiotic in the VAP0960 were compared with two antibiotics in the VAP0960. (NCT3) was compared.2
In conclusion, if the positive results from the phase 2 trial are repeated in the phase 3 trial, murepavadine may be a useful adjuvant to other antibiotics in both conventional and targeted treatment of P. aeruginosa infection in patients with high-risk features. There is an obvious need for efficacy evidence for additional infections, such as bloodstream infections, cUTI, or cIAI.
Cefiderocol
Cefiderocol is an antimicrobial containing a catechin fragment at the 3rd position of the side chain that chelates unbound iron and offers new medications with a new mode of action.65 Bacterial iron transporters that bind to ferric iron and enhance its activity in response to acute infection actively carry the side chains of catechols through the outer membrane. Cefiderocol is also a good carbapenemase enzyme inhibitor. Cefiderocol is particularly effective against the gram-negative bacteria that produce KPC and VIM, P. aeruginosa that produces Stenotrophomonas maltophilia, MBL, and Acinetobacter baumannii OXA-lactamase.66
A phase 2, multicenter, double-blind study compared cepiderochol (2 g every 24 hours) to imipenem/cilastatin (1 g every eight hours) to treat UTIs. In the treatment trial, 183/251 (73%) of patients treated with cepiderochol and 65/118 (55%) of patients treated with imipenem cilastatin achieved primary goals of clinical treatment and microbiological eradication (weighted difference of 18.6%, 95% confidence interval 8.2–28.9).67 Cefiderocol has also been shown to be safe and well-tolerated. Only 5 (2%) discontinued cepiderochol since of C. difficile, anaphylaxis, elevated liver enzymes, or diarrhea. Cefiderochol is also being studied in two further phase 3 clinical studies for hospital-acquired influenza and severe carbapenem-resistant Gram-negative bacteria infections.67 In the APEKSNP study (NCT03032380), all-cause death in adult Gram-negative HP/VAP patients treated with cepiderochol and meropenem was compared (combined with linezolid). In June of 2019, the inquiry is scheduled to be concluded. Another randomised, open-label section three research (CREDIBLECR, NCT02714595) started in 2017 to offer proof of cefiderocol’s efficacy in sufferers with carbapenem-resistant GNB infections (healthcare-related pneumonia [HCAP], cUTI, VAP, HAP, and BSI).68 Cefiderocol is as compared to the quality to be had the remedy for carbapenem-resistant GNB, which incorporates up to a few antibacterial sellers and is both polymyxin-primarily based totally or non-polymyxin-primarily based.69 Despite the absence of clear findings from phase 3 studies, we think Cefiderocol is among the most potential future therapy choices for carbapenem-resistant Gram-negative MDR bacteria such CRPA, CRE, and CRAB infections.
Aztreonam/Avibactam
Since 1986, aztreonam has been the only monobactam antimicrobial licensed to treat gram-negative infections. Ambler class A (KPC and ESBL) and class C (eg AmpC) beta-lactamases hydrolyze them, rendering them worthless against MBL-producing bacteria.66 Consequently, the arrangement of aztreonam and avibactam can prevent cell wall formation by MBL-producing bacteria, even when other beta-lactamases or carbapenemases are coexistent. In vitro, aztreonam/avibactam is 10-fold more active than aztreonam alone against ESBL, lactamase class, MBL, and KPC-producing bacteria. Nevertheless, it showed moderate activity against P. aeruginosa and A. baumannii compared to aztreonam alone.66
A phase III clinical trial is now underway to relate aztreonam/avibactam to meropenem to treat Gram-negative bacteria-caused VAP, HAP, and cIAI, for whom therapeutic options are limited or non-existent (NCT03329092).70 Alternative phase 3 study is initiated to investigate the safety, efficacy, and permissibility of aztreonam/avibactam in treating severe contaminations triggered by MBL-producing gram-negative bacteria (cIAI, cUTI, HAP, NP, BSI) (NCT03580044).71 Aztreonam/avibactam may be a potential therapy option for MBL-producing bacteria infections.
Cefepime/zidebactam Cefepime/zidebactam combines a broad-spectrum antibiotic and diazabicyclooctane (DBO) zidebactam, a second-generation BLI. In vitro, zidebactam was more effective against class C blatamase than abibactam or relebactam.72 When available, Cefepime/zidebactam may become an essential antimicrobial agent in the combat against gram-negative MDR infection. Tolerability, safety, and pharmacokinetic study of the intravenous administration of cefepime/zidebactam (NCT02707107) in healthy volunteers were performed.73
Meropenem/nacubactam
Nacubactam is every other DBO BLI. When used with meropenem, it has validated in vivo efficacy against multidrug-resistant K. pneumoniae, P. aeruginosa, and E. coli.74 A recent study looked at the intrapulmonary absorption of nacubactam and meropenem in fit people (NCT03182504).75
Cefepime/enmetazobactam
Cefepime/enmetazobactam is another promising therapy for β-Lactamase Enterobacterales. When used against ESBL-producing bacteria, enmetazobactam has been demonstrated to restore cefepime and piperacillin activity more efficiently than tazobactam.76 Cefepime/enmetazobactam was as effective in vitro against the same ESBL-producing bacteria as meropenem and imipenem.77
Major Factors to Consider in the Future Therapy of Diseases that are Resistant to Antibiotics
Antimicrobial prudence programs, improved surveillance and infection control programs indicate significant and recommending practices, reduced use of antimicrobials in agriculture, the establishment of innovative antimicrobials, prudent antimicrobial use program, more inclusive availability of drugs, and enhanced antimicrobial use prudence programs are all part of future antimicrobial resistance management programs. Although this is a lengthy and tough list, following these guidelines is crucial for worldwide antibiotic resistance reduction.58
The advent of new diagnostic techniques is one of the most significant projected advancements in treating antibiotic-resistant diseases. Although empirical therapy contributes to antibiotic overuse and resistance, it is still the most extensively utilized approach. Antibiotic sensitivity testing using traditional growth-based techniques is time-consuming and requires clean cultures. Meanwhile, the crude material can be used in new diagnostic procedures using nucleic acid amplification and immunodiagnostic methods.59 These methods promise to enable quick diagnostic and antibiotic susceptibility testing at the service point. This is predicted to shorten treatment times and allow for a change to evidence-based treatment, reducing antibiotic overuse and the spread of antibiotic resistance. Obviating the need to filter and develop cultures may also lower the total cost of therapy.59
Infection specialists should initiate antibiotic management programs, including communication between the management team and crucial maintenance physicians and treatment algorithms for antibiotic medicating and classification. Optimal evidence-based culture and sensation. Because of the continuous high use of carbapenems and the constant rise in carbapenem resistance must be used appropriately, especially in hospitals.78 The World Health Organization’s pathogen priority list provides a framework for researching novel antimicrobial drugs and combinations of new and older medicines, such as those listed below.60 While many progressive medicines display excessive interest ranges in vitro, they may no longer be prepared for medical use, and getting them to the approved degree takes time. Conducting randomized managed research with the specified wide variety of sufferers on time is not always feasible because of the few positive MDR diseases. New agents, in particular, must be compared to established agents to determine their function in treatment algorithms. Newer compounds may be even more efficient and well-tolerated, and they’re much more expensive. Expenses can be decreased by employing de-escalation procedures as needed, and these variables should be addressed in any AMS program. Financial incentives funded by the government are also required to stimulate the development of innovative antimicrobial drugs that might aid in the fight against MDR infections.79 Reimbursement must consider the particular characteristics of emerging antibiotic therapies to promote market acceptance and give pharmaceutical development incentives for these medications.79
Geographical differences in resistance underscore the importance of tailoring empirical treatment to regional epidemiology, patient risk assessment, and regional management measures. Quick diagnoses, carefully tailored treatments, and, where feasible, early de-escalation from broadspectrum medications are all important in guiding management.
Researchers may continue to investigate novel antimicrobials and combinations of prospective and updated medications to limit the spread of Gram-negative MDR infection using the WHO Shortlist of diseases as an incentive and guideline. The success of new antimicrobials will depend on the efforts of governments around the world to support research and development, comprehensive programs for the rational use of antimicrobials, and accurate data on local resistance. In the future, treating severe Gram-negative MDR infection in critically ill patients will require professional and complex clinical judgment, considering the target population and appropriate empirical coverage and the increasingly specific need for new antimicrobial activity on the enzyme level.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
Not Applicable.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
- Knight GM, Costelloe C, Murray KA, Robotham JV, Atun R, Holmes AH. Addressing the unknowns of antimicrobial resistance: quantifying and mapping the drivers of burden. Clin Infect Dis. 2018;66(4):612-616.
Crossref - Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Front Med. 2019;6:74.
Crossref - Watkins RR, Van Duin D. Current trends in the treatment of pneumonia due to multidrug-resistant Gram-negative bacteria. F1000Research. 2019;8:121.
Crossref - Chatterjee A, Modarai M, Naylor NR, et al. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis. 2018;18(12):e368-e378.
Crossref - Garonzik S, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55(7):3284-3294.
Crossref - Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy C. Infectious Diseases Society of America antimicrobial resistant treatment guidance: gram-negative bacterial infections. Practice. 2021;8:72(7):e169-e183.
- Lepape A, Jean A, De Waele J, et al. European intensive care physicians’ experience of infections due to antibiotic-resistant bacteria. Antimicrob Resist Infect Control. 2020;9(1):1-11.
Crossref - Giacobbe DR, Mikulska M, Viscoli C. Recent advances in the pharmacological management of infections due to multidrug-resistant Gram-negative bacteria. Expert Rev Clin Pharmacol. 2018;11(12):1219-1236.
Crossref - Poulakou G, Bassetti M, Righi E, Dimopoulos G. Current and future treatment options for infections caused by multidrug-resistant Gram-negative pathogens. Future Microbiol. 2014;9(9):1053-1069.
Crossref - Giamarellou H. Multidrug-resistant Gram-negative bacteria: how to treat and for how long. Int J Antimicrob Agents. 2010;36:S50-S54.
Crossref - Morris S, Cerceo E. Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics. 2020;9(4):196.
Crossref - Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61(12):e01694-17.
Crossref - Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):e169-e183.
Crossref - Peri AM, Doi Y, Potoski BA, Harris PN, Paterson DL, Righi E. Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn Microbiol Infect Dis. 2019;94(4):413-425.
Crossref - Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: Report of the British society for antimicrobial chemotherapy/healthcare infection society/british infection association joint working party. J Antimicrob Chemother. 2018;73(suppl_3):iii2-iii78.
Crossref - Hughes S, Gilchrist M, Heard K, Hamilton R, Sneddon J. Treating infections caused by carbapenemase-producing Enterobacterales (CPE): a pragmatic approach to antimicrobial stewardship on behalf of the UKCPA Pharmacy Infection Network (PIN). JAC-Antimicrobial Resistance. 2020;2(3):dlaa075.
Crossref - Satlin MJ. Languid uptake of ceftazidime-avibactam for carbapenem-resistant gram-negative infections and continued reliance on polymyxins. Clinical Infection diseases; 2021:622-625.
Crossref - Sutcliffe J, O’brien W, Fyfe C, Grossman T. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother. 2013;57(11):5548-5558.
Crossref - Seifert H, Stefanik D, Sutcliffe JA, Higgins PG. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(1):62-64.
Crossref - Solomkin J, Evans D, Slepavicius A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surgery. 2017;152(3):224-232.
Crossref - Lee YR, Burton CE. Eravacycline, a newly approved fluorocycline. Eur J Clin Microbiol Infect Dis. 2019;38(10):1787-1794.
Crossref - Pharmaceuticals T. Tetraphase announces top-line results from IGNITE3 phase 3 clinical trial of eravacycline in complicated urinary tract infections (cUTI). Globe Newswire. 2018.
- Solomkin JS, Gardovskis J, Lawrence K, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis. 2019;69(6):921-929.
Crossref - Bassetti M, Giacobbe D, Giamarellou H, et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24(2):133-144.
Crossref - Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391-400.
Crossref - Grabein B, Graninger W, Bano JR, Dinh A, Liesenfeld D. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect. 2017;23(6):363-372.
Crossref - Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322-2328.
Crossref - Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133-2143.
Crossref - Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective Pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10-39.
Crossref - Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, et al. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(3):905-913.
Crossref - Bassetti M, Giacobbe D, Giamarellou H, et al. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24(2):133-144.
Crossref - Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest. 2005;128(2):545-552.
Crossref - Pankey GA. Tigecycline. J Antimicrob Chemother. 2005;56(3):470-480.
Crossref - Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943-950.
Crossref - Rao GG, Ly NS, Diep J, et al. Combinatorial pharmacodynamics of polymyxin B and tigecycline against heteroresistant Acinetobacter baumannii. Int J Antimicrob Agents. 2016;48(3):331-336.
Crossref - Wiskirchen DE, Koomanachai P, Nicasio AM, Nicolau DP, Kuti JL. In vitro pharmacodynamics of simulated pulmonary exposures of tigecycline alone and in combination against Klebsiella pneumoniae isolates producing a KPC carbapenemase. Antimicrob Agents Chemother. 2011;55(4):1420-1427.
Crossref - Paul M, Friberg L, Stergiopoulou T, et al. Systematic Review and Meta-Analysis of. 2013;
Crossref - Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67(12):1803-1814.
Crossref - Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527.
Crossref - Shah PJ, Ryzner KL. Evaluating the appropriate use of piperacillin/tazobactam in a community health system: a retrospective chart review. Pharmacy and Therapeutics. 2013;38(8):462-483.
- Zander J, Dobbeler G, Nagel D, et al. Piperacillin concentration in relation to therapeutic range in critically ill patients-a prospective observational study. Crit Care. 2016;20(1):1-11.
Crossref - Bassetti M, Righi E, Russo A, Carnelutti A. New antibiotics for pneumonia. Clin Chest Med. 2018;39(4):853-869.
Crossref - Alosaimy S, Jorgensen SC, Lagnf AM, et al. Real-world multicenter analysis of clinical outcomes and safety of meropenem-vaborbactam in patients treated for serious gram-negative bacterial infections. Open Forum Infect Dis. 2020;7(3):ofaa051.
Crossref - Koulenti D, Song A, Ellingboe A, et al. Infections by multidrug-resistant Gram-negative Bacteria: What’s new in our arsenal and what’s in the pipeline? Int J Antimicrob Agents. 2019;53(3):211-224.
Crossref - Sader HS, Carvalhaes CG, Streit JM, Castanheira M, Flamm RK. Antimicrobial activity of cefoperazone-sulbactam tested against Gram-Negative organisms from Europe, Asia-Pacific, and Latin America. Int J Infect Dis. 2020;91:32-37.
Crossref - Niu T, Luo Q, Li Y, Zhou Y, Yu W, Xiao Y. Comparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control. 2019;8:52.
Crossref - Su J, Guo Q, Li Y, et al. Comparison of empirical therapy with cefoperazone/sulbactam or a carbapenem for bloodstream infections due to ESBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3176-3180.
Crossref - Liu J-W, Chen Y-H, Lee W-S, et al. Randomized noninferiority trial of cefoperazone-sulbactam versus cefepime in the treatment of hospital-acquired and healthcare-associated pneumonia. Antimicrob Agents Chemother. 2019;63(8):e00023-19.
Crossref - Lee Y, Kim J, Trinh S. Meropenem-vaborbactam (Vabomere™): another option for carbapenem-resistant Enterobacteriaceae. Pharmacy and Therapeutics. 2019;44(3):110-113.
- Hackel MA, Lomovskaya O, Dudley MN, Karlowsky JA, Sahm DF. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1):e01904-17.
Crossref - Castanheira M, Doyle TB, Kantro V, Mendes RE, Shortridge D. Meropenem-vaborbactam activity against carbapenem-resistant Enterobacterales isolates collected in US hospitals during 2016 to 2018. Antimicrob Agents Chemother. 2020;64(2):e01951-19.
Crossref - Johnston BD, Thuras P, Porter SB, Castanheira M, Johnson JR. Activity of meropenem/vaborbactam against international carbapenem-resistant Escherichia coli isolates in relation to clonal background, resistance genes, resistance to comparators and region. J Glob Antimicrob Resist. 2021;24:190-197.
Crossref - Kahlmeter G, Brown D, Goldstein F, et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect. 2006;12(6):P501-P503.
Crossref - Liao C-H, Lee N-Y, Tang H-J, et al. Antimicrobial activities of ceftazidime-avibactam, ceftolozane-tazobactam, and other agents against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa isolated from intensive care units in Taiwan: results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan in 2016. Infect Drug Resist. 2019;12:545.
Crossref - Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883-17.
Crossref - Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68(3):355-364.
Crossref - Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis. 2019;68(3):519-524.
Crossref - Vasala A, Hytonen VP, Laitinen OH. Modern tools for rapid diagnostics of antimicrobial resistance. Front Cell Infect Microbiol. 2020:308.
Crossref - O’Meara S. Antimicrobial resistance. Nature. 2020;586(7830):S49-S49.
Crossref - Shrivastava SR, Shrivastava PS, Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Journal of Medical Society. 2018;32(1):76.
Crossref - Srinivas N, Jetter P, Ueberbacher BJ, et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science. 2010;327(5968):1010-1013.
Crossref - Sader HS, Dale GE, Rhomberg PR, Flamm RK. Antimicrobial activity of murepavadin tested against clinical isolates of Pseudomonas aeruginosa from the United States, Europe, and China. Antimicrob Agents Chemother. 2018;62(7):e00311-18.
Crossref - Sader HS, Flamm RK, Dale GE, Rhomberg PR, Castanheira M. Murepavadin activity tested against contemporary (2016-17) clinical isolates of XDR Pseudomonas aeruginosa. J Antimicrob Chemother. 2018;73(9):2400-2404.
Crossref - Martin-Loeches I, Dale GE, Torres A. Murepavadin: a new antibiotic class in the pipeline. Expert Rev Anti Infect Ther. 2018;16(4):259-268.
Crossref - Ito A, Nishikawa T, Matsumoto S, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(12):7396-7401.
Crossref - Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect. 2017;23(10):704-712.
Crossref - Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319-1328.
Crossref - Koren A, Karas A, Echols R. Comment on ‘Cefiderocol, a New Siderophore Cephalosporin for the Treatment of Complicated Urinary Tract Infections Caused by Multidrug-resistant Pathogens: Preclinical and Clinical Pharmacokinetics, Pharmacodynamics, Efficacy and Safety’. Clin Drug Investig. 2021;41(7):659-660.
Crossref - Lucasti C, Vasile L, Sandesc D, et al. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother. 2016;60(10):6234-6243.
Crossref - Karlowsky JA, Kazmierczak KM, de Jonge BL, Hackel MA, Sahm DF, Bradford PA. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother. 2017;61(9):e00472-17.
Crossref - Mo Y, Lorenzo M, Farghaly S, Kaur K, Housman ST. What’s new in the treatment of multidrug-resistant gram-negative infections? Diagn Microbiol Infect Dis. 2019;93(2):171-181.
Crossref - Thomson KS, AbdelGhani S, Snyder JW, Thomson GK. Activity of cefepime-zidebactam against multidrug-resistant (MDR) Gram-negative pathogens. Antibiotics. 2019;8(1):32.
Crossref - Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother. 2017;72(5):1373-1385.
Crossref - Asempa TE, Motos A, Abdelraouf K, Bissantz C, Zampaloni C, Nicolau DP. Meropenem-nacubactam activity against AmpC-overproducing and KPC-expressing Pseudomonas aeruginosa in a neutropenic murine lung infection model. Int J Antimicrob Agents. 2020;55(2):105838.
Crossref - Organization WH. 2019 antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. 2019.
- Morrissey I, Magnet S, Hawser S, Shapiro S, Knechtle P. In vitro activity of cefepime-enmetazobactam against Gram-negative isolates collected from US and European hospitals during 2014-2015. Antimicrob Agents Chemother. 2019;63(7):e00514-19.
Crossref - Lee Y-L, Ko W-C, Lee W-S, et al. In-vitro activity of cefiderocol, cefepime/zidebactam, cefepime/enmetazobactam, omadacycline, eravacycline and other comparative agents against carbapenem-nonsusceptible Enterobacterales: Results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2017-2020. Int J Antimicrobial Agents. 2021;58(3):106377.
Crossref - Plackett B. Why big pharma has abandoned antibiotics. Nature. 2020;586(7830):S50-S50.
Crossref - Morton A, Colson A, Leporowski A, Trett A, Bhatti T, Laxminarayan R. How should the value attributes of novel antibiotics be considered in reimbursement decision making? MDM Policy Pract. 2019;4(2):2381468319892237.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.