ISSN: 0973-7510
E-ISSN: 2581-690X
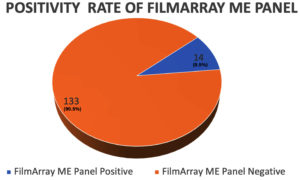
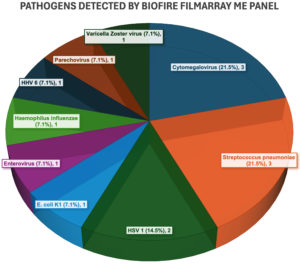
Meningoencephalitis is a severe condition requiring rapid and accurate diagnosis for effective management. Traditional culture methods often fail to detect certain pathogens, leading to delayed treatment. This study was conceptualized with an aim to assess the BioFire FilmArray Meningitis/Encephalitis (ME) Panel as a first-line diagnostic tool. A total of 147 patients (91 males and 56 females) with suspected meningoencephalitis were included. Cerebrospinal fluid (CSF) samples were analyzed using the FilmArray ME Panel and results were compared with traditional culture methods. The BioFire FilmArray ME Panel detected pathogens in 14 out of 147 CSF samples, yielding a positivity rate of 9.52%. Detected pathogens included Cytomegalovirus (03), Streptococcus pneumoniae (03), HSV1 (02), E. coli K1 (01), Enterovirus (01), Haemophilus influenzae (01), HHV6 (01), Parechovirus (01), and Varicella Zoster Virus (01). In contrast, traditional culture methods detected pathogens in only 7 cases, highlighting the panel’s superior sensitivity and underscoring the limitations of traditional methods. The ME Panel’s ability to deliver accurate results from just 200 microliters of CSF and the rapid turnaround time of just 1 hour highlights its efficiency in providing critical diagnostic information from small sample volumes. The study also assessed the impact of CSF volume, with nearly one-third of samples being 0.5 mL, noting that the panel requires only 200 microliters to provide comprehensive results. The BioFire FilmArray ME Panel significantly outperformed traditional culture methods in detecting a wide range of pathogens in CSF samples, even with minimal sample volume. Its rapid and accurate diagnostics improve early diagnosis and patient management in meningoencephalitis, supporting its integration into routine clinical practice.
Meningoencephalitis, BioFire FilmArray Meningitis/Encephalitis (ME) Panel, Cerebrospinal fluid (CSF)
Meningoencephalitis (ME) represents a significant global clinical challenge, characterized by high morbidity and mortality rates.1-5 In India, the condition is particularly concerning due to the diverse epidemiology and high burden of infectious diseases. ME is a condition which involves inflammation of both the meninges and brain, with etiological agents including bacteria, viruses, fungi, and parasites.6,7 The Indian subcontinent experiences a substantial disease burden due to factors such as overcrowding, poor sanitation, and frequent outbreaks of infections.8
Epidemiological data on ME in India is limited, with many cases going unreported or undiagnosed, creating challenges in accurately assessing the disease’s impact and developing effective public health interventions. Reliable information on the burden of this endemic disease is hindered by limited surveillance, insufficient laboratory resources, frequent misdiagnoses, and widespread antibiotic use, which is common in India.9 Reports indicate that certain regions, especially in northern and eastern India, experience seasonal outbreaks of ME, with viral infections like enterovirus contributing significantly to the burden.8 Additionally, bacterial pathogens like Streptococcus pneumoniae and Neisseria meningitidis are frequently involved, particularly affecting pediatric population.6,9
In numerous cases of suspected central nervous system (CNS) infections, diagnostic testing requires large volumes of CSF and often involves lengthy turnaround times. The accuracy of these results can be influenced by factors such as prior antimicrobial therapy, timing of lumbar puncture, and the volume of CSF analyzed.10-13
In spite of extensive efforts to determine a cause, the diagnosis remains obscure in about 25-50% of patients with acute meningoencephalitis, leaving uncertainty regarding whether these cases stem from infectious agents or other origins.10-18
Multiplex molecular assays, which allow for the simultaneous detection of multiple microbial targets, have become essential tools and are now widely used for diagnosing various infections. The BioFire FilmArray ME panel can detect fourteen (14) meningoencephalitis-related pathogens – 06 bacteria, 07 viruses, and 01 yeast – requiring only 200 µL of cerebrospinal fluid (CSF) and providing results in about an hour. By leveraging multiplex PCR technology, this panel enables rapid, simultaneous pathogen detection directly from CSF, supplying clinicians with crucial information to make timely therapeutic decisions (BioFire FilmArray ME panel Performance: 94.2% sensitivity and 99.8% specificity).19,20
By detecting 14 different pathogens, including Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, and various viral agents, this multiplex FilmArray panel provides a valuable tool for managing central nervous system (CNS) infection. Its rapid turnaround time significantly reduces the time to diagnosis, enabling earlier and more appropriate therapeutic interventions, which can improve clinical outcomes in resource-constrained settings like India.5,21,22
The present study aims to assess the clinical utility of the BioFire FilmArray ME Panel in a tertiary/quaternary care setting in North India, assessing its impact on diagnostic accuracy and patient management in cases of suspected meningoencephalitis.
This retrospective study was conducted over 15 months at a tertiary/quaternary care hospital in North India. A total of 147 patients, both pediatric and adult, presenting with clinically suspected meningoencephalitis were included. CSF samples were analyzed using the BioFire FilmArray ME Panel, and pathogen detection was compared with results from traditional microbiological methods, including CSF culture.
Standard of care CSF testing
In addition to the FilmArray ME Panel, routine diagnostic tests were performed on all CSF samples, including: (i) Gram Stain: Used for bacterial identification and guiding initial antibiotic therapy. (ii) CSF Culture: For isolation and identification of bacterial, fungal, and mycobacterial pathogens. (iii) Cytological Examination: including white blood cell (WBC) and red blood cell (RBC) counts and differential counts. (iv) Biochemical Analysis: assessing glucose and protein levels to differentiate between bacterial and viral infections. (v) India Ink Staining: used to detect Cryptococcus species, particularly in immunocompromised patients. (vi) PCR for Specific Pathogens: applied for the detection of organisms not included in the FilmArray panel.
These standard tests were performed alongside the FilmArray ME Panel to ensure comprehensive diagnostic coverage. FilmArray ME Panel Testing Procedure (Reference Instructions for Use (IFU) for BioFire ME Panel. BioFire Diagnostics, LLC. Available at: BioFire IFU).
The study population consisted of 147 patients, out of which 91 were males and 56 were females. The male-female ratio was 1.625:1. The study population comprised of pediatric and adult participants, with ages ranging from 6 months to 86 years.
The FilmArray ME Panel identified pathogens in 14 of the 147 samples, resulting in a positivity rate of 9.52% (Figure 1). Pathogens detected included Cytomegalovirus (03), Streptococcus pneumoniae (03), HSV1 (02), E. coli K1 (01), Enterovirus (01), Haemophilus influenzae (01), HHV6 (01), Parechovirus (01), and Varicella Zoster Virus (01). The distribution of various pathogens detected is shown in Table 1 and Figure 2.
Table (1):
Pathogens detected by FilmArray ME Panel
Pathogens Detected with FilmArray ME Panel |
Number of pathogens detected |
|---|---|
Cytomegalovirus |
3 (21.5%) |
Streptococcus pneumoniae |
3 (21.5%) |
HSV1 |
2 (14.4%) |
E. coli K1 |
1 (7.1%) |
Enterovirus |
1 (7.1%) |
Haemophilus influenzae |
1 (7.1%) |
HHV6 |
1 (7.1%) |
Parechovirus |
1 (7.1%) |
Varicella Zoster Virus |
1 (7.1%) |
Total |
14 |
In contrast, traditional culture methods detected pathogens in only 7 cases. Out of three cases where the BioFire panel detected Streptococcus pneumoniae, whereas only 33% (1 out of 3) could be detected by culture. Additionally, Haemophilus influenzae and Escherichia coli K1 were not detected by culture. The patient with E. coli K1 had a concurrent culture growth of Acinetobacter baumannii, which is not included in the BioFire panel. Another case identified HSV-1 along with Acinetobacter baumannii. Furthermore, of the BioFire-negative cases, cultures revealed the presence of Pseudomonas aeruginosa in two instances and Acinetobacter baumannii in two additional cases. The comparison of BioFire FilmArray ME Panel and conventional culture is shown in Table 2.
Table (2):
Comparison of Pathogen Detection between BioFire ME Panel and Culture Results
Organism |
Filmarray ME Panel Result |
No. of detections |
Culture Result |
Remark |
|---|---|---|---|---|
Streptococcus pneumoniae |
Positive |
3 |
Only in 1 out of 3 samples detected by Filmarray ME panel |
67% missed by culture |
Hemophilus influenzae |
Positive |
1 |
No Growth |
100% missed by culture |
E. coli K1 |
Positive |
1 |
Concurrently detected Acinetobacter baumannii |
E. coli K1 missed by culture Acinetobacter baumannii: off-panel target for Film Array ME panel |
HSV-1 |
Positive |
1 |
Concurrently detected Acinetobacter baumannii |
Acinetobacter baumannii: off-panel target for Film Array ME panel |
Pseudomonas aeruginosa |
Negative |
2 |
Positive |
Pseudomonas aeruginosa: off-panel target for Film Array ME panel |
Acinetobacter baumannii |
Negative |
2 |
Positive |
Acinetobacter baumannii: off-panel target for Film Array ME panel |
Traditional microbiological methods, including routine bacterial cultures, Gram staining, and phenotypic identification, are essential tools for guiding the treatment of bacterial meningitis. However, these methods have limitations, including a low positive rate in clinical diagnosis. CSF cultures are time-intensive, restricted to bacteria that grow on specific media, and may not always align with findings from microscopy. For example, only 25% of cases with bacterial concentrations <10³ CFU/mL yield positive microscopy results, while 60% are detected when concentrations range from 10³ to 10u CFU/mL.23 A 27-year study also found that CSF culture could miss bacterial meningitis diagnoses in more than 10% of cases.3,24
CSF culture remains the gold standard, showing 70-85% positivity in patients who have not received prior antibiotic treatment. While antibiotics typically do not alter CSF cell counts, glucose, or protein, they reduce Gram stain sensitivity to 40-60% and culture positivity to below 50%. Administering antibiotics before CSF sampling can significantly hinder diagnosis by reducing microbial load or eliminating pathogens entirely, resulting in sterile CSF samples.25,26
Sulaiman et al. identified the etiology in only about 32% of cases, with similar findings reported in other studies. This limited detection is likely due to the low sensitivity of CSF cultures for non-bacterial pathogens and the underuse of molecular and serologic testing for viral agents.15,27,28
This diminished sensitivity due to the aforementioned factors complicate timely diagnosis and optimal treatment. Consequently, clinicians often face a diagnostic dilemma when CSF cultures return negative despite a high suspicion of infection, particularly in patients who have received prior antimicrobial therapy.
The introduction of multiplex PCR-based assays, such as the BioFire FilmArray Meningitis/Encephalitis (ME) Panel, has significantly enhanced the diagnostic landscape for CNS infections by offering rapid, sensitive, and comprehensive pathogen detection. These tools enable early and targeted therapy, which is crucial for life-threatening conditions like meningoencephalitis.5,22
The FilmArray ME Panel, which is US FDA approved, provides a comprehensive, accurate, and time-efficient solution for pathogen detection.
Despite certain challenges, such as small sample volumes, the FilmArray ME Panel demonstrated robust diagnostic performance by utilizing just 200 µL of CSF, significantly enhancing pathogen detection through multiplex PCR. Its rapid turnaround time and comprehensive pathogen coverage facilitate early therapeutic interventions in critical clinical settings and also in antimicrobial stewardship.21,29,30
In this study, the BioFire FilmArray ME panel detected Streptococcus pneumoniae in three cases, with a culture-confirmed detection rate of only 33%, highlighting the superior sensitivity of the BioFire panel over traditional culture methods.30,31 Additionally, culture methods failed to detect Haemophilus influenzae and Escherichia coli K1, further underscoring the limitations of conventional microbiological techniques in identifying pathogens in CSF samples.32,33
Notably, one patient with E. coli K1 also exhibited concurrent growth of Acinetobacter baumannii in culture, a nosocomial pathogen not included in the BioFire panel’s repertoire. This case emphasizes the need for a comprehensive diagnostic approach, integrating both the BioFire panel and conventional culture methods, particularly for nosocomial pathogens like Acinetobacter and Klebsiella species, which are not part of the panel’s design.34
Additionally, certain studies have highlighted the FilmArray ME Panel’s superior diagnostic yield compared to traditional methods. For example, a study conducted at the Kerala Institute of Medical Sciences found a 23.6% positivity rate with the FA-ME panel, compared to just 3% with standard culture methods.5 Other studies similarly reported that enteroviruses were the most commonly detected pathogens, corroborating the findings of this study.5,13
In line with Infectious Diseases Society of America (IDSA) guidelines, which recommend the use of molecular diagnostics in the evaluation of encephalitis, this study supports the integration of rapid molecular diagnostics into routine clinical practice for optimal management of CNS infections.3
The enhanced performance of PCR-based diagnostics over conventional microbiological testing, including patients who have already received antibiotics, where traditional culture-based methods may not be diagnostic, further solidifies the role of these rapid diagnostics in clinical microbiology.
While CSF culture is considered the gold standard to determine antimicrobial susceptibility, WHO also strongly recommended CSF be assayed using PCR-based molecular tests for relevant pathogens in individuals with suspected acute meningitis. This guideline was supported by an extensive meta-analysis of CSF molecular testing.5,22,35,36
Finally, the minimal CSF volume requirement (200 µL) and rapid turnaround time (approximately 1 hour) of the FilmArray ME Panel make it a valuable tool in clinical practice, particularly in scenarios requiring prompt diagnosis and intervention.
The FilmArray ME Panel significantly outperformed traditional culture methods in detecting a broad spectrum of pathogens in cerebrospinal fluid (CSF) samples, demonstrating its superior sensitivity even with minimal sample volumes of just 200 µL. This rapid and accurate diagnostic capability enhances early diagnosis and improves patient management in cases of meningoencephalitis, allowing for timely interventions and potentially better clinical outcomes. Furthermore, the integration of the BioFire panel into routine clinical practice aligns with antimicrobial stewardship efforts by facilitating targeted therapy and reducing unnecessary broad-spectrum antibiotic use. This is particularly crucial in a clinical landscape where misdiagnosis and delays can lead to detrimental patient outcomes. As we continue to face the challenges of central nervous system infections, ongoing research and investment in rapid and innovative diagnostic technologies like the FilmArray ME Panel are essential. Such advancements not only promise to enhance the diagnostic landscape but also aim to improve the yield and overall clinical outcomes for patients affected by CNS infections.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PB, VS, JT, SK conceptualized the study. PB, VS, JT performed literature review. PB, VS, JT performed experiments. PB, VS, JT, SK performed results analysis. PB, VS, JT wrote the manuscript. PB, SK reviewed the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Army Hospital (R & R), Delhi Cantt., vide regn. no. 14/2025.
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317-328.
Crossref - Misra UK, Kalita J, Bhoi SK. Spectrum and outcome predictors of central nervous system infections in a neurological critical care unit in India: a retrospective review. Trans R Soc Trop Med Hyg. 2014;108(3):141-146.
Crossref - Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, McArthur JC, Geocadin RG, Venkatesan A. Predictors of outcome in acute encephalitis. Neurology. 2013;81(9):793-800.
Crossref - World Health Organization. Meningitis. 2023. https://www.who.int/news-room/fact-sheets/detail/meningitis
- Chandran S, Arjun R, Sasidharan A, Niyas VK, Chandran S. Clinical Performance of FilmArray Meningitis/Encephalitis Multiplex Polymerase Chain Reaction Panel in Central Nervous System Infections. Indian J Crit Care Med. 2022;26(1):67-70.
Crossref - Jayaraman Y, Veeraraghavan B, Purushothaman GKC, et al. Burden of bacterial meningitis in India: Preliminary data from a hospital-based sentinel surveillance network. PLoS ONE. 2018;13(5):e0197198.
Crossref - Dutta AK, Swaminathan S, Abitbol V, Kolhapure S, Sathyanarayanan S. A Comprehensive Review of Meningococcal Disease Burden in India. Infect Dis Ther. 2020;9(3):537-559.
Crossref - Sapra H, Singhal V. Managing Meningoencephalitis in Indian ICU. Indian J Crit Care Med. 2019;23(Suppl 2):S124-S128.
Crossref - Narain JP. Public Health Challenges in India: Seizing the Opportunities. Indian J Community Med. 2016;41(2):85-88.
Crossref - Cizman M, Jazbec J. Etiology of acute encephalitis in childhood in Slovenia. Pediatr Infect Dis J. 1993;12(11):903-908.
Crossref - Sivertsen B, Christensen PB. Acute encephalitis. Acta Neurol Scand. 1996;93(3):156-159.
Crossref - Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin Infect Dis. 2002;35(2):175-182.
Crossref - Shukla B, Aguilera EA, Salazar L, Wootton SH, Kaewpoowat Q, Hasbun R. Aseptic meningitis in adults and children: diagnostic and management challenges. J Clin Virol. 2017;94:110-114.
Crossref - George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000-2010. PLoS ONE. 2014;9(9):e104169.
Crossref - Hasbun R, Rosenthal N, Balada-Llasat JM, et al. Epidemiology of meningitis and encephalitis in the United States, 2011-2014. Clin Infect Dis. 2017;65(3):359-363.
Crossref - Hasbun R, Wootton SH, Rosenthal N, et al. Epidemiology of meningitis and encephalitis in infants and children in the United States, 2011-2014. Pediatr Infect Dis J. 2019;38(1):37-41.
Crossref - Bloch KC, Glaser CA. Encephalitis surveillance through the emerging infections program, 1997-2010. Emerg Infect Dis. 2015;21(9):1562-1567.
Crossref - Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology. 2014;82(5):443-451.
Crossref - Fleischer E, Aronson PL. Rapid Diagnostic Tests for Meningitis and Encephalitis-BioFire. Pediatr Emerg Care. 2020;36(8):397-401.
Crossref - BioFire Diagnostics. FilmArray Meningitis/Encephalitis (ME) Panel: Instruction Booklet. Salt Lake City (UT): BioFire Diagnostics; 2016.
- Radmard S, Reid S, Ciryam P, et al. Clinical Utilization of the FilmArray Meningitis/Encephalitis (ME) Multiplex Polymerase Chain Reaction (PCR) Assay. Front Neurol. 2019;10:281.
Crossref - Tarai B, Das P. FilmArray® meningitis/encephalitis (ME) panel, a rapid molecular platform for diagnosis of CNS infections in a tertiary care hospital in North India: one-and-half-year review. Neurol Sci. 2019;40(1):81-88.
Crossref - La scolea Jr LJ, Dryja D. Quantification of bacteria in CSF and blood of children with meningitis and its diagnostic significance. J Clin Microbiol. 1984;19:187-9024.
Crossref - Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults, a review of 493 episodes. N Engl J Med. 1993;328(1):21-28.
Crossref - Benninger F, Steiner I. CSF in acute and chronic infectious diseases. Handb Clin Neurol. 2017;146:187-206.
Crossref - Men X, Zhao G, Zhao W, Zhang J, Yang Y, Chen C. Pathogen identification in culture-negative cerebrospinal fluid specimens of patients with purulent meningitis using next-generation sequencing technology. Int J Clin Exp Pathol. 2020;13(9):2427-2438.
- Sulaiman T, Salazar L, Hasbun R. Acute versus subacute community-acquired meningitis: analysis of 611 patients. Medicine (Baltimore). 2017;96(36):e7984.
Crossref - McGill F, Heyderman RS, Michael BD, et al. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J Infect. 2016;72(4):405-438.
Crossref - Wootton SH, Aguilera E, Salazar L, Hemmert AC, Hasbun R. Enhancing pathogen identification in patients with meningitis and a negative Gram’s stain using the BioFire FilmArray® Meningitis/Encephalitis panel. Ann Clin Microbiol Antimicrob. 2016;15(1):26.
Crossref - Mina Y, Schechner V, Savion M, et al. Clinical benefits of FilmArray meningitis-encephalitis PCR assay in partially-treated bacterial meningitis in Israel. BMC Infect Dis. 2019;19:713.
Crossref - Arora HS, Asmar BI, Salimnia H, Agarwal P, Chawla S, Abdel-Haq N. Enhanced Identification of Group B Streptococcus and Escherichia Coli in Young Infants with Meningitis Using the BioFire Filmarray Meningitis/Encephalitis Panel. Pediatr Infect Dis J. 2017;36(7):685-687.
Crossref - Du B, Hua C, Xia Y, et al. Evaluation of the BioFire FilmArray meningitis/encephalitis panel for the detection of bacteria and yeast in Chinese children. Ann Transl Med. 2019;7(18):4.
Crossref - Dien Bard J, Naccache SN, Bender JM. Use of a Molecular Panel To Aid in Diagnosis of Culture-Negative Meningitis. J Clin Microbiol. 2016;54 (12):3069-3070.
Crossref - Kar M, Dubey A, Singh R, Sahu C, Patel SS, Fatima N. Acinetobacter Meningitis: A Retrospective Study on its Incidence and Mortality Rates in Postoperative Patients at a Tertiary Care Centre in Northern India. J Clin of Diagn Res. 2023;17(1):DC01-DC06.
Crossref - Myint T, Soria J, Castillo MRC, Gao Y, Conejo Castillo MR, Arora V, Ribes JA. Comparison of positive BioFire FilmArray meningitis/encephalitis (ME) panels, CSF cultures, CSF parameters, clinical presentation and in-patient mortality among patients with bacterial and fungal meningitis. Microbiol Spectr. 2025;13(2):e0001424.
Crossref - World Health Organization. WHO guidelines on meningitis diagnosis, treatment and care. Geneva: World Health Organization; 2025.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.