ISSN: 0973-7510
E-ISSN: 2581-690X
Traditional microbiological techniques, while effective, are often time-consuming and labour-intensive. Machine learning and deep learning, enable rapid and accurate identification of microbial pathogens from complex datasets such as whole-genome sequencing, mass spectrometry, and clinical laboratory reports. Artificial Intelligence (AI) revolutionizes medical microbiology by enhancing pathogen detection, antimicrobial resistance prediction, and clinical decision-making. AI facilitates automated image analysis for culture-based diagnostics, improving the speed and accuracy of colony identification and antimicrobial susceptibility testing. One of the most impactful applications of AI is in antimicrobial resistance (AMR) surveillance. Machine learning models can analyse genetic determinants of resistance and predict antimicrobial susceptibility patterns, allowing for early detection of multidrug-resistant organisms. Moreover, AI-integrated clinical decision support systems (CDSS) enhance antimicrobial stewardship by providing real-time recommendations on appropriate antibiotic use, thereby reducing the spread of resistance. Natural language processing (NLP) further optimizes data extraction from electronic health records, improving diagnostic workflows and patient outcomes. Despite its transformative potential, challenges such as data standardization, model interpretability, and integration into routine laboratory workflows must be addressed. Ethical considerations, including data privacy and algorithmic bias, also warrant careful attention. As AI continues to evolve, its synergy with microbiology will pave the way for precision diagnostics, personalized treatment strategies, and global AMR mitigation. Leveraging AI-driven innovations will be crucial in shaping the future of infectious disease diagnostics and public health microbiology.
Artificial Intelligence, Medical Microbiology, Antimicrobial Resistance, Machine Learning, Deep Learning
Medical microbiology, a foundation of modern healthcare, has long played an important role in diagnosing, preventing, and treating infectious diseases. From identifying pathogens to directing antimicrobial treatments, it offers the basis for battling diseases that pose a threat to the health of the world. However, conventional methods find it difficult to keep up with the increasing complexity of microbiological investigation as pathogens change and mutate. Artificial intelligence (AI), a revolutionary technology has the ability to improve the health care sector.1,2
AI’s contribution to medical microbiology is based on its unmatched speed and accuracy in analysing large datasets. AI can understand complicated microbial patterns, such as identifying antibiotic resistance mechanisms from genomic sequences, predicting pathogen virulence, and classifying microbial communities from metagenomic data, and improve diagnostic accuracy due to machine learning algorithms, neural networks, and predictive analytics. For instance, convolutional neural networks (CNNs) have been used to detect antimicrobial resistance genes from raw sequencing data,3 while machine learning models have accurately predicted Clostridioides difficile infection outcomes based on patient microbiome profiles.4 These capabilities surpass traditional rule-based methods by uncovering hidden, non-linear relationships in large datasets. Advanced AI models, can analyse genetic sequences to identify mutations linked to antibiotic resistance, offering vital information for precision healthcare. AI-powered image recognition technology also helps in automated microscope image analysis, which lessens the strain on human knowledge while reducing errors.5
AI also provides a proactive method for managing disease. AI can forecast epidemics, monitor pathogen progression, and direct public health actions by combining data from environmental sources, electronic health records, and real-time monitoring systems. This skill lessens the impact of epidemics by enhancing response times and assisting in the development of focused control measures.6
The potential of personalized treatment is being further expanded by the collaboration between microbiology and artificial intelligence. AI-powered systems can examine microbiological profiles and unique patient data, allowing for customized treatment regimens that optimize therapeutic effectiveness while reducing adverse effects. In situations where standard protocols might not be sufficient, this method can help in managing chronic infections. In the future, microbial risks will be addressed with creative, data-driven solutions with the help of artificial intelligence (AI), which is improving diagnostic accuracy, optimizing workflows, and allowing predictive insights. As this shift takes place, it has the potential to reshape the fields of public health and healthcare, ushering in a new era of accuracy and readiness.1,2,5,6
Nevertheless, there are certain difficulties in incorporating AI into medical microbiology. Careful thought must be given to issues like algorithmic biases, data privacy, and the requirement for strong validation criteria. To guarantee the ethical and appropriate use of AI in healthcare, collaboration between microbiologists, data scientists, and legislators is crucial.1,2,5,6
The current literature review used a variety of reliable resources, such as PubMed, Google Scholar, Scopus, and Web of Science, to find relevant research on the use of artificial intelligence in the field of medical microbiology. The key phrases used in the literature search for this review article were “AI in medical microbiology and its types”, “ML in medical microbiology”, “DL in medical microbiology”, “AI in Drug discovery”, “AI and AMR”, “AI and infection control”, “AI and medical diagnostics”, “AI in Vaccine Development”, “AI in HAI”, “AI in Epidemiology and Outbreak Prediction”, “AI in Personalised Treatment”, “Benefits and Challenges of AI in Healthcare” and “AI and Ethical issues”.
The criteria for inclusion were peer-reviewed papers published between 2013 and 2024. Non-peer-reviewed articles, editorials, and publications that did not specifically discuss AI in medical microbiology were excluded.
Initial keyword searches were conducted as part of the search process, and these were then refined using inclusion and exclusion criteria. Titles and abstracts were reviewed to determine their significance, and full-text articles were gathered for further study. Data on the specific AI technology, its application, benefits, and challenges were categorized.
Artificial Intelligence (AI)
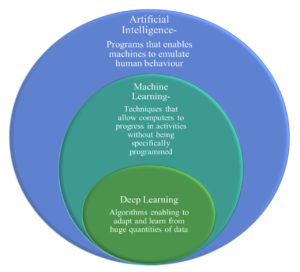
Artificial intelligence (AI) is the creation of systems with cognitive functions including reasoning, meaning-finding, generalization, and/or experience-based learning that are comparable to those of humans.2
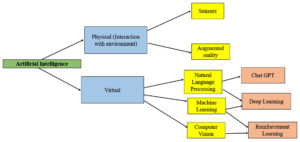
Artificial Intelligence is divided into two broad categories (Figure 1):
Physical (Interaction with environment)
Sensors
Devices that collect real-world data (e.g., temperature, motion, sound) for AI systems to interpret.
Augmented Reality (AR)
Enhances the physical environment using digital overlays, often guided by AI for real-time interaction.
Virtual (Digital Intelligence and Processing)
Natural Language Processing (NLP): Allows AI to understand and generate human language (e.g., ChatGPT).
Machine Learning (ML)
Enables AI to learn from data and improve over time (e.g., Deep Learning, a subset that uses neural networks).
Computer Vision
Allows AI to interpret and understand visual data (e.g., Reinforcement Learning can be used to improve visual recognition systems over time).1,2
Machine learning and deep learning are commonly employed in clinical microbiology laboratories.
Machine Learning (ML)
One aspect of AI that enables systems to learn and enhance procedures without the need for explicit programming is machine learning2 (Figure 2).
Deep Learning (DL)
A subset of machine learning known as “deep learning” makes use of multi-layered neural networks, or “deep architectures,” to learn complex patterns in large datasets, often in image or sequence form2 (Figure 2).
Application
Automated image analysis of microbiological slides, such as identifying bacterial morphology or colony characteristics.
Example
Convolutional neural networks (CNNs) used for automated identification and classification of Gram-stained slides or microbial colonies.2
Machine learning mechanism
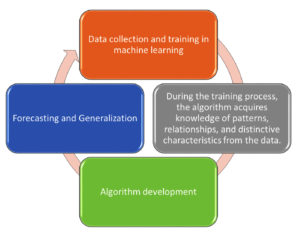
Figure 3 illustrates the fundamental mechanism of machine learning as a continuous cycle. It begins with data collection and training, where large volumes of relevant data are gathered and used to train machine learning models. During this phase, the algorithm goes through a learning process, in which it identifies patterns, relationships, and unique features within the data. This acquired knowledge forms the basis for the next stage, which is algorithm development. Here, the algorithm is refined and optimized based on the insights gained during training. Once developed, the model moves to the forecasting and generalization phase, where it is applied to new or unseen data to make predictions or generate insights. This cycle can be repeated with new data or improved algorithms to continuously enhance the model’s performance and accuracy.2
Types of machine learning
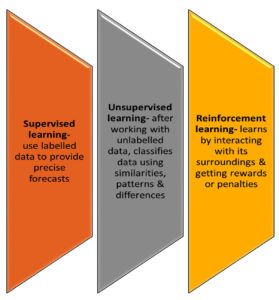
In clinical microbiology laboratories, machine learning (ML) is increasingly being applied to improve the speed, accuracy, and efficiency of diagnostics, analysis, and decision-making processes. Here are some key machine learning mechanisms and their applications (Figure 4):
Supervised learning
Mechanism
Involves training models on labelled data (e.g., known pathogen identification from culture results) to predict outcomes for new, unseen data.
Application
Pathogen identification from blood cultures or PCR results using labelled datasets to train ML models for rapid bacterial or fungal identification.
Example
ML models trained on bacterial genome sequences can predict antibiotic resistance profiles, helping clinicians choose the most effective antibiotics.2,5
Unsupervised Learning
Mechanism
Identifies patterns in data without predefined labels, allowing the discovery of hidden relationships or clusters.
Application
Microbial community profiling from metagenomic data to detect novel or rare pathogens.
Example
Clustering of bacterial species from patient samples to identify infection sources or track outbreaks.2,5
Natural Language Processing (NLP)
Mechanism
NLP involves the processing of unstructured text data to extract meaningful information, such as clinical notes, lab reports, and research papers.
Application
Automated extraction of clinical information from microbiology reports (e.g., identifying pathogen names, susceptibility results) for faster integration into the patient’s electronic health record (EHR). Example: NLP tools used to extract and categorize antibiotic resistance data from laboratory reports to support surveillance and treatment decision-making.2,5
Reinforcement Learning
Mechanism
A type of ML where models learn by interacting with an environment and receiving feedback to improve performance over time.
Application
Optimizing diagnostic workflows by learning from lab activities, adjusting processes, and recommending the best course of action (e.g., adjusting diagnostic tests or treatment options based on outcomes).
Example
Reinforcement learning algorithms can optimize the sequencing of diagnostic tests or identify the most efficient lab processes, reducing wait times for microbiological results.2,5
Predictive analytics
Mechanism
ML algorithms analyze historical data to predict future outcomes or trends.
Application
Antimicrobial resistance (AMR) prediction by analyzing patient data and microbial patterns over time to predict which antibiotics will be most effective for future infections.
Example
ML models predicting AMR patterns based on trends in laboratory data, helping clinicians make proactive treatment decisions.2,5,6
Key Benefits of ML in Clinical Microbiology
Faster diagnostics
Automates pathogen identification and antibiotic susceptibility testing, reducing the time to diagnosis.
Improved accuracy
Reduces human error in diagnostic interpretation, especially in complex cases.
Real-Time Surveillance
Facilitates early detection of infection outbreaks and AMR patterns, allowing for a quicker response.1,2,6
Machine learning is revolutionizing clinical microbiology by automating complex tasks, improving accuracy, and supporting real-time decision-making.
Laboratory applications
AI can be used in laboratory medicine to improve or automate human-based procedures and make operational choices. These comprise automation of instruments, detection of errors, predictions, interpretation of result, utilization of tests, genomics and analysis of image. AI-powered image analysis presently only supports human labour; it cannot take the place of human knowledge6 (Figure 5).
Diagnosis in Medical Microbiology
Image analysis
Diagnostic lab procedures could be drastically altered by machine learning-based image analysis, which could transform agar plate examination and microscopy (Table 1). AI diagnostics can be applied to clinical microbiology data sets such as digital pictures, mass spectra, metagenomic results, and genomic information. To advance clinical microbiology, researchers must investigate, create, and apply AI and computer vision.2,7
Additionally, food safety and environmental protection, as well as water quality monitoring, use AI imaging technologies.
Table (1):
Applications of AI in Diagnostics Microbiology
Author |
Purpose |
Input- Using AI Approach |
|---|---|---|
Dey et al.8 |
Automated detection of malarial parasites |
Microscopy of thick blood films-using ResNet 152 model Deep Greedy network |
Holmstrom et al.9 |
AI-assisted identification of Schistosoma haematobium |
Digital images from mobile and slide-scanner microscopy of stool stained with and soil-transmitted helminths Iodine stain-using Sequential algorithms |
Ibrahim et al.10 |
AI-assisted detection of Mycobacterium tuberculosis |
Sputum-AFB staining images using AlexNet model (using transfer learning) |
Xiong et al.11 |
AI-assisted detection of acid-fast TB bacilli |
Tissue sections-AFB staining images using CIFAR-10 CNN |
Smith et al.12 |
Automated interpretation of gram-stains from blood culture |
Blood culture- Gram stain images using Inception v3 CNN, TensorFlow, Python |
Hoorali et al.13 |
AI-based detection and segmentation of Bacillus anthracis |
Cutaneous anthrax-Tissue slide images using UNet and UNet++, Keras, TensorFlow |
Kang et al.14 |
Using deep learning to identify non-O157 Shiga toxin- producing Escherichia coli (STEC) |
Hyperspectral images- using Linear Discriminant Analysis (LDA), Support Vector Machine (SVM), and soft-max regression (SR) |
Oyamada et al.15 |
To diagnose leptospirosis by determining agglutination in microscopic pictures |
MAT microscopic images-using Support Vector Machine (SVM) |
Tong et al.16 |
Raman spectroscopy for the detection of hepatitis B virus infection |
Raman spectroscopy of serum samples- using Principal component analysis (PCA), support vector machine (SVM) |
Tabarov et al.17 |
SERS coupled with ML to detect A and B influenza viruses |
Surface-enhanced Raman scattering spectroscopy (SERS)-using Support Vector Machine (SVM) |
Liu et al.18 |
Identification of fungal species in stool samples |
Stool specimen-using ANN-1 & ANN-2 |
Example
In less than nine hours, time-lapse coherent imaging can identify bacterial development without the need for a culture and quickly detect and classify live bacteria, including E. coli.
Automated culture analysis
Artificial intelligence (AI) systems can identify bacterial species by analysing growth patterns in culture media. AI-enabled automated systems can track cultures in real time, yielding faster outcomes than conventional techniques. To find specific patterns or genetic markers connected to certain infections, these algorithms are trained (Table 2). The analysis of cultures has been enhanced by automated methods like PhenoMATRIX and the automated plate assessment system (APAS) Independence (Figure 6).2,19
Table (2):
AI-Driven Approaches for Microbial Culture and Colony Identification
Author |
Purpose |
Input- Using AI Approach |
|---|---|---|
Rattray et al.20 |
Identification using data on colony images |
P. aeruginosa-Colony images using ResNet-50, VGG-19, MobileNetV2 and Xception |
Zhang et al.21 |
Bacterial colony detection using Deep Learning |
E. coli-Colony images using Random cover targets algorithm (RCTA), YOLOv3 |
Ma et al.22 |
Assessing a new method for Aspergillus |
Colony images in dissecting microscopy- detection using stereomicroscopy using Xception |
Meeda et al.23 |
Using colony fingerprinting to distinguish between different fungal species |
Fungal cultures, confocal microscopy images-using Support vector machine (SVM) and Random Forest (RF) |
Figure 6.The APAS Independence: Intelligent imaging and machine learning technology to read and interpret the presence of significant bacteria in culture plates. (Source: https://cleverculturesystems.com/technology/intelligent-automation/apas-independence)
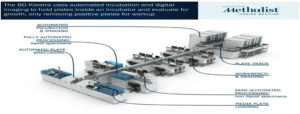
Examples of AI in total laboratory automation is Kiestra Total Laboratory Automation (TLA). From sample processing and incubation to digital imaging, the BD KiestraTM Total Laboratory Automation (TLA) system automates several parts of microbiology lab workflows, increasing productivity and perhaps cutting down on turnaround times for culture findings (Figure 7).
Figure 7. Examples of AI in total laboratory automation- Kiestra Total Laboratory Automation (TLA) and WASP Lab (Source: https://bd.com/en-us/products-and-solutions/products/product-families/bd-kiestra-tla)
Molecular tests
AI improves the interpretation of molecular diagnostic procedures like Next-Generation Sequencing (NGS) and Polymerase Chain Reaction (PCR). The complex information produced by these methods may be processed by machine learning algorithms, resulting in more rapid and precise pathogen detection and resistance profiling. Rapid molecular testing can also speed up the process of identifying infections and locating important resistance factors.24
Drug discovery and antimicrobial resistance prediction
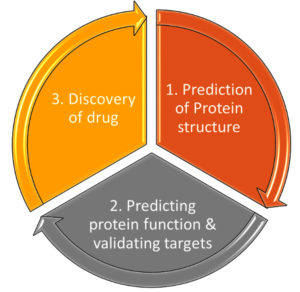
By analysing microbial genomes, proteomes, and metabolic pathways, artificial intelligence makes it easier to identify potential treatment targets. Predicting the microbial targets and compounds having affinity and binding to microbial targets improves the drug development process and expedites the choice of potential drugs for experimental validation as shown in Figure 8. AI has a significant impact on predicting pharmacokinetic and pharmacodynamic aspects of medications. With the help of AI-powered models, we can reduce side effects, optimise dosage levels, ensure compatibility, efficacy, and safety of clinical trials.25-28
Example
Predicting Drug- Target Interactions
Artificial Intelligence (AI) accelerates malaria drug discovery by analyzing large datasets, predicting drug-target interactions, and identifying potential antimalarial compounds faster and more cost-effectively than traditional methods. Example: DeepMind’s AlphaFold, an AI system, predicted the 3D structures of Plasmodium falciparum proteins (malaria parasite), helping researchers identify potential drug targets.25-27
Drug repurposing
AI algorithms can identify new uses for existing drugs by analyzing patterns in molecular data. This speeds up the drug development process, especially in urgent cases like pandemics. Example: During the COVID-19 pandemic, AI was used to identify potential repurposed drugs for treating COVID-19, such as Remdesivir, by screening vast databases of existing compounds.27
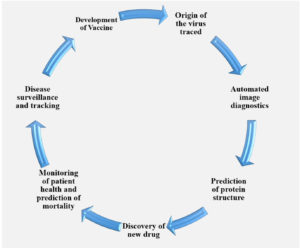
By analyzing the genetic sequences of pathogens, artificial intelligence (AI) can predict patterns of antimicrobial resistance and detect mutations linked to resistance, facilitating the timely detection, treatment, and containment of drug-resistant infections and resistant strains. By utilizing huge amounts of data, artificial intelligence (AI), particularly DL and ML, is being utilized to address issues in the field of antimicrobial resistance (AMR). AI has paved the way for new developments in AMR, including the discovery of novel AMR genes and mutations and the reduction of diagnostic time from days to hours as shown in Figure 9.25-28
Development of vaccine
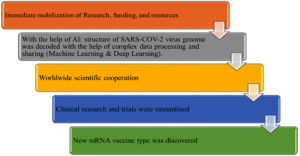
Traditional vaccine development methods are time-consuming and frequently fail to keep up with evolving challenges. In addition, the unpredictable nature of pathogen mutations and the immune system’s reaction to novel vaccines complicate the process. In this context, artificial intelligence (AI) is emerging as a strong tool, providing novel solutions that improve the efficiency and precision of vaccine research. The phenomenon of viral escape mutations, in which viruses adapt to avoid the immune response brought on by vaccination or a natural infection, presents a challenge to the creation of vaccines. Hie et al.29 offer a novel method for comprehending and forecasting these escape mutations by using machine learning techniques, particularly language models. Using only sequencing information, this technique effectively predicts escape mutations in the viral proteins of influenza, HIV, and SARS-CoV-2 (Figure 10). These predictive tools are extremely useful because they enable scientists to create vaccines that are more resistant to virus evolution, which could result in immunity that lasts longer.5
Epidemiology and prediction of outbreaks
Epidemiological data can be analysed with the help of AI in order to forecast outbreaks and monitor the transmission of infectious diseases. ML models use data from multiple sources, such as weather trends, travel databases and social media to predict outbreaks of disease and guide public health actions. Real-time clinical and epidemiological data analysis by AI can help with contact tracking and assess how well containment strategies are working. Application of AI technology in microbial detection revolutionizes detection of epidemics and their management, thus helping in reducing infectious diseases impact on health globally and saving lives30,31 (Figure 11).
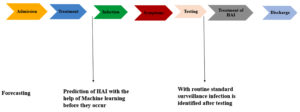
Surveillance of Hospital Acquired Infections & Prevention and Control of Infections
AI and ML have the potential to be used in the creation of HAI monitoring algorithms that will help identify transmission pathways, improve patient risk stratification, comprehend HAI risk factors, and detect infections in real time (Figure 12). Monitoring infection patterns and assessing therapeutic choices require the use of complex dataset analysis from electronic health records (EHRs). The term “forecasting” refers to the large number of AI and ML models that have been created that can predict the occurrence of an event in advance. To predict VAP, CLABSIs, and SSIs, the risk of colonization or infection with an MDR pathogen, and consequences in the hospital context, a growing number of machine learning models have been built thus far. But the discipline has been dominated by predicting sepsis and/or septic shock, with the majority of studies falling into this category.32-35
Benefits and Challenges of AI
The dual-edged nature of Artificial Intelligence presents both opportunities for advancement and challenges that require thoughtful solutions. Table 3 shows Benefits of Artificial intelligence, encountered challenges and with possible solutions.1,2
Table (3):
Artificial intelligence: Benefits, Challenges and their Solutions
Category |
Benefits |
Challenges & Examples |
Solutions |
|---|---|---|---|
Diagnostic Accuracy |
AI improves accuracy in identifying pathogens and resistance genes, reducing diagnostic errors. |
Algorithm biases can lead to unreliable outcomes, requiring rigorous validation across diverse datasets.
E.g. MALDI-TOF Spectra Classification: An ML model trained only on spectra from E. coli strains collected in Europe may misidentify strains from Asia or Africa due to regional genetic variations. So, outcome will be misidentification or low confidence scores when applied to geographically diverse isolates.19 |
• Use training datasets from multiple geographic regions, patient populations, instruments, and lab protocols to enhance generalizability. • Collaborate with global networks or open-access databases to build robust datasets.19 |
Speed and Efficiency |
AI accelerates sample analysis, providing quicker turnaround times and improving patient outcomes. |
Integration into existing workflows demands significant investment in infrastructure and training. E.g. Automated Image Analysis of Culture Plates: • ML-based colony counters or plate readers require high-resolution imaging systems, stable lighting conditions, and powerful GPUs to run real-time analysis. • Many microbiology labs, especially in smaller hospitals, lack the infrastructure to support these systems, resulting in slow or failed adoption.21,23 |
Phased or Modular Implementation: • Begin with cloud-based or modular ML solutions that don’t require full system overhauls (e.g., SaaS-based colony counting or resistance prediction tools). • Integrate these tools as decision-support systems before full automation.21,23 |
Predictive Capabilities |
Enables forecasting of pathogen trends, resistance development, and outbreak risks for proactive management. |
Variability in laboratory methods and inconsistent data quality hinder reliable AI development. E.g. 1. Antimicrobial Susceptibility Testing (AST) Data: • Different labs may use different AST methods (e.g., disk diffusion vs. broth microdilution), breakpoints (e.g., CLSI vs. EUCAST), and interpretation criteria. • AI models trained on data from one method may misinterpret or inaccurately predict resistance when applied to data from another lab or standard.24,28 |
Standardization of Laboratory Protocols: • Encourage adoption of standardized testing methods and guidelines (e.g., use of CLSI/EUCAST harmonized data). • Implement lab-wide standard operating procedures (SOPs) for generating and labelling data used in AI training.24,28 |
Personalized Medicine |
Facilitates tailored treatment plans by integrating patient data with microbial profiles. |
Trust issues and fear of job displacement may lead to resistance from clinicians and healthcare staff. E.g. AI-Guided Antibiotic Selection: • When an AI tool recommends a specific antimicrobial based on pathogen genome and patient data, clinicians may distrust the suggestion, especially if it contradicts standard guidelines or clinical intuition. • Clinicians may ignore AI recommendations or revert to empirical therapy, limiting the benefit of personalized treatment.36,37 |
Human-in-the-Loop Approach: • Design AI systems to support, not replace, human decision-making—providing recommendations that clinicians can accept, modify, or reject. • Include override options with traceable justification to maintain clinician autonomy.36,37 |
Data Handling |
AI handles large datasets efficiently, uncovering patterns that are difficult to detect manually. |
Concerns over data privacy, security, and consent require robust governance frameworks. E.g. Sharing Patient Microbial Genomic Data: • Whole-genome sequencing (WGS) data linked with patient metadata is highly informative but also identifiable. • Sharing such data with external AI developers or international databases without clear consent may violate privacy laws.38 |
Implement Robust Data Governance Frameworks: • Establish institutional policies on data access, sharing, and storage that align with national and international data protection regulations. • Include clear audit trails and role-based access control to monitor who accesses what data and when.38 |
Recommended Ethical Frameworks for Implementation of AI
The rapid adoption of Artificial Intelligence (AI) across sectors necessitates the development of robust ethical frameworks to guide its responsible implementation. Following are the recommended ethical frameworks for implementation of AI.38,39
FAT-ML Principles (Fairness, Accountability, and Transparency in ML)
Advocates building fair and accountable systems with clear documentation and bias checks.
AI Ethics Guidelines from WHO (2021)
Emphasizes inclusiveness, human oversight, privacy, and equitable access to AI technologies in health.
Bioethics Framework (Principles of Autonomy, Beneficence, Non-maleficence, and Justice)
A foundational guide for integrating AI ethically into patient care and diagnostics.
RE-AIM Framework (Reach, Effectiveness, Adoption, Implementation, and Maintenance)
Helps assess how ethically and practically an AI tool is deployed across clinical settings.38,39
Limitation of the study
This article only covered key uses of AI in medical microbiology. Additional applications might not have been included.
Artificial Intelligence is redefining the landscape of medical microbiology by enabling faster, more accurate diagnostics, streamlining workflows, and enhancing disease surveillance and outbreak prediction. Its applications-from automated image analysis and antimicrobial resistance prediction to personalized treatment strategies and vaccine development-are transforming laboratory and clinical practices. However, the successful integration of AI requires overcoming challenges related to data quality, ethical use, and infrastructure. Moving forward, collaborative efforts among clinicians, microbiologists, data scientists, and policymakers will be essential to ensure AI is implemented ethically, effectively, and equitably in advancing infectious disease management and public health.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not Applicable.
- Alsulimani A, Akhter N, Jameela F, et al. The Impact of Artificial Intelligence on Microbial Diagnosis. Microorganisms. 2024;12(6):1051.
Crossref - Gurajala S. Artificial intelligence (AI) and medical microbiology: a narrative review. Indian J Microbiol Res. 2024;11(3):156-162.
Crossref - Arango-Argoty G, Garner E, Pruden A, Heath LS, Vikesland P, Zhang L. DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome. 2018;6(1):23.
Crossref - Fedarko MW, Simeone DM, Whitney AK, et al. Predicting recurrent Clostridioides difficile infection using the microbiome. Open Forum Infect Dis. 2020;7(7):ofaa146.
- Luo M, Yang W, Bai L, et al. Artificial intelligence for life sciences: a comprehensive guide and future trends. The Innovation Life. 2024;2(4):100105.
Crossref - Baddal B, Taner F, Ozsahin DU. Harnessing artificial intelligence for the diagnosis and prevention of hospital-acquired infections: a systematic review. Diagnostics. 2024;14(5):484.
Crossref - Rhoads DD. Computer vision and artificial intelligence are emerging diagnostic tools for the clinical microbiologist. J Clin Microbiol. 2020;58(6):e00511-20.
Crossref - Dey S, Nath P, Biswas S, Nath S, Ganguly A. Malaria detection through digital microscopic imaging using deep greedy network with transfer learning. J Med Imaging. 2021;8(5):054502.
Crossref - Holmstrom O, Linder N, Ngasala B, et al. Point-of-care mobile digital microscopy and deep learning for the detection of soil-transmitted helminths and Schistosoma haematobium. Glob Health Action. 2017;10(1):1337325.
Crossref - Ibrahim AU, Guler E, Guvenir M, Suer K, Serte S, Ozsoz M. Automated detection of Mycobacterium tuberculosis using transfer learning. J Infect Dev Ctries. 2021;15(5):678-686.
Crossref - Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of Mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
Crossref - Smith KP, Kang AD, Kirby JE. Automated interpretation of blood culture Gram stains by use of a deep convolutional neural network. J Clin Microbiol. 2018;56(3):e01521-17.
Crossref - Hoorali F, Khosravi H, Moradi B. Automatic Bacillus anthracis bacteria detection and segmentation in microscopic images using UNet++. J Microbiol Methods. 2020;177:106056.
Crossref - Kang R, Park B, Chen K. Identifying non-O157 Shiga toxin-producing Escherichia coli (STEC) using deep learning methods with hyperspectral microscope images. Spectrochim Acta A Mol Biomol Spectrosc. 2020;224:117386.
Crossref - Oyamada Y, Ozuru R, Masuzawa T, et al. A machine learning model of microscopic agglutination test for diagnosis of leptospirosis. PLoS One. 2021;16(10):e0259907.
Crossref - Tong D, Chen C, Zhang J, et al. Application of Raman spectroscopy in the detection of hepatitis B virus infection. Photodiagn Photodyn Ther. 2019;28:248-252.
Crossref - Tabarov A, Vitkin V, Andreeva O, et al. Detection of A and B influenza viruses by surface-enhanced Raman scattering spectroscopy and machine learning. Biosensors. 2022;12(12):1065.
Crossref - Liu L, Yuan Y, Zhang J, et al. Automatic identification of fungi under complex microscopic fecal images. J Biomed Opt. 2015; 20(7):076004.
Crossref - Burton RJ, Albur M, Eberl M, Cuff SM. Using artificial intelligence to reduce diagnostic workload without compromising detection of urinary tract infections. BMC Med Inform Decis Mak. 2019; 17(1):78.
Crossref - Rattray JB, Lowhorn RJ, Walden R, et al. Machine learning identification of Pseudomonas aeruginosa strains from colony image data. PLoS Comput Biol. 2023;19(3):e1011699.
Crossref - Zhang B, Zhou Z, Cao W, Qi X, Xu C, Wen W. A new few-shot learning method of bacterial colony counting based on the edge computing device. Biology. 2022;11(2):156.
Crossref - Ma H, Yang J, Chen X, et al. Deep convolutional neural network: a novel approach for the detection of Aspergillus fungi via stereomicroscopy. J Microbiol. 2021;59(5):563-572.
Crossref - Maeda Y, Sugiyama Y, Lim TK, et al. Rapid discrimination of fungal species by the colony fingerprinting. Biosens Bioelectron. 2019;146:111747.
Crossref - Maurer FP, Christner M, Hentschke M, Rohde H. Advances in rapid identification and susceptibility testing of bacteria in the clinical microbiology laboratory: implications for patient care and antimicrobial stewardship programs. Infect Dis Rep. 2017;9(1):6839.
Crossref - Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekad RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26(1):80-93.
Crossref - Qureshi R, Irfan M, Gondal TM, et al. AI in drug discovery and its clinical relevance. Heliyon. 2023;9(7):e17575.
Crossref - Yin Z, Wong STC. Artificial intelligence unifies knowledge and actions in drug repositioning. Emerg Top Life Sci. 2021;5(6):803-813.
Crossref - Ali T, Ahmed S, Aslam M. Artificial intelligence for antimicrobial resistance prediction: challenges and opportunities towards practical implementation. Antibiotics. 2023;12(3):523.
Crossref - Hie B, Zhong ED, Berger B, Bryson B. Learning the language of viral evolution and escape. Science. 2021;371(6526):284-288.
Crossref - Nia NG, Kaplanoglu E, Nasab A. Evaluation of artificial intelligence techniques in disease diagnosis and prediction. Discov Artif Intell. 2023;3(1):5.
Crossref - Colubri A, Silver T, Fradet T, Retzepi K, Fry B, Sabeti P. Transforming clinical data into actionable prognosis models: machine learning framework and field-deployable outcome of Ebola patients. PLoS Negl Trop Dis. 2016;10(3):e0004549.
Crossref - Cusumano-Towner M, Li DY, Tuo S, Krishnan G, Maslove DM. A social network of hospital-acquired infection built from electronic medical record data. J Am Med Inform Assoc. 2013;20(3):427-434.
Crossref - Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
Crossref - Meric G, Mageiros L, Pensar J, et al. Disease-associated genotypes of the commensal skin bacterium Staphylococcus epidermidis. Nat Commun. 2018;9(1):5034.
Crossref - Drew RJ, Murphy T, Broderick D, O’gorman J, Eogan M. An interpretation algorithm for molecular diagnosis of bacterial vaginosis in a maternity hospital using machine learning: proof-of-concept study. Diagn Microbiol Infect Dis. 2020;96(2):114950.
Crossref - Schork N. J. Artificial Intelligence and Personalized Medicine. In: Von Hoff, D., Han, H. (eds) Precision Medicine in Cancer Therapy. Cancer Treatment and Research, vol 178. Springer, Cham.
Crossref - Taherdoost H, Ghofrani A. AI’s role in revolutionizing personalized medicine by reshaping pharmacogenomics and drug therapy. Intelligent Pharmacy. 2019;2(5):643-650.
Crossref - Farhud DD, Zokaei S. Ethical Issues of Artificial Intelligence in Medicine and Healthcare. Iran J Public Health. 2021; 50(11):i-v.
Crossref - Singhal A, Neveditsin N, Tanveer H, Mago V. Toward Fairness, Accountability, Transparency, and Ethics in AI for Social Media and Health Care: Scoping Review. JMIR Med Inform. 2024;12:e50048.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.