ISSN: 0973-7510
E-ISSN: 2581-690X
Encystment (resting cyst formation), a state of dormancy with extreme tolerance of environmental stresses, is the standard survival strategy used by protists in response to unfavorable environmental changes. Here, we investigated the relative tolerances of resting cysts and vegetative cells of the colpodid ciliate Colpoda cucullus to exposure to surfactants. The effects of four different types of surfactant, namely, anionic surfactant (SDS), cationic surfactant (benzalkonium chloride), zwitterionic surfactant (CHAPS), and non-ionic surfactant (NP-40), were tested on Colpoda resting cysts and vegetative cells. We found that resting cysts showed tolerance levels to the surfactants that were more than 100 times higher than those of vegetative cells. This study provides updated information on the tolerance of resting cysts with regard to how they can adapt to environmental stresses and human influences. Additionally, our results highlight the importance of considering resting cysts when using detergents for cleaning, which is crucial for preventing infectious diseases and promoting the One Health initiative in our daily lives.
Dormancy, Detergent, Toxic Resistance, Infectious Disease, One Health, SDGs, Public Health
Protists are ubiquitous,1,2 and their typical strategy for surviving unfavorable environments is encystment; the formation of resting cysts3 is a reversible cell differentiation process that requires an antagonistic excystment.4 Resting cysts are in a state of dormancy and demonstrate extreme tolerance to various environmental stresses. The encystment process involves significant morphogenetic and physiological changes, which are regulated by genes.5 Previous research has focused on the tolerance of resting cysts in colpodid ciliates, i.e., the protist species forming resting cysts,6 to desiccation,7,8 high and low temperatures,9 freezing,10,11 UV irradiation,12,13 acids and alkalis,14,15 electrostatic exposure,16 high salinity,17 and gamma irradiation.18,19 However, their tolerance to surfactants is largely unknown. Although some studies have examined the toxicity of surfactants on protists and ciliates, including the genus Colpoda,20,21 these did not consider resting cysts.
Soil protists such as Colpoda are ubiquitous in the environment (approximately 170,000 cells per gram of soil)22 and are an important component of soil ecosystems.23,24 Resting cyst formation is an evolutionary strategy used in unfavourable environments as well as for protection against parasites and microbial infections. While protists generally play important and beneficial roles, some are infectious and harmful to human health, making them a global problem. For example, Giardia,25 Toxoplasma,26 and Entamoeba27 cause infectious diseases worldwide by infecting animals, including humans, while in a cystic state. The cyst-forming Naegleria fowleri is also known to cause the fatal disease primary amoebic meningoencephalitis.28 These microorganisms are present worldwide in many aquatic environments including the soil surface, puddles, ponds, lakes, rivers, and occasionally tap water and sewerage. In our daily lives, surfactants are used to clean our bodies and foods to inactivate and eliminate dirt. Therefore, understanding the tolerances of resting cysts to surfactants is essential for supporting disease prevention.
Chemicals
Four types of surfactant were used in our bioassay experiment (Table 1): an anionic surfactant, sodium dodecyl sulfate (SDS, Fujifilm Wako Pure Chemical Corporation, Osaka, Japan); a cationic surfactant, benzalkonium chloride (BAC, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan); a zwitterionic surfactant, 3-{dimethyl[3-(3α,7α,12α-trihydroxy-5β-cholan-24-amido) propyl]azaniumyl}propane-1-sulfonate (CHAPS; Dojindo Laboratories, Kumamoto, Japan); and a non-ionic surfactant, and polyethylene glycol mono (tert-octylphenyl) ether (Nonidet P-40, NP-40; Sigma Aldrich Japan, Tokyo, Japan). The critical micelle concentration (CMC) was taken from previous reports (Table 2).29-39
Culture and sample preparation
For the experiments, the Colpoda cucullus R2TTYS strain was grown in culture medium [0.05% (w/v) dried rice leaf infusion supplemented with 0.05% (w/v, final conc.) Na2HPO4]16 for 1.0-2 days, then washed twice by centrifugation (1500 g, 1 min) with the experiment medium [1 mM Tris-HCl (pH 7.2); Ex-medium]. Cell concentration was adjusted to a low cell density (<2000 cells/ml) for the toxicity test of vegetative cells. Encystment was induced by suspending cells at a high density (>10,000 cells/ml) in encystment-inducing medium [1 mM Tris-HCl (pH 7.2), 0.1 mM CaCl2, En-medium],40 while excystment was induced by replacing the En-medium with an excystment-inducing medium [0.2% (w/v) rice leaf infusion medium supplemented with 0.05% (w/v) NaH2PO4; Ex-medium].16 Cysts more than 1-week-old were used for when testing toxicity on resting cysts.
Toxicity testing of vegetative cells
Vegetative cell samples were prepared by suspending vegetative cells with Ex-medium at a low cell density, and 1 mL aliquots of the vegetative cell suspension were placed on watch glasses. Cell density was evaluated by counting the number of moving cells in a 10 µL aliquot of the sample under a stereo microscope (STEMi 305; Carl Zeiss Japan Co. Ltd., Tokyo, Japan). This procedure was carried out three times, and the average was taken as the cell density before exposure. The samples were incubated for 1 hour after dripping 100 × concentrated surfactant. After exposure to each surfactant, the cell density in the samples was evaluated, as described above. The survival rate was calculated as follows: mean cell density after exposure × (mean cell density before exposure)-1 × 100 (%).
Toxicity test for resting cysts
Resting cyst samples were prepared on glass depression slides. Samples were washed twice with En-medium and then incubated for 1 hour in En-medium containing a surfactant at various concentrations. After exposure, the samples were washed with Ex-medium and then incubated in Ex-medium for 12 hours. The toxicity of surfactants on resting cysts was determined as the excystment rate (%) after exposure, according to the method of Saito et al.41 Viable cysts excyst after excystment induction, but nonviable cysts do not. The excystment rate was calculated as follows: excystment (%) = (number of excysted cysts) × (number of excysted and non-excysted cysts)-1 × 100. Samples of excysted and non-excysted cells (>100 cells) were directly counted under the stereo microscope (Stemi 305).
Fluorescence microscope observation
Resting cyst samples were incubated for 1 hour in En-medium containing different concentrations of surfactant and also fluorescein (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) dissolved in dimethyl sulfoxide (DMSO) (final conc. 10-2 mg/mL fluorescein, 1% DMSO). After incubation, they were washed with En-medium and then analyzed under a fluorescence microscope (Axio Scope A1; Carl Zeiss Japan Co. Ltd.) using a 475 nm laser.
In this study, the tolerances of vegetative cells and resting cysts to four surfactants (Table 1) were examined. The CMCs of these four surfactants (Table 2) were obtained from previous reports. Note that the CMCs of surfactants vary depending on experimental conditions such as temperature.
Table (1):
Surfactants used in this study
Name |
Abbreviation |
Charge |
Structural formula |
|---|---|---|---|
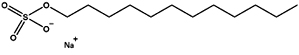
Sodium dodecyl sulfate |
SDS |
Anionic |
|
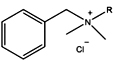
Benzalkonium chloride |
BAC |
Cationic |
|
3-{Dimethyl[3-(3α,7α,12α-trihydroxy-5β-cholan-24-amido)propyl]azaniumyl} propane-1-sulfonate |
CHAPS |
Zwitterionic |
|
Polyethylene glycol mono (tert-octylphenyl) ether |
NP-40 |
Non-ionic |
Table (2):
CMCs of the four surfactant types used in this study, including references. Molecular weight (MW) values with double asterisks were reported by Coligan et al.31 Values indicated by asterisks are taken from the matching reference papers, whereas those without asterisks were converted into units using the referenced values and MW. When a reference gives multiple values for different conditions, minimum and maximum values are indicated
| Surfactants | MW | CMC (mM) | CMC (w/v %) | *CMC Ref. |
|---|---|---|---|---|
| BAC | – | – | *1.0 × 10-3 – 2.0 × 10-2 | 29 |
| *<0.15 | – | 30 | ||
| SDS | **288 | 8.0 | *2.3 × 10-1 | 31 |
| *8.0 – 9.2 | 2.3 × 10-1 – 2.6 × 10-1 | 32 | ||
| *7.9 – 9.6 | 2.3 × 10-1 – 2.8 × 10-1 | 33 | ||
| *2.6 × 10-1 | 7.5 × 10-3 | 34 | ||
| *2.6 | 7.4 × 10-2 | 35 | ||
| NP-40 | **603 | 2.8 × 10-1 | *1.7 × 10-2 | 31 |
| 3.8 × 10-3 – 2.1 | *2.3 × 10-4 – 1.3 × 10-1 | 36 | ||
| CHAPS | **615 | 8.0 | *4.9 × 10-1 | 31 |
| *7.0 | 4.2 × 10-1 – 4.7 × 10-1 | 37 | ||
| *6.8 | 4.2 × 10-1 | 38 | ||
| *4.6 – 7.1 | 2.8 × 10-1 – 4.3 × 10-1 | 39 |
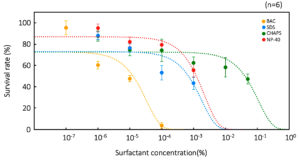
The effects of the surfactants on vegetative cells and resting cysts are shown in Figures 1 and 2, respectively. BAC had the largest effect on Colpoda vegetative cells, followed by SDS, NP-40, and CHAPS (Figure 1). The surfactant concentrations that inactivated 50.0% and 99.9% of the vegetative cells (In 50 and In 99.9, respectively) were calculated from the experimental data in Figure 1, as shown in Table 3. A previous study20 reported that the median effective concentration (EC50) of BAC in Colpoda aspera vegetative cells was 2.9 × 10-6 %, while In 50 in C. cucullus vegetative cells was 1.3 × 10-5 % (Table 3). Comparing the present study with the previous study,20 C. cucullus vegetative cells tolerate approximately a 4.5 times higher BAC concentration than those of C. aspera. On the other hand, the minimum inhibition concentration in A. faecealis is 1.5 × 10-4 %20 and is approximately 11.8 times higher than the In 50 of C. cucullus vegetative cells.
Table (3):
Summary of the tolerances of C. cucullus vegetative cells (‘C. cucullus veg’), C. cucullus resting cysts (‘C. cucullus cyst’), C. aspera vegetative cells (‘C. aspera veg’), and A. faecalis (‘A. faecalis’). The surfactant concentrations for inactivation of 50% of cells (‘In 50’), that for inactivation of 99.9% of cells (‘In 99.9’), and that for effects in 50% of cells were affected (‘EC 50’, defined by Kakiichi et al.) are shown, respectively. The values of In 50 and In 99.9 of C. cucullus vegetative cells and resting cysts were calculated from the data in Figure 1 and Figure 2, respectively (‘A’ – ‘D’). The data of C. aspera (‘F’) and A. faecalis and (‘G’) data were reported previously20. In addition, the values of C/A, D/B, A/F, A/G, and C/G are given
| Surfactants | C. cucullus veg (this study) | C. cucullus cyst (this study) | Kakiichi et al.20 | (C)/(A) |
(D)/(B) | (A)/(F) | (A)/(G) | (C)/(G) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) In 50 | (B) In 99.9 | (C) In 50 | (D) In 99.9 | (F) C. aspera veg EC50 | (G) A. faecalis EC50 | ||||||
| BAC (w/v %) | 1.3 × 10-5 | 2.2 × 10-4 | 1.8 × 10-3 | 4.4 × 10-3 | 2.9 × 10-6 | 1.5 × 10-4 | 1.4 × 102 | 19.8 | 4.4 | 8.5 × 10-2 | 11.7 |
| SDS (w/v %) | 7.0 × 10-4 | 1.2 × 10-2 | 1.5 | 8.7 | – | – | 2.2 × 103 | 7.2 × 102 | – | – | – |
| NP-40 (v/v %) | 1.2 × 10-3 | 1.5 × 10-2 | 1.8 × 104 | 5.2 × 1011 | – | – | 1.5 × 107 | 3.5 × 1013 | – | – | – |
| CHAPS (w/v %) | 4.1 × 10-2 | 7.2 × 10-1 | 10.8 | 6.8 × 102 | – | – | 2.6 × 102 | 9.5 × 102 | – | – | – |
Figure 1. Tolerances of vegetative cells to four surfactants: BCA, CHAPS, SDS, and NP-40. Points and error bars correspond to the means and standard errors, respectively, of six measurements. The lines in the figure show the equations of the approximation lines. The horizontal axis of graph is a logarithmic scale
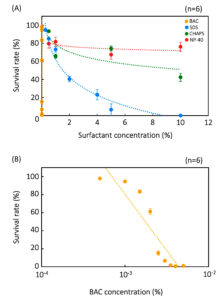
BAC is also the most effective surfactant on Colpoda resting cysts, followed by SDS, CHAPS, and NP-40 (Figure 2). The effects of BAC on resting cysts are too large to show in single graph with data from the other three surfactants (Figure 2A); hence, it is shown in a separate graph using a log scale horizontal axis (Figure 2B). Values for In 50 and In 99.9 of the resting cysts were also calculated from the experimental data in Figure 2, as shown in Table 3. By comparing the tolerances of vegetative cells and cysts, it is evident that the latter have a more than 102 fold greater tolerance than vegetative cells (Table 3). These results clearly indicate that Colpoda cells show surfactant tolerance through resting cyst formation.
Figure 2. Tolerance of resting cysts to four surfactants. The effects of BCA, CHAPS, SDS, and NP-40 are shown as single graph (A) and data of ‘BAC’ is magnified and displayed as another graph (B). Points and error bars correspond to the means and standard errors, respectively, of six measurements. The lines in the figure show the equations of the approximation lines. The horizontal axis of graph (B) is a logarithmic scale
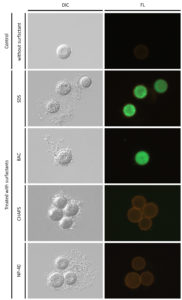
In a previous study,34 the toxicity effects of nonionic surfactants (Triton X-100 and monolaurin) and zwitterionic (DDPS), anionic (SDS), and cationic (CnTAB, C12PB, and C12BZK) surfactants on eukaryotic cells were examined. All the surfactants except cationic surfactants were cytotoxic at about their CMCs but cationic surfactants were cytotoxic at concentrations far below their CMCs. In this study, the four types of surfactant showed adverse effects on vegetative cells at less than their CMCs, i.e., In 50 values of the four surfactants were less than CMCs (Tables 2 and 3). In vegetative cells, the surfactants caused serious damage even at concentrations less than their CMCs, possibly due to cytological damage caused by surfactant absorption into the cell. On the other hand, resting cysts were affected at higher concentrations of CMCs. The values of In 50 were higher than their CMCs, although BAC affected resting cysts at similar levels of concentrations to CMCs (Table 2 and 3). These results suggest that absorption of surfactants by cell membranes and cyst walls is difficult, and that these membranous structures may be disrupted by the effect of micellar surfactants. The damage to cell membranes by the four types of surfactants was visualized using the fluorescent marker, fluorescein (Figure 3). Fluorescein passed through cell membranes in the presence of SDS and BAC but not in the presence of NP-40 and CHAPS, nor in the control without surfactants. This observation also supports the idea that the loss of tolerance in cysts is due to membrane destruction. Damage to the cell membrane likely occurred, with at least one factor involved in the loss of tolerance.
Figure 3. Morphological effects of four surfactants (5% SDS, 1 × 10-3 % BAC, 10% CHAPS, 10% NP-40) to Colpoda resting cysts. ‘Control’ is resting cysts suspended in the En-medium without any surfactants. The bar at bottom right is 50 µm. ‘DIC’ and ‘FL’ represent microphotographs by differential interference contrast microscopy and fluorescent microscopy
BAC, a cationic surfactant, showed effects on both vegetative cells and resting cysts at fairly low concentrations as the cell surface is generally negatively charged due to the presence of glycoproteins.42 On the other hand, the different membrane structures of vegetative cells and resting cysts17 possibly raised the tolerance of resting cysts to BAC.
Our results here showed that the efficacy of surfactants differs between vegetative cells and resting cysts. Ciliates have been used in toxicity tests as environmental or toxicity indicators,43 including for surfactants; however, these studies did not consider the possibility of higher tolerances of resting cysts compared to vegetative cells. Thus, the efficacy of surfactants on microorganisms, including protists, may be affected during cleaning of soiled items, such as vegetables or hands. This is of importance as some pathogenic species such as Giardia,25 Toxoplasma,26 Entamoeba,27 and N. fowleri,28 cause serious infectious diseases worldwide. For example, Entamoeba histolytica, which causes diarrheal disease, leads to 50 million infections and 100,000 deaths annually, often due to a lack of clean water and poor sanitation.44 The obligate intracellular protist parasite Toxoplasma gondii is found in one-third of the world population and causes toxoplasmosis, one of the most common food-borne parasitic zoonoses.45-47 Humans are infected by ingesting tissue cysts from meat, such as pork,46-48 or oocysts from inadequately washed vegetables or from the environment.45,47,49 Hence, this study’s findings are a crucial contribution to public health by improving water quality and sanitation, aligning with SDGs Goal 6. They also contribute to the One Health initiative in controlling disease, particularly those induced by cyst-forming protists. Our study also show that ciliates can be a useful tool for toxicity assessments beyond surfactants and environmental indicators, as their effectiveness against heavy metals has been demonstrated.50,51
In this study, we demonstrated that the efficacy of surfactants differs significantly between vegetative cells and resting cysts of Colpoda cucullus. We tested four different types of surfactants: SDS, BAC, CHAPS, and NP-40. For vegetative Colpoda cells, BAC had the largest effect, followed by SDS, NP-40, and CHAPS. For resting cysts, BAC is also the most effective surfactant, followed by SDS, CHAPS, and NP-40. Comparing the tolerances, resting cysts exhibited a greater than 100-fold higher tolerance than vegetative cells across all four surfactants. Morphological microscopic observation using fluorescent marker fluorescein supported that the lethal effect of high surfactant concentrations on resting cysts is likely due to cell membrane destruction. Our results highlight the importance of considering resting cysts when using detergents for cleaning. This is crucial for preventing infectious diseases and promoting the One Health initiative in our daily lives. Furthermore, these findings are a significant contribution to public health by improving water quality and sanitation, directly aligning with SDG Goal 6.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
YoiS conceptualized and supervised the study. HY, YutS, KY, TakS, SK, TatS, TaiS, RS and YoiS performed data curation. HY, SH, YoiS, YutS, KY, TakS, SK, TatS, TaiS, and RS performed formal analysis. HY, SH, NY, FS, YutS, KY, TakS, SK, TatS, TaiS, RS and YoiS performed Investigation and data validation. NY and SH performed visualization. FS and YoiS performed funding acquisition. YoiS wrote the original draft. HY, SH, and YutS contributed equally to this work. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by JSPS KAKENHI through grant numbers 19K16193, 22K06326, 23K27393, and 25K09711.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Finlay BJ, Esteban GF, Fenchel T. Protozoan Diversity: Converging Estimates of the Global Number of Free-Living Ciliate Species. Protist. 1998;149(1):29-37.
Crossref - Foissner W. Protist Diversity: Estimates of the Near-Imponderable. Protist. 1999;150(4):363-368.
Crossref - Li Y, Wang Y, Zhang S, Maurer-Alcala XX, Yan Y. How ciliated protists survive by cysts: some key points during encystment and excystment. Front Microbiol. 2022;13:785502.
Crossref - Venrni F, Rosati G. Resting cysts: A survival strategy in Protozoa Ciliophora. Ital J Zool. 2011;78(2):134-145.
Crossref - Sogame Y, Saito R, Hakozaki S. Resting Cyst Formation as a Strategy for Environmental Adaptation in Colpodid Ciliates. Zool Sci. 2025;42(1):96-106.
Crossref - Lynn DH. The ciliated protozoa 3rd Eds. Springer, Dordrecht Heidelberg London New York. 2008.
- Corliss JO, Esse SC. Comments on the role of the cyst in the life cycle and survival of free-living protozoa. Trans Am Micros Soc. 1974;93(4):578-593.
Crossref - Goodey T. Note on the remarkable retention of vitality by protozoa from old stored soils. Ann Appl Biol. 1915;1(3-4):395-399.
Crossref - Taylor CV, Strickland AGR. Effects of high vacua and extreme temperatures on cysts of Colpoda cucullus. Physiol Zool. 1936;9(1):15-26.
- Uspenskaya ZI, Lozina-Lozinsky LK. Antigen rearrangements in Colpoda maupasi cells after freezing at -196 °C, and after shortwave ultraviolet irradiation. Cryobiology. 1979;16(6):542-549.
Crossref - Matsuoka T, Sogame Y, Nakamura R, Hasegawa Y, Arikawa M, Suizu F. Antifreeze water-rich dormant cysts of the terrestrial ciliate Colpoda cucullus Nag-1 at -65 °C: Possible involvement of ultra-antifreeze polysaccharides. Acta Protozool. 2020;59(3-4):141-147.
Crossref - Matsuoka K, Funadani R, Matsuoka T. Tolerance of Colpoda cucullus resting cysts to ultraviolet irradiation. J Protozool Res. 2017;27(1-2):1-7.
- Yamane S, Watanabe M, Funadani R, et al. Tolerance of Colpoda cucullus Nag-1 resting cysts and presumed structure for protection against UV light. Acta Protozool. 2020;59(1):55-60.
Crossref - SogameY, Kida A, Matsuoka T. Possible involvement of endocyst in tolerance of the resting cyst of Colpoda cucullus against HCl. Afr J Microbiol Res. 2011;5(25):4316-4320.
Crossref - Nakamura, R, Sogame Y, Arikawa M, Suizu F, Matsuoka T. Tolerance of Colpoda cucullus Nag-1 wet resting cysts to extreme pH (pH 1 and 13): Implications of less permeability of the cyst membrane to H+ and OH–. J Protozool Res. 2020;30(1-2):38-46.
- Saito T, Yabuki Y, Saito Y, et al. Comparison of the relative tolerance of Colpoda resting cysts and vegetative cells to electrostatic exposure. J Protozool Res. 2023;33:32-42.
- Saito R, Yamanobe H, Yabuki K, et al. Salinity tolerance in resting cysts of colpodid ciliates: Comparative transcriptomics analysis and chemical analysis of cyst walls to investigate their tolerance capability. Curr Res Microbial Sci. 2025;8:100371.
Crossref - Saito R, Koizumi R, Sakai T, Ono T, Sogme Y. Gamma radiation tolerance and protein carbonylation caused by irradiation of resting cysts in the free-living ciliated protist Colpoda cucullus. Acta Protozool. 2020a;59(2):67-75.
Crossref - Saito R, Sakai T, Koizumi R, et al. Comparison of the morphology and viability of gamma irradiated vegetative cells, wet cysts, and dry cysts of the soil ciliate Colpoda cucullus. J Protozool Res. 2020b;30:20-30.
- Kakiichi N, Saito M, Yayamoto T, et al. Toxicity Test for Invert Soap against Ciliate Colpoda aspera. Nihon Chikusan Gakkaiho. 1991;62(12):1113-1119.
Crossref - Kakiichi N, Yamamoto A, Kamata S, Otsuka H, Uchida K. Toxicity Compounds of Research Note Amphoteric Surfactant, and Orthodichlorobenzene Biguanide Preparation Colpoda aspera against Ciliate Anim. Sci Technol. 1993;64(10):1013-1016.
Crossref - Vargas R, Hattori T. The distribution of protozoa among soil aggregates. FEMS Microbiol Lett. 1990;74(1):73-77.
Crossref - Singer D, Seppey CVW, Lentendu G, et al. Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems. Environ Int. 2021;146:106262.
Crossref - Li H, Wu K, Feng Y, et al. Integrative analyses on the ciliates Colpoda illuminate the life history evolution of soil microorganisms. mSystems. 2024;9(6):e01379-23.
Crossref - Lujan HD, Mowatt MR, Nash TE. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol Mol Biol Rev. 1997;61(3):294-304.
Crossref - Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11(2):267-299.
Crossref - Manna D, Ehrenkaufer GM, Lozano-Amado D, Singh U. Entamoeba stage conversion: progress and new insights. Curr Opin Microbiol. 2020;58:62-68.
Crossref - Maciver SK, Pinero JE, Lorenzo-Morales J. Is Naegleria fowleri an Emerging Parasite? Trends in Parasitol. 2020;36(1):19-28.
Crossref - Georgiev GA, Yokoi N, Koev K, et al. Surface chemistry study of the interactions of benzalkonium chloride with films of meibum, corneal cells lipids, and whole tears. Invest Ophthalmol Vis Sci. 2011;52(7):4645-4654.
Crossref - Mathioudakis GN, Soto Beobide A, Bokias G, Koutsoukos PG, Voyiatzis GA. Surface-enhanced Raman scattering as a tool to study cationic surfactants exhibiting low critical micelle concentration. J Raman Spectrosc. 2020;51(3):452-460.
Crossref - Coligan, JE, Commonly Used Detergents. Curr Protoc Protein Sci. 1998;11: A.1B.1-A.1B.3.
Crossref - Marcolongo JP, Mirenda M. Thermodynamics of Sodium Dodecyl Sulfate (SDS) Micellization: An Undergraduate Laboratory Experiment. J Chem Edu. 2011; 88(5):629-633.
Crossref - Shah SS, Jamroz NU, Sharif QM. Micellization parameters and electrostatic interactions in micellar solution of sodium dodecyl sulfate (SDS) at different temperatures. Colloids Surf A. 2001;178(1-3):199-206.
Crossref - Inacio AS, Mesquita KA, Baptista M, Ramalho-Santos J, Vaz WL, Vieira OV. In vitro surfactant structure-toxicity relationships: implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One. 2011;6(5):e19850.
Crossref - Brito RM, Vaz WL. Determination of the critical micelle concentration of surfactants using the fluorescent probe N-phenyl-1-naphthylamine. Anal Biochem. 1986;152(2):250-255.
Crossref - Malik WU, Chand P, Saleem SM. Determination of critical micelle concentration of non-ionic surfactants by electrocapillary curves. Talanta. 1968;15(1):133-136.
Crossref - Kroflie A, Sarac B, Bester-Rogae M. Thermodynamic Characterization of 3-[(3-Cholamidopropyl)-dimethylammonium]-1-propanesulfonate (CHAPS) Micellization Using Isothermal Titration Calorimetry: Temperature, Salt, and pH Dependence. Langmuir. 2012;28(28):10363-10371.
Crossref - Merchan MD, Velazquez MM. Properties of CHAPS micelles modulated by different polyelectrolytes. Colloids Surf A Physicochem Eng Asp. 2020;366(1-3):12-17.
Crossref - Giacomelli CE, Vermeer AWP, Norde W. Micellization and Adsorption Characteristics of CHAPS. Langmuir. 2000;16(11)4853-4858.
Crossref - Hakozaki S, Yamanobe H, Yabuki K, et al. ATP accumulation in early resting cyst formation towards cryptobiosis in Colpoda cucullus. Acta Protzool. 2023;62:39-44.
Crossref - Saito R, Koizumi R, Sakai T, et al. Recovery of proliferative capability in gamma irradiated Colpoda cucullus (ciliated protist) resting cysts and its radiation hormesis. Protistology. 2020;14(3):160-171.
Crossref - Jones MN. Surfactants in membrane solubilisation. Int J Pharm. 1999;177(2):137-159.
Crossref - Persoone G, Dive D. Toxicity tests on ciliates-A short review. Ecotox Environ Safe. 1978;2(2):105-114.
Crossref - Gupta P, Singh KK, Balodhi A, Jain K, Deeba F, Salam N. Prevalence of amoebiasis and associated complications in India: A systematic review. Acta Parasitol. 2022;67(2):947-961.
Crossref - Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12-13):1217-1258.
Crossref - Moudgil P, Pandita S, Kumar R, et al. Seroprevalence and risk factors associated with Toxoplasma gondii in pigs in Haryana, India. 2024;71(2) 136-143.
Crossref - Deshmukh AS, Hebbar BK, Mitra P, et al. Seroprevalence and risk factors of Toxoplasma gondii infection among veterinary personnel and abattoir workers in Central India. Parasitol Int. 2021;84:102402.
Crossref - Thakur R, Sharma R, Aulakh RS, Gill JPS, Singh BB. Prevalence, molecular detection and risk factors investigation for the occurrence of Toxoplasma gondii in slaughter pigs in North India. BMC Vet Res. 2019;15(1):431.
Crossref - Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634-640.
Crossref - Somasundaram S, Abraham JS, Maurya S, Toteja R, Gupta R, Makhija S. Molecular characterization and transcriptional modulation of stress-responsive genes under heavy metal stress in freshwater ciliate, Euplotes aediculatus. Ecotoxicology. 2022;31(2):271-288.
Crossref - Varatharajan GR, Calisi A, Kumar S, Bharti D, Dondero F, La Terza A. Cytotoxicity and Antioxidant Defences in Euplotes aediculatus exposed to single and binary mixtures of heavy metals and nanoparticles. Appl Sci. 2024;14(12):5058.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.