ISSN: 0973-7510
E-ISSN: 2581-690X
Plant growth-promoting rhizobacteria (PGPR), which were isolated from the plant rhizosphere, decrease the addition of chemical fertilizer (N) and promote plant growth. Some PGPR isolates can fixate nitrogen, solubilize phosphate, produce phytohormones, and control soil pathogens. This study has focused on isolating rhizobacteria from root nodules of Edamame, Glycine max L, Dieng Peanut, Solanum sp. and Peanut root. Eleven isolates were assayed to examine their activities, including the ability of nitrogen fixation, produce Indol Acetic Acid hormone, siderophore, ammonia, and catalase activity. Furthermore, 11 isolates were tested to promote soybean growth in pot experiments using sterile sand media and the test of symbiotic capacity. The results showed that six isolates (RhizE2, RhizE3, RhizE4, RhizKdKbm1, Bio2DW, and Bio3DW) could form root nodules, and four isolates (RhizE2, RhizE3, RhizKdKbm1, and Bio2DW) showed a symbiotic capacity of more than 90%. RhizE2 significantly increased the number of nodules and dry weight of the entire plant (24.08 g) in comparison with plants without inoculation and added N fertilizer (11.59 g) and added N fertilizer (19.60 g). RhizE2 can be further developed as a biological fertilizer agent, especially for soybean plants. From all parameters observed, the plants inoculated with RhizE2 showed the best growth result.
Nitrogen Fixation, Nodulation, Rhizobacteria Soybean, Sterile Sand Medium
Soybeans are one of the plants included in the family of legumes with high nutritional value, as these plants contain high vegetable protein that ranges from around 38-42%. However, soybean production only fulfilled 35.1% of the community’s demands.1 Therefore, productivity needs to be improved to foster increased production. Further, providing chemical fertilizers can improve soybeans’ quality, but it comes with higher money, thus an alternative fertilizer that is more affordable is sought after, and this can be the use of biological fertilizers. It has many benefits besides being affordable, harmless, having no side effects, and does not bring pollution to the environment. It can also maintain soil fertility, which can increase crop production.2,3 Soybean plants are able to carry out symbiosis with plants. Effective symbiosis between rhizobia and legume plants depends on Nod factors, flavonoids, bacterial surface polysaccharides and plant lectins. Symbiotic nodulation genes can be identified and used as genetic markers to determine host specificity and symbiotic diversity. The abundance of rhizobacteria in native soil samples can play an important role in nodulation and also act as an indicator of the influence of rhizobial populations on their symbiotic plants. There is a serious need to explore biological fertilizers with more varied specifications such as geographical acclimatization to optimize crop yields while maintaining efficacy and shelf life to reduce the use of chemical fertilizers.4
Plant-growth-promoting rhizobacteria (PGPR) are a group of bacteria that can be used as a biological fertilizer. These bacteria are found in the area of the rhizosphere or on the root surface, or associated with the roots of the plant.5,6 They can support plant growth directly through Nitrogen fixing from the atmosphere, mineral dissolution, such as phosphate, siderophores production, and growth hormone syntheses, such as Indole Acetic Acid (IAA), gibberellin acid, cytokines, and ethylene,7 besides that the PGPR application is useful in accelerating seed germination and seedling growth.8
Rhizobium bacteria is one of the bacteria that can live in the soil or inside a root nodule formed from the symbiotic mutualism with the host plant.9 One Rhizobium species is incapable of spinning in every legume plant. As such, Rhizobium is categorized into the cross-inoculation group, where each group consists of Rhizobium species capable of forming root nodules, with legume species originating from the same group.10 Rhizobium associated with legume crops can be fixed at 100-300 kg N/ha in one growing season and would leave a substantial amount of nitrogen for the next crop. Also, it can supply 80% of the legume plant nitrogen requirement and increase production by 10-25%. Rhizobium varies greatly depending on the soil condition, the environment, and the effectiveness of the population of microorganisms in the soil.11,12 The growth of Rhizobium bacteria is influenced by the availability of nutrients in the rooting environment. Nutrient excess and deficiency will also affect the growth of Rhizobium and nitrogen fixation.13,14 The successful use of Rhizobium on soybean is dependent on the effective formation of symbiosis with bacterial root nodules, resulting in a mutualistic symbiosis, which will be able to meet the nitrogen needs for growth and ultimately improve yield.11,15 In addition, inoculation of legume seeds with effective Rhizobium strains is an advantage of using biological nitrogen fertilizers, it is expected to reduce chemical fertilizers and increase yields.16
This research aimed to screen the Rhizobium isolates capable of forming the root nodule and fixing Nitrogen effectively and efficiently, which can further increase growth and ultimately lead to an increase in the crop yield, particularly in the case of soybean.
Bacterial isolation
Eleven Rhizobacteria were collected from various plants, that included: RhizE1, RhizE2, RhizE3, RhizE4, and RhizE5, which were root nodules of Edamame plant from Cisarua, Bogor, West Java, Indonesia (S 6° 41’18,2″, E 106° 56′ 55,1″), RhizKdKbm which was root nodules of Glycine max L. plant from Kebumen, Central Java, Indonesia (S 7° 44’41,1″, E 109° 33′ 01,1″), Bio20R which was root nodules of Glycine max L. plant from Jember, East Java, Indonesia (S 8° 09’39,7″, E 113° 39′ 41,3″), Bio22R which was root nodules of Glycine max L plant from Citayam, Bogor, West Java, Indonesia (S 6° 26’26,5″, E 106° 47′ 48,0″), Bio1DW which was Rhizosphere of Dieng Peanut plant, Bio2DW rhizosphere of Solanum plant, as well as Bio3DW which was Dieng Peanut root nodules from Dieng, Central Java, Indonesia (S 7° 12’58,2″, E 109° 54′ 17,7″).
Characterization of rhizobium
The Rhizobacteria isolates were first characterized and tested for their activity as PGPR (Plant Growth Promoting Rhizobacteria). Characterization was performed by growing bacterial isolates on a YEMA (Yeast Extract Mannitol Agar) medium with the composition of: 0.5 gL-1 K2HPO4, 0.2 gL-1MgSO4.7H2O, 0.1 gL-1 NaCl, 3 gL-1 CaCO3, 10 gL-1 Mannitol, 3 gL-1 Yeast extract, 20 gL-1 Agar, 1000 mL dH2O, pH 6.8.17 The growth media was added by BTB (Brom Thymol Blue) on a Petri dish plate. Then incubated at room temperature (27-28°C), the yellow colonies were considered fast-growing group colonies, while the blue was slow growing group colony.18
Production of siderophores
Siderophore production test that used selective media was Chrome Azurol Sulfate (CAS) Agar consisting of: blue dye (0.06 gL-1 chrome azurol in 50 mL dH2O), 0.0027 gL-1 FeCl3 – 6H2O (in 10 mM HCl), 0.073 gL-1 HDTMA (in 40 mL dH2O), D-glucose (20 g L-1 in 100 mL dH2O), casamino acid (5 gL-1 in 45 mL dH2O + 1.35 gL-1 hydroxyquinoline in 45 mL chloroform), MM9 Medium (5 gL-1 KH2PO4, 25 gL-1 NaCl, 50 gL-1 NH4Cl in 500 mL dH2O). The CAS medium (75 mL dH2O + 10 mL MM9 Medium + 3.024 gL-1 PIPES + 1.5 gL-1 bacto agar in autoclave) + 3 mL casamino + 1 mL glucose + 10 mL blue dye).
Bacterial isolates were inoculated on the Petri dish containing CAS media agar with the bacteria, which was then incubated at room temperature (27-28°C). The formation of a zone with the color of orange or brown around bacterial colonies will indicate the positive result of forming a siderophore.19
Indole acetic acid production
The IAA production test was done using TSA (Tryptone soya agar), consisting of 10 gL-1 peptones, 2.5 gL-1 NaCl, 22 gL-1 Agar, and 1000 mL dH2O. Bacterial isolates were inoculated at the center of the Petri dish, which had been filled with the media mentioned above, then incubated at room temperature (27-28°C) for 2-5 days, the colony color to white.
Bacterial colonies that had grown in TSA media were dripped by approximately 1 mL of Salkowvsky solution (consisting of 1 mL 0.5 M FeCl3 + 50 mL 50% HClO4) to cover the colonies, then were incubated in the dark room for approximately 1 hour. A positive (+) result was indicated by a change from color white to color pink, indicating that these isolates were able to produce IAA. Positive isolates were then further assessed quantitatively for their ability to produce IAA using a spectrophotometer.20
Ammonia production
Bacterial isolates were tested for their ability to produce ammonia by the following methods.21 Isolates were grown in a liquid NB medium (5 g of bacto peptone and 3 g of beef extract) in the reaction tube and along with a blank NB medium (NB medium without the isolates) incubated for 48 hours, then added 1.25 mL Nessler reagent, with a medium color change containing bacterial isolates to brownish orange.
Catalase test
One loopful bacterial isolate was placed in the glass object, dropped with 3% H2O2 solution, and mixed gently. A positive catalase was characterized by the formation of bubbles of oxygen (O2) as a result of a breakdown of the H2O2 by the enzyme catalase produced by bacteria.22
The activity test of nitrogen fixation
The ability of the bacteria to fix nitrogen was tested using a semi-solid NFB (Nitrogen Free Medium),23 which consisted of: 5.0 gL-1 malic acid, 4.0 gL-1 KOH, 0.5 gL-1 K2HPO4, 0.5 gL-1 FeSO4 – 7H2O, 0.01 gL-1 MnSO4 – H2O, 0.01 gL-1 MgSO4 – 7H20, 0.1 gL-1 NaCl, 0.02 gL-1 CaCl2, Na2MoO4 – 2H2O 0.002 gL-1, 4 mL 1.64% Fe-EDTA, 4 gL-1 KOH, 1 mL Vit solution, 2 mL microelements, 2 mL BTB (0.5% alcoholic solution), 22 gL-1 agar and 1000 mL dH2O. Bacterial isolates were grown in the semi-solid NFB media in a small test tube and incubated at room temperature (27-28°C) for 2-7 days. A positive result was characterized by forming a white ring on the surface of the media, indicating that these isolates were able to fix the nitrogen.
Effect of rhizobium bacteria on the growth of Glycine max L in greenhouse
The study was conducted in the greenhouse field of Microbiology, Research Center for Biology-LIPI. This study used sand as plant growth media from Pelabuhan Ratu (Ratu Harbour), West Java. The sand media is washed repeatedly until clean, then sterile, put in 0.5 gL-1 gallon plastic pots, and a total of 1.8 kg of sterile sand as a medium grows. The seeds were then grown on top of it. As the seed cover was planted, sterile sand mixed with paraffin and benzol (sand: paraffin: benzol = 20 kg:100 gL-1:100 mL) as high as 2 cm was added as the seed cover has planted the seeds used were soybean from the Anjasmoro varieties.
The eleven bacterial isolates were used in the research. There were two variable controls, plants without inoculation and without added by N fertilizer (K1); and plants without inoculation and added by N fertilizer (dosage 100 Kg/ha) (K2). The design used was a Completely Randomized Design, with each treatment going through three replications.
The bacterial isolate in the test tube was added with 10 ml of sterile distilled water, then mixed until dissolved. Then transferred to another sterile test tube and vortexed until homogeneous. After that, the bacterial isolate solution was injected to the germinated soybean seeds and soaked for one hour. Then the soybean seeds were planted.
Plants were harvested at 45 days old. The observed parameters included: height, leaves, dry weight of the canopy, roots, wet weight of nodules, and dry weight of total plants. To maintain moisture (24%), plants were watered every day using the nutrient solution without N bound, as done by Saono et al.24 The composition of nutrient solution (10 liters of solution A + 10 mL of solution B + 10 mL of solution C + 10 mL solution of D) are shown in Table 1. To determine the symbiotic capacity (Sc) on inoculation of Rhizobium, the following is applied Brockwell et al.25 Equation.
Sc = I – U / N – U
Note:
Sc: symbioses ability, I: averages shoot dry weight of the plants were inoculated, U: the average dry weight of plants without inoculated and without N (K1), N: average plant dry weight without inoculated and N (K2),
Sc values were divided into four categories: VE (very effective) if Sc > 0.67, e (effective) if 0.33 < Sc < 0.67, le (less effective) if Sc < 0.33 and I (not effective) if Sc < 0.
The effectiveness can be measured by comparing the total plant dry weight tested with what the plant dry weight control plus with N fertilizer (K2) was, and this was expressed through a percentage as proposed by Date.26 Data Analysis Values performed using Duncan Test followed by the same letter in each column are not significantly different (p=0.05).
Table (1):
The composition of nutrient solution.
Solution |
The element solution contained |
Amount |
|---|---|---|
A (Standard solution of Calcium Sulfur) |
CaSO42H2O MgSO47H2O dH2O |
2.5 g 2.5 g 10 L |
B (Standard solution of Ferric Citrate) |
Ferric Citrate Sterile distilled water |
30 g 1 L |
C (Standard solution of Fosfat) |
KH2PO4 KOH dH2O |
34 g 1.96 g 1 L |
D (Standard solution of Trice element) |
MnSO4H2O ZnSO45H2O CuSO45H2O NaMoO22H2O H3BO3 CaCl26H2O dH2O |
1 g 0.25 g 0.25 g 0.06 g 0.50 g 0.05 g 1 L |
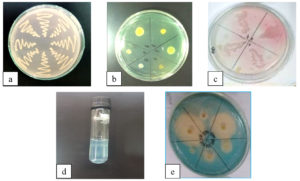
Images of the test carried out, including the control culture Rhizobium + CR are shown in Figure. Of the eleven isolates, two isolates were included in the slowly growing group characterized by blue colonies, and nine isolates belonged to the fast-growing group indicated with yellow color (Figure B). Isolates grown in siderophores showed that eight isolates showed positive results with clear zones (Figure D), indicating that the isolates were capable of producing siderophores.
For isolates grown in Tryptone Soya Agar (TSA) media for IAA, assays showed that six isolates were positive (+), indicating that the isolates could produce IAA characterized by colony color, which changed from white to pink (Figure C). Isolates grown in NB liquid media for ammonia production showed that all reacted positively, and there was a change of media color to brownish shades. The colony color changed from white to brownish-yellow isolates, indicating that they were capable produce ammonia. All isolates reacted positively, which showed they could produce catalase enzyme. Whereas for the ability of N isolates in Nitrogen Free Medium (NFB) Media, there were four isolates capable of forming a white ring under the media surface, and there was a change of media color from green to bluish-green color (Figure E). This indicates that the isolates can fix nitrogen (Table 2).
Table (2):
Characterization and test results of production activities siderophores, IAA, ammonia, catalase activity and the ability to qualitatively nitrogen fixative of eleven isolates of Rhizobium.
| Strain of bacteria | BTB | Siderophores production | IAA production | Ammonia production | Catalase activity | N Fixation test | |
|---|---|---|---|---|---|---|---|
| growth | colour | ||||||
| Rhiz E 1 | fast | yellow | – | – | + | + | + |
| Rhiz E 2 | fast | yellow | – | – | + | + | + |
| Rhiz E 3 | fast | yellow | + | – | + | + | + |
| Rhiz E 4 | fast | yellow | + | – | + | + | – |
| Rhiz E 5 | fast | yellow | + | – | + | + | – |
| Rhiz Kd.Kbm | fast | yellow | + | + | + | + | + |
| Bio 20 R | fast | yellow | + | + | + | + | – |
| Bio 12 R | fast | yellow | + | + | + | + | – |
| Bio 1 DW | slow | blue | + | + | + | + | – |
| Bio 2 DW | slow | blue | + | + | + | + | – |
| Bio 3 DW | fast | yellow | – | + | + | + | + |
Note: + = has the ability to produce siderophores, IAA, ammonia, having catalase activity and is able to perform nitrogen fixation.
Figure. Pure culture of Rhizobium + CR (a), Rhizobium+BTB (b), qualitative test IAA hormone (c), N fixation (d), and sidorophore (e)
Tests for symbiotic capacity showed that the inoculated Rhizobium isolates had a very effective effect (plants inoculated with Rhiz2, Rhiz3, RhizKdKbm1, and Bio2DW isolates), and which were effective (plants inoculated with RhizE1, Rhiz4 isolates, Rhiz5, Bio20R, Bio12R, Bio1DW, and Bio3DW). This shows that these isolates can infect plant roots, to form root nodules which ultimately can increase plant growth (Table 3).
Table (3):
Value “Symbiotic Capacity” and the percentage of inoculated Rhizobium isolates the effectiveness of the Glycine max L crop.
| Treatment | Symbiotic Capacity | The level of effectiveness (%) | |
|---|---|---|---|
| Real | Relative | ||
| Rhiz E 1 | e | 0.42 | 76.36 |
| Rhiz E 2 | VE | 1.08 | 122.85 |
| Rhiz E 3 | VE | 0.90 | 108.92 |
| Rhiz E 4 | e | 0.42 | 100.28 |
| Rhiz E 5 | e | 0.42 | 76.77 |
| Rhiz Kd Kbm | VE | 0.87 | 106.37 |
| Bio 20 R | e | 0.58 | 83.43 |
| Bio 12 R | e | 0.47 | 79.52 |
| Bio 1 DW | e | 0.66 | 86.51 |
| Bio 2 DW | VE | 0.70 | 91.22 |
| Bio 3 DW | e | 0.62 | 84.05 |
| K1 | 0 | 0 | 0 |
| K2 | 0 | 0 | 0 |
Note: VE = very effective, e = effective
One of the morphological responses was plant height. The measured heights were: the highest plants height in the first week were those inoculated with Bio1DW, the highest plants height at the second week were those inoculated with Bio1DW, the highest plants height at the third week were those inoculated with Bio12R, the highest plants height at fourth and fifth week were which inoculated with RhizE2, and the highest plants height at sixth week were which inoculated with Bio2DW (Table 4).
Table (4):
Average height of plant growth soybean plants inoculated with Rhizobium isolates.
| Treatment | High of plants (Age) (weeks) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Rhiz E 1 | 11.00 cd | 31.67 de | 55.67 ab | 83.00 ab | 88.67 b | 96.00 bc |
| Rhiz E 2 | 10.00 cd | 29.67 cde | 58.00 bc | 92.67 c | 99.33 e | 96.67 bc |
| Rhiz E 3 | 11.00 cd | 25.33 ab | 59.00 bc | 79.00 ab | 90.33 bc | 97.00 bcd |
| Rhiz E 4 | 11.33 cd | 28.67 bcde | 59.33 c | 82.00 ab | 92.67 bcd | 99.00 bcde |
| Rhiz E 5 | 9.00 ab | 25.00 a | 54.33 a | 78.33 a | 88.33 b | 101.67 cde |
| Rhiz Kd Kbm | 9.67 bc | 29.00 cde | 58.67 bc | 89.67 c | 94.00 cd | 102.00 cde |
| Bio 20 R | 10.67 bcd | 28.00 abcd | 59.00 bc | 82.33 ab | 92.67 bcd | 103.00 def |
| Bio 12 R | 11.00 cd | 29.00 cde | 59.67 c | 84.00 ab | 91.00 bc | 103.33 ef |
| Bio 1 DW | 11.67 d | 32.00 e | 58.67 bc | 90.00 c | 94.00 cd | 104.33 ef |
| Bio 2 DW | 10.00 bcd | 30.00 de | 59.33 c | 84.00 ab | 96.00 de | 109.00 f |
| Bio 3 DW | 9.00 ab | 29.00 cde | 58.00 bc | 81.67 ab | 93.67 bcd | 116.67 g |
| K1 | 7.67 a | 26.33 abc | 53.33 a | 80.00 ab | 83.67 a | 85.00 a |
| K2 | 11.00 cd | 31.33 de | 59.67 c | 84.33 b | 93.67 bcd | 105.00 ef |
Note: Values followed by the same letter in each column are not significantly different at the 0.05 level test performed by Duncan
In addition to plant height, the morphological response was the number of leaves. The number of leaves at one week of age, the highest value in plants inoculated were those with Bio1DW, Bio2DW, and Bio3DW, at age 2, 3, and 4 weeks of the highest value in plants inoculated were those with Bio3DW, at age five weeks of the highest value in plants inoculated were those with RhizE2 and RhizE3. At six weeks of age, the highest value in plants inoculated were those with RhizE2 (Table 5).
Table (5):
Average number of leaves, chlorophyll and diameter growth leaves of soybean plants inoculated with Rhizobium isolates.
| Treatment | The number of leaves (age) (weeks) | Chlorophyll |
Leaf diameter |
|||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | |||
| Rhiz E 1 | 7.33 abc | 7.67 abc | 9.67 b | 13.67 cd | 20.43 a | 5.50 abc |
| Rhiz E 2 | 8.00 cd | 8.00 abc | 9.67 b | 14.67 d | 34.07 d | 6.83 c |
| Rhiz E 3 | 7.67 bcd | 8.00 abc | 9.67 b | 14.67 d | 31.77 cd | 6.83 c |
| Rhiz E 4 | 7.67 bcd | 8.67 abc | 10.00 bc | 13.00 bcd | 25.69 b | 5.67 abc |
| Rhiz E 5 | 7.00 ab | 8.67 abc | 10.33 bcd | 11.67 abc | 21.69 a | 5.33 ab |
| Rhiz Kd Kbm | 8.00 cd | 8.67 abc | 10.67 bcd | 12.00 bc | 27.03 b | 5.83 bc |
| Bio 20 R | 6.67 a | 9.00 bc | 10.67 bcd | 11.33 ab | 21.17 a | 5.33 ab |
| Bio 12 R | 7.67 bcd | 9.00 bc | 10.67 bcd | 12.00 bc | 20.45 a | 5.33 ab |
| Bio 1 DW | 8.33 d | 9.00 bc | 11.33 cde | 12.67 bcd | 20.11 a | 5.33 ab |
| Bio 2 DW | 8.33 d | 9.33 bc | 11.67 de | 11.33 ab | 21.51 a | 6.00 b |
| Bio 3 DW | 8.33 d | 9.67 c | 12.33 e | 12.33 bc | 25.33 b | 5.83 bc |
| K1 | 6.67 a | 6.67 a | 8.00 a | 9.67 a | 19.15 a | 5.17 a |
| K2 | 7.67 bcd | 7.33 ab | 9.33 ab | 14.67 d | 30.63 c | 7.17 c |
Note: The values followed by the same letter in each column are not significantly different at the 0.05 level test of Duncan
For the highest dry weight of canopy in the plant inoculated with RhizE2, the highest dry weight of roots in plants inoculated were those with RhizKdKbm1, the wet weight of root nodules in the plants inoculated with RhizE2, and the dry weight of whole plants were those inoculated with RhizE2. Of the 11 isolates of Rhizobium inoculated, six isolates were able to form root nodules. This indicated that the isolates included were highly effective and capable of increasing the growth of soybean crops (Table 6).
Table (6):
Average value shoot dry weight (sdw), roots (r), wet weight of nodules (wwn) and total plant dry weight (dw) growth of soybean plants inoculated with Rhizobium isolates.
Treatment |
sdw |
r |
wwn |
dw |
|---|---|---|---|---|
Rhiz E 1 |
12.79 b |
2.18 ab |
0.00 a |
14.97 b |
Rhiz E 2 |
17.29 h |
3.12 de |
3.67 c |
24.08 f |
Rhiz E 3 |
16.08 gh |
2.53 bcde |
2.63 b |
21.35 e |
Rhiz E 4 |
15.42 efg |
2.41 bc |
1.81 b |
19.66 e |
Rhiz E 5 |
12.80 b |
2.24 abc |
0.00 a |
15.05 b |
Rhiz Kd Kbm |
15.62 fg |
3.17 e |
2.05 b |
20.85 e |
Bio 20 R |
13.86 bcd |
2.48 bcd |
0.00 a |
16.35 bcd |
Bio 12 R |
13.13 bc |
2.24 abc |
0.00 a |
15.59 bc |
Bio 1 DW |
14.44 cdef |
2.16 ab |
0.00 a |
16.94 cd |
Bio 2 DW |
14.66 def |
2.69 bcde |
0.52 a |
17.88 d |
Bio 3 DW |
14.17 cde |
2.30 abc |
0.72 a |
16.47 bcd |
K1 |
9.89 a |
1.67 a |
0 00 a |
11.59 a |
K2 |
16.733 gh |
2.86 cde |
0 00 a |
19.60 e |
Note: Values followed by the same letter in each column are not significantly different at the 0.05 level test of Duncan
Fast-growing Rhizobium, characterized by a change in color to yellow, is caused by the production of acids or bases in the media. Yema + BTB media is used to classify bacteria that have fast growth (yellow color) and slow growth (blue color) based on the production of acids and bases in the media.27 Slow-growing isolates were not always ineffective, and fast-growing isolates did not always prove effective. As such, this requires further research. Based on the results of the siderophore detection test were able to form a clear zone formed around the colony, which shows that the colony is capable of producing a siderophore. Siderophore production can also majorly affect the host’s resilience to disease attacks. Siderophore is classified by ligands used for iron rods. Siderophore is a complex compound of Fe3+ or specific iron chelating produced by several types of microbes to hide iron elements in the rhizosphere, so it is not available for the development of pathogenic microbes.28
Isolates were capable of producing IAA hormone, which was indicated by the color change of isolates to faded pink. IAA is a phytohormone that has a vital role as a regulator of plant development. IAA production capability of each microbe is very different, depending on the conditions of microbial culture, growing media, and environment.29 Isolates were grown in NB liquid media for ammonia production. It showed that all reacted positively, a change of media color which already contained brownish yellow isolates meaning that the isolates indicated capable of producing ammonia, for the catalase test showed that all reacted positively which showed that the enzyme catalase produced by bacterial isolates was able to break down H2O2 (hydrogen peroxide) into water (H2O) and produce bubbles. The bubble was the formation of oxygen gas (O2), which means that all of these isolates are capable of producing catalase enzymes, which the catalase enzymes are essential for growth.30
Whereas for the ability of N isolates in NFB (Nitrogen Free Medium) media (media does not contain nitrogen), there are five isolates capable of forming colonies in the form of a white ring under the surface of media, and there is a change of media color from blue color to dark blue, this indicates that the isolates are capable of fixing nitrogen.27 As reported by Zgadzaj et al.,31 root nodules formed in the root area are indicators of the success of inoculation. More root nodules formed to indicate that microbial inoculation can cause symbiosis with plants, thus leading to an increase in growth and ultimately increase yields. The results showed that the inoculated Rhizobium of soybean plants were not all able to the root nodule, isolates suitable for soybean plants will form nodules.
Based on the morphological responses (plant height), there are different effects from each treatment where if the isolates inoculated had a match. They would be able to increase growth. As reported by Kumar et al.32 inoculation will have a significant effect if the isolates given have a match for the host plant. This shows that there are different effects from each treatment where if the isolates inoculated have a match, they will be able to increase growth.
Rhizobacteria isolates that can form the root nodule are a very effective type of Rhizobium to those used in soybean plants.33 Inoculated plants with RhizE2 showed the most number of nodules compared with other treatments; each Rhizobium has different suitability for certain legume types, and biological research results support this by Alam et al.34 which states that every kind of legume has different genetic variations in forming a symbiosis with a particular Rhizobium strain. Rhizobium strain also has different abilities in infecting host plants, and multiple strains can infect a host plant, but some strains can symbolize more than one legume plant species.
From all parameters observed, the plants inoculated with RhizE2 showed the best growth results, and this is because the isolates were isolated from fellow legumes, held the possibility of having synergistic and matching properties, and were capable of increasing growth. Host plants in the association Rhizobium with Leguminosae obtain the result of nitrogen fixation in amino acids that are translocated through the xylem. In contrast, the Rhizobium bacteria get photosynthetic carbon compounds from the host plant.35
ACKNOWLEDGMENTS
The authors would like to thank All Agricultural Microbiology Laboratory Colleagues for the Technical Assistance and Implementation of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by DIPA grant 2015-2016, Indonesian Institute of Sciences (LIPI).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Ali A, Khan SA, Khan E, Ali N, Hussain I, Ahmad F. Genetic studies among diverse soybean (Glycine max L. Merrill) genotypes for variability and correlation at Swat. Int J Biosci. 2015;6(4):165-169.
Crossref - Zimmer S, Messmer M, Haase T, et al. Effect of soybean variety and Bradyrhizobium strains on yield, protein content and biological nitrogen fixation under cool growing conditions in Germany. Eur J Agron. 2016;72:38-46.
Crossref - Kuntyastuti H, Lestari SAD, Sutrisno S. Effects of organic fertilizer and plant spacing on early-medium maturity soybean. J Degrad Min Lands Manag. 2018;5(3):1171-1179.
Crossref - Pindi PK, Satyanarayana SDV, Kumar KS. Rhizobium-Legume Symbiosis: Molecular Determinants and Geospecificity. J Pure Appl Microbiol. 2020;14(2):1107-1114.
Crossref - Saharan BS, Nehra V. Plant growth promoting rhizobacteria: A critical review. Life Sci Med Res. 2011;21:1-30.
- Backer R, Rokem JS, Ilangumaran G, et al. Plant growth-promoting Rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1473.
Crossref - Shetta ND, Al-Shaharani TS, Abdel-Aal M. Identification and characterization of Rhizobium associated with woody legume trees grown under Saudi Arabia condition. Am Eurasian J Agric Environ Sci. 2011;10:410-418.
- Divyanshu K, Yadav M, Shukla V, Kumar S, Tripathi YN, Upadhyay RS. Molecular Identification and Characterization of Plant Growth Promoting Rhizobacteria and their Effect on Seed Germination and Vigour Index of Barley (Hordeum vulgare L.) J Pure Appl Microbiol. 2022;16(2):974-989.
Crossref - Mohammadi K, Sohrabi Y. Bacterial biofertilizers for sustainable crop production: A review. ARPN J Agric Biol Sci. 2012;7:307-316.
- Rechiatu A, Ewusi-Mensah N, Abaidoo RC. Response of soybean (Glycine max L.) to Rhizobia ınoculation and molybdenum application in the Northern Savannah Zones of Ghana. J Plant Sci. 2015;3(2):64-70.
Crossref - Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe condition and in an arid climate. Microbol Mol Biol Rev. 1999;63(4):968-989.
Crossref - Usman M, Nangere MG, Musa I. Effect of three levels of NPK fertilizer on growth parameters and yield of maize-soybean inter crop. Int J Sci Res Publ. 2015;5:1-6.
- O’Hara G. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust J Exp Agric. 2001;41:417-433.
Crossref - Reghuvaran A, Jacob KK, Ravindranath AD. Isolation and characterization of nitrogen fixing bacter from raw coir pith. Afr J Biotechnol. 2012;11(27):7063-7071.
Crossref - Thilakarathna MS, Raizada MN. A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field condition. Soil Biol Biochem. 2017;105:177-196.
Crossref - Berrada H, Ibijbijen. J, Benbrahim KF. Determination of Symbiotic Effectiveness of Rhizobium Strains Isolated from Food Legumes (Bean) Collected from Fez, Morocco . J Pure Appl Microbiol. 2019;13(1):247-255.
Crossref - Vincent JM. A manual for the practical study of the root-nodule bacteria, International Biological Program. IBP Handbook. 1970:1-3
- Somasegaran P, Hoben H. Methods in Legume Rhizobium Technology. Springer-Verlag New York, Inc., New York. 1984.
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47-56.
Crossref - Gravel V, Antoun H, Tweddell RJ. Effect of indole-acetic acid (IAA) on the development of symptoms caused by Pythium ultimum on tomato plants. Eur J Plant Pathol. 2007;119(4):457-462.
Crossref - Cappuccino JG, Sherman N. Microbiology: A Laboratory Manual, 9th ed. Benjamin Cummings, Boston. 2011.
- Jamet A, Sigaud S, Van de Sype G, Puppo A, Herouart D. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol Plant Microbe Interact. 2003;16(3):217-225.
Crossref - Dobereiner J. The Genera of Azospirillum and Herbaspirillum in the Prokaryotes. Springer-Verlag, New York. 1991.
- Saono S, Karsono H, Suseno D. Studies on the effect of different rhizobial strains on Phaseolus lunatus in sand culture. Annales Bogorience. 1976;6:143-154.
- Brockwell J, Hely FW, Neal-Smith CA. Some symbiotic characteristics of rhizobia responsible for spontaneous, effective field nodulation of Lotus hispidus. Aust J Exp Agric Anim Husb. 1965;6(23):365-370.
Crossref - Vincent JM. Nitrogen Fixation in Legumes: Based on Proceedings of an İnternational Seminar Sponsored by the Australian Development Assistance Bureau and the University of Sydney. Academic Press, New York. 1982.
- Harpreet K, Sharma P, Kaur N, Gill BS. Phenotypic and biochemical characterization of Bradyrhizobium and Ensifer spp. isolated from soybean rhizosphere. Biosci Discov. 2012;3:40-46.
- Dhull S, Gera R. Siderophore production by Rhizobia isolated from cluster bean [Cyamopsis tetragonoloba (L.) Taub.] Growing in semi-arid regions of Haryana, India. Int J Curr Microbiol App Sci. 2018;7(3):3187-3191.
Crossref - Kallimath G, Patil CR. An exploration of Rhizobium from green gram root nodules in the three Agroclimatic Zones of Karnataka, India. Int J Curr Microbiol Appl. 2018;7(3):2118-2130.
Crossref - Simon Z, Mtei K, Gessesse A, Ndakidemi PA. Isolation and characterization of nitrogen fixing rhizobia from cultivated and uncultivated soils of Northern Tanzania. Am J Plant Sci. 2014;5(26):4050-4067.
Crossref - Zgadzaj R, James EK, Kelly S, et al. A legume genetic framework controls ınfection of nodules by symbiotic and endophytic bacteria. PLoS Gen. 2015;11(6):e1005280.
Crossref - Kumar A, Devi S, Patil S, Payal C, Negi S. Isolation, screening and characterization of bacteria from Rhizosperic soils for different plant growth promotion (PGP) activities: An in vitro study. Recent Res Sci Technol. 2012;4(1):1-5.
- Dudeja SS, Giri R, Saini R, Suneja-Madan P, Kothe E. Interaction of endophytic microbes with legumes. J Basic Microbiol. 2011;52:248-260.
Crossref - Alam F, Bhuiyan MAH, Alam SS, Waghmode TR, Kim PJ, Lee YB. Effect of Rhizobium sp. BARIRGm901 inoculation on nodulation, nitrogen fixation and yield of soybean (Glycine max) genotypes in gray terrace soil. Biosci Biotechnol Biochem. 2015;79(10):1660-1668.
Crossref - de Souza R, Ambrosini A, Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015;38(4):401-419.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.