ISSN: 0973-7510
E-ISSN: 2581-690X
Early diagnosis of dengue is crucial to prevent the progression to severe dengue (SD) leading to mortality rate reduction. This study aimed to determine the role of the CXCL10 in dengue and its potential utilization as one of the biomarkers for the early diagnosis of dengue. A case-control study was conducted involving healthy subjects as control (n = 10) and 193 subjects as dengue cases. The cases were categorized into dengue without warning signs (DwoWS: n = 70; 34.5 %), dengue with warning signs (DWWS: n = 108; 23.2 %), and severe dengue (SD: n = 15; 7.4 %). The socio-demographic characteristics, clinical presentations, and laboratory parameters (platelet and hematocrit) were documented. Serum CXCL10 quantification was performed using an enzyme-linked immunosorbent assay (ELISA). The descriptive analysis and Pearson’s correlation test were used to analyze demographic data and the correlation between CXCL10, hematocrit, and platelet respectively. The difference in age (p = 0.02) and ethnicity (p = 0.02) were significant between cases and control. Males more frequently had SD in contrast to females (4:1). The frequent warning signs were abdominal pain (42.0 %), severe vomiting (38.3 %), bleeding tendency (15.0 %), and fluid accumulation (7.2 %). The increase in hematocrit (p = 0.039) and platelet reduction (p = 0.0005) were significant in SD. The mean of CXCL10 in control (134.85 ± 48.52 rg/mL) was significantly lower than in cases (545.22 ± 76.33 rg/mL, p = 0.0005). The CXCL10 is evident to be a potential biomarker in the early diagnosis of dengue.
CXCL10, Dengue, Early Diagnosis, Severe Dengue, Hematocrit, Biomarker
Dengue has been classified into dengue and severe dengue (SD), with dengue further categorized into dengue without warning signs (DwoWS) and dengue with warning signs (DWWS) attributed to its severity .1 Dengue virus (DENV) has three structural proteins which are core (C) nucleocapsid protein, membrane-associated protein (prM), envelope protein (E), and seven non-structural (NS) proteins including NS1 which is essential for viral replication in the acute phase.2 The DENV-2 was reported to be more virulent and highly associated with dengue hemorrhagic fever (DHF) in contrast to other serotypes.3,4 On a similar note, the introduction of a new genotype or lineage in the population could lead to SD, as DENV-3 was reported in India causing a DHF outbreak in 2001, replacing DENV-2.5
Immunopathogenesis of dengue incorporates antibody-dependent enhancement and activation of cross-reactive memory T cells causing cytokines overproduction leading to increased capillary permeability and plasma leakage.6,7 The cytokine includes inflammatory cytokines, chemokines, adhesion molecules, and growth factors.8,9 The CXCL10 is a chemokine whose production is induced by IFN-g and is highly expressed on primary cells that have been infected by DENV.10 The CXCL10 interacts with heparin and heparan sulphate, competing with the virus thus impede viral binding.11 This serves as an innate immunity in the early course of dengue.
To date, the diagnosis of dengue commonly involves NS1 antigen detection whereby the highest percentage were identified on day one to three of illness.2,12 The NS1 antigen is a highly conserved glycoprotein that appears to be crucial for virus viability. The sensitivity of NS1 antigen reduces from day four of illness onwards and becomes undetectable in the convalescence phase.13 While IgM or IgG antibody detection is preferred once a patient comes at day five of illness or more. Meanwhile, a combination assay that detects both antigen and antibody is frequently used as a rapid test. This test has a longer detection window as it detects both virus and antibodies, thus lessening the false negative results.14
In providing better management of dengue, early diagnosis of dengue is crucial to prevent the progress towards severe disease. With the knowledge of CXCL10 increased in the early phase of dengue, hence, this study was conducted with the aim to determine the involvement of the CXCL10 in all dengue categories, which potentially contribute to the biomarker development for an early diagnosis of dengue and SD detection.
Study Design, Specimen Collection, and Selection Criteria
A case-control study was conducted with the definition of the cases based on the World Health Organization (WHO) 2009 criteria including DwoWS, DWWS, and SD. The control is defined as a population who has not been exposed to dengue, and was recruited from healthy adults (n=10) with the age ranges from 25 to 39-year-old to obtain population-based normal ranges. While the case group involved 193 specimens collected from patients who were admitted to a tertiary hospital in Selangor, Malaysia. The demographic characteristics were gathered along with clinical presentations (e.g., pleural effusion, abdominal pain, severe vomiting, and significant bleeding) and laboratory parameters (platelet and hematocrit).
The laboratory diagnosis of dengue was conducted using NS1 antigen using Platelia Dengue NS1 Antigen Strip (Bio-Rad Laboratories, France) or DENV specific antibodies IgM or IgG using PanBio Dengue IgM and IgG Capture ELISA (PanBio, Brisbane, Australia). Clinically diagnosed dengue patients with positive NS1 antigen or serological tests for either IgM or IgG detection or both were included in this study. Patients who had negative NS1 antigen with non-detected IgM or IgG, or those infected with other etiology were excluded.
Serum CXCL10 Quantification
Sera from the case and control groups were subjected for CXCL10 measurement using quantitative ELISA which was performed in duplicate (R&D Systems, USA). All sera from the case group were collected within the first week of illness (day 3 to 7 of fever). The enzyme immunoassay technique involves the use of a monoclonal antibody specific for CXCL10 which has been pre-coated onto a microplate. The CXCL10 that is existing in the samples is bound to the immobilized antibody. The second detector antibody, an enzyme-linked polyclonal antibody specific for CXCL10 is added after the unbound substances are washed. Following that, a substrate solution is introduced and reacts with the enzyme-antibody-target complex. This reaction generates color changes that are equal to the amount of CXCL10 bound at the initial steps. The reaction is terminated, and the color intensity is measured.
A 75 µL of Assay Diluent RD 1-56, was initially added to each microtiter well followed by 75 µL of samples, controls, and standards. It was then incubated for two hours at room temperature. The microtiter plate was washed four times using 400 µL of wash buffer. The wells were then filled with 200 µL CXCL10 conjugate and incubated at room temperature for two hours. Similar wash steps were performed. A total of 200 µL substrate solution, later loaded to the wells and again incubated for 30 minutes at room temperature. The color changed from blue to yellow after 50 µL of stop solution was introduced. The optical densities were measured using a microplate reader at the wavelength of 450 nm and 570 nm. Subsequently, a standard curve was constructed by plotting the mean absorbance for each standard to acquire the value of CXCL10.
Statistical Analysis
The descriptive analysis was performed to analyze the demographic data. Meanwhile, the relationship between CXCL10 and the dengue severity was analyzed using ANOVA, followed by Tukey HSD post hoc tests. The probability value of p < 0.05 was considered significant. The correlation between CXCL10, hematocrit, and platelet level was analyzed using Pearson’s correlation test.
Demographic Characteristics
There were 70 (34.5 %) subjects classified as DwoWS, 108 (53.2 %) categorized into DWWS, and 15 (7.4 %) subjects grouped as SD. These were further analyzed according to their age, gender, and ethnicity as shown in Table 1.
Table (1):
The demographic analysis including age, gender, and ethnicity between control and cases.
n (%) |
Control 10 (4.9 %) |
Total Cases 193 (95.1 %) |
DwoWS 70 (34.5 %) |
DWWS 108 (53.2 %) |
SD 15 (7.4 %) |
|---|---|---|---|---|---|
Age |
|||||
Mean ± SD* |
30.0 ± 5.1 |
30.7 ± 12.9 |
32.3 ± 14.4 |
28.5 ± 11.3 |
38.5 ± 13.1 |
Range |
25 – 39 |
6 – 79 |
6 – 79 |
7 – 76 |
13 – 60 |
Gender [n (%)] |
|||||
Male |
0 |
118 (58.1 %) |
47 (23.2 %) |
59 (29.1 %) |
12 (5.9 %) |
Female |
10 (4.9 %) |
85 (41.9 %) |
23 (11.3 %) |
49 (24.1 %) |
3 (1.5 %) |
Ethnicity [n (%)] |
|||||
Malay |
10 (4.9 %) |
103 (50.7 %) |
36 (17.7 %) |
62 (30.5 %) |
5 (2.5 %) |
Chinese |
0 |
21 (10.3 %) |
7 (3.4 %) |
10 (4.9 %) |
4 (2.0 %) |
Indian |
0 |
15 (7.4 %) |
11 (5.4 %) |
2 (1.0 %) |
2 (1.0 %) |
Non-Malaysian |
0 |
54 (26.6 %) |
16 (7.9 %) |
34 (16.7 %) |
4 (2.0 %) |
DwoWS: dengue without warning signs, DWWS: dengue with warning signs, SD: severe dengue, SD*: standard deviation.
Age
The subjects were ranged from 6 to 79 years of age with a mean of 30.7 ± 12.9 years. The youngest age in DwoWS was 6 years and the oldest age was 79 with a mean of 32.3 ± 14.4 years. Those who had DWWS were between 7 to 76 years old with a mean of 28.5 ± 11.3 years. The age range for subjects with SD was 13 to 60 years and the mean was 38.5 ± 13.1 years, as shown in Table 1. There was a significant difference in the mean age between dengue cases and control (p = 0.02).
Gender
The majority of the subjects were male (n = 118, 58.1 %) while the remaining 85 (41.9 %) were female. Males predominated in all dengue cases including DwoWS (n= 47, 23.2 %), DWWS (n= 59, 29.1 %), and SD (n = 12, 5.9 %) respectively. Precisely, the female was having a higher percentage of DWWS (n = 49, 24.1 %) as compared to DwoWS (n = 23, 11.3 %) and SD (n = 3, 1.5 %), as shown in Table 1. The control consists of all females (n = 10, 4.9 %). The difference between male and female subjects in cases was significant (p = 0.0005).
Ethnicity
Of all dengue cases, most of the subjects were of Malay ethnicity, as shown in Table 1. The Malay subjects were categorized into DWWS (n = 62, 30.5 %) followed by DwoWS (n = 36, 17.7 %) and SD (n = 5, 2.5 %). Similarly, Chinese subjects were having a greater percentage of DWWS (n = 10, 4.9 %) as compared to DwoWs (n = 7, 3.4 %) and SD (n = 4, 2.0 %). Meanwhile, Indian subjects had contracted more for DwoWS (n = 11, 5.4 %) in contrast to other categories. The percentage for non-Malaysian subjects was raised in DWWS (n = 34, 16.7 %) followed by DwoWS (n = 16, 7.9 %) and SD (n = 4, 2.0 %). The difference in ethnicity was significant between cases and control (p = 0.02).
Clinical Presentations
In DWWS, most of the subjects complained of abdominal pain (n = 72, 66.7 %) and severe vomiting (n= 67, 62.0 %). Twenty-five subjects (23.1 %) presented with gum bleeding, two subjects (1.9 %) had epistaxis, and one (0.9 %) with heavy menses. In SD, most subjects (60 %) experienced abdominal pain, and 46.7 % were having persistent vomiting with one subject having gum bleeding (6.7 %). Of 15 subjects with SD, 13 suffered from pleural effusion (86.7 %) and one had ascites (6.7 %), as shown in Table 2. The difference in all warning signs between DwoWS, DWWS, and SD was significant (p = 0.0005).
Table (2):
The distribution of warning signs within dengue cases.
Clinical Symptoms |
Total [n (%)] |
DwoWS [n (%)] |
DWWS [n (%)] |
SD [n (%)] |
|---|---|---|---|---|
Abdominal pain |
||||
No |
112 (58.0 %) |
70 (100 %) |
36 (33.3 %) |
6 (40.0 %) |
Yes |
81 (42.0 %) |
0 |
72 (66.7 %) |
9 (60.0 %) |
Severe vomiting |
||||
No |
119 (61.7 %) |
70 (100 %) |
41 (38.0 %) |
8 (53.3 %) |
Yes |
74 (38.3 %) |
0 |
67 (62.0 %) |
7 (46.7 %) |
Bleeding tendency |
||||
No |
164 (85.0 %) |
70 (100 %) |
80 (74.1 %) |
14 (93.3 %) |
Gum bleeding |
26 (13.5 %) |
0 |
25 (23.1 %) |
1 (6.7 %) |
Epistaxis |
2 (1.0 %) |
0 |
2 (1.9 %) |
0 |
Heavy menses |
1 (0.5 %) |
0 |
1 (0.9 %) |
0 |
Fluid accumulation |
||||
No |
179 (92.7 %) |
70 (100 %) |
108 (100 %) |
1 (6.7 %) |
Pleural effusion |
13 (6.7 %) |
0 |
0 |
13 (86.7 %) |
Ascites |
1 (0.5 %) |
0 |
0 |
1 (6.7 %) |
DwoWS: dengue without warning signs, DWWS: dengue with warning signs, SD: severe dengue.
Laboratory Parameters
Hematocrit and Platelet
The mean for hematocrit was 42.88 ± 5.57% with a minimum level of 22.40 % and a maximum of 58.40 %. The mean hematocrit in SD was elevated (46.36 ± 5.68%) as compared to DwoWS (42.73 ± 5.43 %) and DWWS (42.49 ± 5.68 %). The lowest hematocrit level in SD was 35.10 %, while the highest was 58.40 %. Meanwhile, the mean platelet of all dengue cases was 49.79 ± 38.01 × 109/L with a range of 2 to 230 × 109/L. The mean platelet was reduced in SD (12.47 ± 12.65 × 109/L) with the lowest level was 2 × 109/L and the greatest level of 51 × 109/L. These findings revealed that subjects with SD had a significant increase in the mean of hematocrit (p = 0.039) and a decreased mean of platelet as compared to those with DwoWS and DWWS (p = 0.0005).
Association of CXCL10 with Control and Dengue Cases
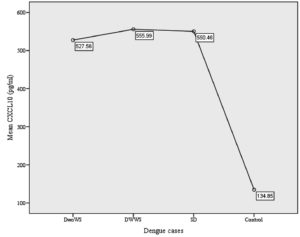
The control group had CXCL10 level ranging from 90.44 to 253.51 pg/mL. The mean for CXCL10 was significantly lower in control group (134.85 ± 48.52 pg/mL) in comparison to dengue cases (545.22 ± 76.33 pg/mL), DwoWS (527.56 ± 91.17 pg/mL), DWWS (555.99 ± 66.81 pg/mL), and SD (550.46 ± 48.43 pg/mL), with p = 0.0005, as shown in Figure. However, there was no significant correlation for the mean of CXCL10 between DwoWS versus (vs) DWWS (p = 0.65), DwoWS vs SD (p = 0.7) and DWWS vs SD (p = 0.99).
Association of CXCL10 with Hematocrit and Platelet
There was a significant but weak positive correlation between the circulating level of CXCL10 and hematocrit (r = 0.2, p = 0.007). However, there was no significant correlation found between CXCL10 level and platelet count (r = -0.108, p = 0.14).
Dengue ranks as the most important mosquito-borne viral disease in the world and remains endemic in Malaysia, despite the current COVID-19 pandemic. The WHO reported that the largest number of dengue cases occurred in 2019, and continues affecting more than 15 countries worldwide, which made the combined impact of COVID-19 and dengue epidemics potentially result in devastating consequences for the populations at risk. Almost 3 billion people live in areas with a risk of dengue and each year up to 400 million people get infected with dengue with 22,000 died from severe dengue.15 Although SD was seen in a small proportion of dengue, it does contribute to significant mortality. Five percent of hospitalized dengue patients in Vietnam progressed to SD,16 while 6.6% of SD was documented by a former study performed in Vitoria, Brazil.17 In Malaysia, Ahmad et al.18 reported that SD contributed up to 4.9% of all other categories of dengue, with two other studies showing the percentage of SD cases were 4.6%.18-20 In the present study, the prevalence of SD was 7.4% which was slightly higher in contrast to previous reports. This finding suggests a true reflection of Malaysia as one of the endemic countries for dengue.
Dengue affects all ages and is commonly seen in adults. A systematic literature review conducted from the year 2000 to 2012 found that the number of dengue cases was stable in adults as compared to children.20,21 This was concurred by Ahmad et al.18 that revealed 75% of dengue cases were frequently occurring in subjects of more than 15 years old. The prevalence of dengue was found to be higher in the age group of 20 to 29 years.21 Similarly, the mean age for current dengue cases in our study was 30.7 years, which categorized the obtained mean age as a young adult. This is most probably linked to a productive age group and increasing outdoor activities leading to a high incidence of dengue exposure. Furthermore, the relationship of age with dengue cases was significant in the present study, as SD had a higher mean age (38.5) as compared to DwoWS (32.3) and DWWS (28.5). These findings corresponded with the previous reported study.22 Our findings indicate that older subjects were at high risk for SD as increased age is associated with the presence of comorbidities and altered level of immunity.23,24
Males were reported to contract dengue more, in contrast to females. The percentage of males infected with dengue was higher in several studies ranging from 60.0% to 63.0%.19-21 Based on Malaysia’s surveillance data, adult males had a 4.17 times greater risk of contracting dengue in contrast to females.24,25 Our analysis revealed that the male and female ratio in dengue cases was 1.57:1, while in SD the ratio was increased to 4:1. The difference in gender was significant
(p = 0.0005) and these were in agreement with other former studies.21,24
Racial allocation and density are possible contributions to the ethnicity aspect of dengue distribution in the country. It was previously reported that Malay was more susceptible to dengue in contrast to other ethnicities.26 Our findings revealed that 103 (50.7 %) subjects were Malay followed by non-Malaysian (n = 54, 26.6 %), Chinese (n = 21, 10.3 %), and Indian (n = 15, 7.4 %), with Malay dominating all the dengue cases. These findings were in parallel to previous studies.20,21
A small number of dengue cases may progress to SD despite most cases being self-limiting, thus attention should be given to dengue patients with warning signs as they would be susceptible to SD. The presence of warning signs (abdominal pain, severe vomiting, bleeding tendency, and clinical fluid accumulation) in this study was significantly associated with dengue cases (p = 0.0005) which corresponded to findings reported by previous studies.18,27 The frequent warning signs from our analysis were abdominal pain (42.0 %), followed by severe vomiting (38.3 %), bleeding tendency (15.0 %), and fluid accumulation (7.2 %). These were concurrent with a study conducted in Brazil,22 whereby 59.8 % of the subjects were having abdominal pain, 44.3 % vomiting, 29.9 % mucosal bleeding, and 28.9 % with pleural effusion.
Increased vascular permeability leads to plasma leakage causing hemoconcentration and manifests as a high hematocrit level. The level of hematocrit is associated with volume loss and severity of the disease.18,27 Our findings demonstrated a significant elevation of mean hematocrit (46.36% ± 5.68) in SD compared to DwoWS (42.73 % ± 5.43) and DWWS (42.49 % ± 5.52) (p = 0.039). This is consistent with other studies that had raised hematocrit levels in DHF/DSS as compared to dengue fever.28,29 Our analysis proved that high hematocrit is linked to SD, and its measurement has been the most common method used to identify hemoconcentration.
Thrombocytopenia in dengue occurs due to complex formation, following secretion of P-selectin which enables platelet binding to monocytes.30 The present study demonstrated a significant (p = 0.0005) reduction of platelet count in SD (12.47 ± 12.65 × 109/L) vs DwoWS (54.56 ± 44.09 × 109/L) and DWWS (51.88 ± 33.21 × 109/L). The results were in agreement with Chen et al.29 and Md-Sani et al.23 who reported that thrombocytopenia was associated with plasma leakage and mortality in SD respectively.
The mean of CXCL10 for the control group in the present study was significantly lower (p = 0.0005) (134.85 ± 48.52 pg/mL) than all dengue cases (545.22 ± 76.33 pg/mL), and these were in parallel to the previous studies that had similar findings.31,32 This can be explained as the production of CXCL10 was augmented in the presence of DENV, causing raised levels of CXCL10 in all dengue cases in contrast to healthy control.31,32 Furthermore, CXCL10 competed with DENV in the early course of the disease, thus reducing viral uptake and replication.11
There were studies reported CXCL10 level was significantly elevated in DHF in contrast to dengue fever which implied that CXCL10 has the ability to serve as one of the biomarkers for dengue severity.31,32 In the present study, CXCL10 demonstrated a significant relationship with hematocrit level, however, there was a weak positive correlation between CXCL10 and hematocrit level (r = 0.2, p = 0.007). Our finding suggests that CXCL10 is an essential factor (chemokine) causing raised hematocrit levels leading to vascular permeability despite the presence of weak correlation. This implied that CXCL10 is evident to be one of the crucial biomarkers that can be employed in diagnosing dengue in the early stage, as it has the ability to distinguish between dengue and non-dengue cases. However, our finding was inadequate to discriminate between DwoWS, DWWS, and SD. This was probably due to a limited number of sample sizes for SD with an absence of sequential blood collected for remaining days of fever in all dengue cases, which restricted us in reporting the effects of cytokine.
The CXCL10 involvement in all dengue categories with its association with demographic characteristics and selected laboratory parameters were ascertained in the present study. A crucial finding in the study substantiated the essential role of CXCL10 in the early diagnosis of dengue, whereby CXCL10 has the capacity to differentiate dengue from non-dengue cases, thus potentiated to be utilized as one of the supplementary tests in the current diagnosis of dengue. The other roles of CXCL10 discerning dengue versus severe dengue particularly as a marker for dengue severity remain to be explored.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Abu Thalhah from IMMB, Faculty of Medicine, Universiti Teknologi MARA, and Dr. Adilahtul Bushro from Hospital Sungai Buloh, Selangor, Malaysia for their technical assistance and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All the authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by the Malaysia Ministry of Education under the Research Development Grant Scheme 600-RMI/RAGS 5/3 (51/2014).
AVAILABILITY OF DATA
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Universiti Teknologi MARA (UiTM) (RAGS/1/2014/SKK04/UITM/4) and the Medical Research & Ethics Committee (MREC), Ministry of Health, Malaysia, NMRR-17-417-34493.
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control – New Edition 2009. https://www.who.int. Accessed 29 January, 2022.
- Solanke VN, Karmarkar, Mehta PR. Early dengue diagnosis: Role of rapid NS1 antigen, NS1 early ELISA, and PCR assay. Trop J Res. 2015;18(2):95-99.
Crossref - Basu P, Bhattacharya S. A new dimension in the dengue epidemiology with special reference to the genetic diversity of the virus: A review. Int J Fauna Biol Stud. 2016; 3:29-41.
- Dong T, Moran E, Vinh Chau N, et al. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS ONE. 2007;2(12):e1192.
Crossref - Dash PK, Parida MM, Saxena P, et al. Re-emergence of dengue virus type-3 (subtype-III) in India: implications for increased incidence of DHF & DSS. Virol J. 2006;3:55. doi: 10.1186/1743-422X-3-55.
Crossref - Martina BEE, Koraka P, Osterhaus ADME. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564-581.
Crossref - Mongkolsapaya J, Duangchinda T, Dejnirattisai W, et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176(6):3821-3829.
Crossref - Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue Virus Infection of Human Endothelial Cells Leads to Chemokine Production, Complement Activation, and Apoptosis. J Immunol. 1998; 161(11):6338-6346.
- Rathakrishnan A, Wang SM, Hu Y, et al. Cytokine Expression Profile of Dengue Patients at Different Phases of Illness. PLoS ONE. 2012;7(12):e52215.
Crossref - Becerra A, Warke RV, Martin K, et al. Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo. J Med Virol. 2009;81(8):1403-1411.
Crossref - Chen JP, Lu HL, Lai SL, et al. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol. 2006;177(5):3185-3192.
Crossref - Adikari TN, Gomes L, Wickramasinghe N, et al. Dengue NS1 antigen contributes to disease severity by inducing interleukin (IL)-10 by monocytes. Clin Exp Immunol. 2016;184(1):90-100.
Crossref - Sekaran SD, Lan EC, Mahesawarappa KB, et al. Evaluation of dengue NS1 capture ELISA assay for the rapid detection of dengue. J Infect Dev Ctries. 2007;1(2):182-188.
- Gan VC, Tan LK, Lye DC, et al. Diagnosing dengue at the point-of-care: utility of a rapid combined diagnostic kit in Singapore. PLoS One. 2014;9(3):e90037.
Crossref - Centres for Disease Control & Prevention. Dengue. 2021. https://www.cdc.gov/dengue/about/index.html. Accessed 29 January, 2022.
- Jaenisch T, Tam DTH, Kieu NTT, et al. Clinical evaluation of dengue and identification of risk factors for severe disease: protocol for a multicentre study in 8 countries. BMC Infect Dis. 2016;16(1):120.
Crossref - Vicente CR, Herbinger KH, Froschl G, et al. Serotype influences on dengue severity: a cross-sectional study on 485 confirmed dengue cases in Vitoria, Brazil. BMC Infect Dis. 2016;16:320.
Crossref - Ahmad MH, Ibrahim MI, Mohamed Z, et al. The sensitivity, specificity and accuracy of warning signs in predicting severe dengue, the severe dengue prevalence and its associated factors. Int J Environ Res Public Health. 2018;15(9):2018.
Crossref - Low GKK, Papapreponis P, Isa RM, et al. Geographical distribution and spatio-temporal patterns of hospitalization due to dengue infection at a leading specialist hospital in Malaysia. Geospat Health. 2018;13(1):642.
Crossref - Mohd-Zaki AH, Brett J, Ismail E, L’Azoo M. Epidemiology of dengue disease in Malaysia (2000-2012): A systematic literature review. PLoS Negl Trop Dis. 2014;8(11):e3159.
Crossref - Chew MH, Rahman Md. M, Salleh SA. Dengue in Malaysia: An epidemiological perspective study. Pak J Med Sci. 2012;28(4):643-647.
- Amancio FF, Heringer TP, de Oliveira Cda C, et al. Clinical Profiles and Factors Associated with Death in Adults with Dengue Admitted to Intensive Care Units, Minas Gerais, Brazil. PLoS ONE. 2015;10(6): e0129046.
Crossref - Md-Sani SS, Md-Noor J, Han WH, et al. Prediction of mortality in severe dengue cases. BMC Infect Dis. 2018;18(1):232.
Crossref - Siregar D, Djadja IM, Arminsih R. Analysis of the Risk Factors of Dengue Hemorrhagic Fever (DHF) In Rural Populations in Panongan Subdistrict, Tangerang 2016. KnE Life Sci. 2018;4(1):119-128.
Crossref - Anker M, Arima Y. Male-female differences in the number of reported incident dengue fever cases in six Asian countries. Western Pac Surveill Response J. 2011;2(2):17-23.
Crossref - Appanna R, Ponnampalavanar S, See LLC, Sekaran SD. Susceptible and Protective HLA Class 1 Alleles against Dengue Fever and Dengue Hemorrhagic Fever Patients in a Malaysian Population. PLoS ONE. 2010;5(9):e13029.
Crossref - Raza FA, Rehman Su, Khalid R, et al. Demographic and Clinico-Epidemiological Features of Dengue Fever in Faisalabad, Pakistan. PLoS ONE. 2014;9(3):e89868.
Crossref - Sam S-S, Omar SFS, Teoh B-T, Abd-Jamil J, AbuBakar S. Review of Dengue Hemorrhagic Fever Fatal Cases Seen Among Adults: A Retrospective Study. PLoS Negl Trop Dis. 2013;7(5):e2194.
Crossref - Chen RF, Yang KD, Wang L, Liu J-W, Chiu C-C, Cheng J-T. Different clinical and laboratory manifestations between dengue haemorrhagic fever and dengue fever with bleeding tendency. Trans R Soc Trop Med Hyg. 2007;101(11):1106-1113.
Crossref - Malavige GN and Ogg GS. Pathogenesis of vascular leak in dengue virus infection. Immunology. 2017;151(3):261-269.
- Masood KI, Jamil B, Rahim M, Islam M, Farhan M, Hasan Z. Role of TNF α, IL-6 and CXCL10 in dengue disease severity. Iran J Microbiol. 2018;10(3):202-207.
- Ferreira RA, de Oliveira SA, Gandini M, et al. Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta Trop. 2015;149:138-147.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.