ISSN: 0973-7510
E-ISSN: 2581-690X
In order to fight the coronavirus disease 2019 (COVID-19) pandemic, the AstraZeneca vaccine was introduced in the Saudi Arabian vaccination campaign. AstraZeneca was rapidly developed, and side effects have been reported following vaccination. This review aims to evaluate the adverse side-effects of the AstraZeneca vaccine within the Saudi population. A literature search of the national and international databases PubMed, Scopus, Google Scholar, Embase databases, and Cochrane Library using the search terms “Covid-19 vaccine”, “AstraZeneca post-vaccination,” and “Covid-19 vaccine Saudi Arabia” in combination with the terms “side effects,” “adverse effects,” and “Covid-19 AstraZeneca” was performed. Articles published before 12 January 2022 were eligible for screening. A total of seven articles met the inclusion criteria, totaling 4838 participants. The most common side effects were fever and either pain or redness at the site of the injection. Furthermore, systemic reactions to AstraZeneca accounted for approximately 80% of adverse effects following vaccination. In sum, AstraZeneca appears to have mild to moderate side effects.
COVID-19, Vaccine, Side Effects, AstraZeneca
At the end of December 2019, a newly emerging virus, now known as Coronavirus Disease 2019 (COVID-19), was detected in Wuhan, China.1 COVID-19 virus is currently designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a recently found member of the human coronavirus family.2 Following its first appearance, SARS-CoV-2 began spreading over the globe, causing a global pandemic3 that has had a significant global economic impact and caused societal disruption; these factors encouraged countries to take preventive measures to fight the disease.4,5Saudi Arabia was one country that rapidly established several preventative measures to limit the spread of the disease, including closing schools, colleges, and mosques, suspending international flights, and putting the country into lockdown.2,4
One of the most effective methods for preventing the spread of potentially fatal infectious diseases is vaccination.3 As such, significant efforts have been implemented to develop a safe, effective COVID-19 vaccine since the beginning of the pandemic.6,7 As the need for authorized COVID-19 vaccines increased both globally and locally, Saudi Arabia established a vaccine distribution plan that involved three phases, initially targeting the populations at high risks, such as healthcare workers, individuals with comorbid disorders and the elderly.8 The second and third phases were implemented by making the vaccine available to the rest of the population; the goal was to vaccinate at least 70% of the population.2,4 Various COVID-19 vaccines have been distributed worldwide, and many countries have granted emergency permission for certain vaccines to be used.3 Such examples include a vaccine that utilizes human adenovirus 26 as a vector (Ad26.COV2.S-Johnson & Johnson/Janssen), a chimpanzee adenovirus vector vaccine carrying the genetic information required for the synthesis of the coronavirus spike protein (SARS-CoV-2 S gene) (ChAdOx1- AstraZeneca), and vaccines based on the mRNA technology to express the SARS-CoV-2 spike (S) gene, including BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna).2,8-10 According to the Saudi Food and Drug Authority (SFDA), Pfizer-BioNTech was approved for vaccination protocols on 10 December 2020, followed by AstraZeneca on 18 February 2021, and Moderna on 9 July 2021.3
Usually, vaccines must be preserved at low temperatures; the Oxford–AstraZeneca vaccine, however, can be maintained in an ordinary refrigerator.11 The efficacy rate of the AstraZeneca vaccine is 70%, which is considered too low in contrast to other vaccines due to the inadequate T-cell response shown in patients who received the vaccine.12-15 In addition, the AstraZeneca vaccination program is divided into two 0.5 mL doses, with an interval ranging from four to twelve weeks between the two doses.9
Although vaccination is currently considered optimal method for controlling the COVID-19 pandemic, side effects may occur after receiving the vaccine, including fever, fatigue, headache, and pain at the injection site.1,16,17 Generally, the vaccine’s side effects can in hesitancy toward receiving the vaccine at all. Evaluating the side effects that might appear after receiving the vaccine and establishing the absence of severe side effects of the AstraZeneca vaccine, could significantly reduce this hesitancy.18,19 The intensity of the pandemic will then be reduce; people who are at higher risk of developing severe illnesses from COVID-19 will be protected and the spread of the infection will be halted.20-22To our knowledge, studies on the side effects of the AstraZeneca vaccine, on the Saudi Arabian population specifically, remain limited. Therefore, this narrative review aims to examine and evaluate the most common side effects of the AstraZeneca COVID-19 vaccine among the Saudi population.
Online articles that investigated the side effects of the AstraZeneca COVID-19 vaccine in Saudi Arabia were collected through PubMed, Scopus, Google Scholar, Embase databases, Cochrane Library, and LILACS. Articles published from after the pandemic started in Saudi Arabia, 2 March 2020 until 12 January 2022 were included in the study. In addition, reference lists from extracted articles were manually searched for additional reliable sources. Both Arabic and English papers were reviewed, and keywords were used to extract reports that meet the inclusion criteria using the advanced search bar. Keywords included ‘oxford AstraZeneca’ OR AstraZeneca vaccine’ OR ‘ChAdOx1- AstraZeneca’ AND ‘Saudi Arabia’ AND ‘COVID-19’ OR ‘coronavirus’ OR ‘COVID-19 Vaccine’ OR ‘SARS-CoV-2’ AND ‘side effect’ OR ‘adverse events’ OR ‘post-vaccination symptoms.
Study selection
Six authors independently reviewed articles to assess their eligibility. The reviewers individually screened the titles; this was followed by abstract evaluation and an examination of the result section to study the quantitative results. The inclusion and exclusion criteria used were as follows:
- Inclusion: free, full-text articles that examine the side effects of the AstraZeneca vaccine in Saudi Arabia will be selected.
- Exclusion criteria: Reviews, letters to the editor, personal opinions, book chapters, case reports, congress abstracts, studies with animals, studies on vaccines other than the AstraZeneca COVID-19 vaccine and countries other than Saudi Arabia will be excluded.
In case of doubt or disagreement, the final decision was made by consensus. Articles were collected using reference manager software EndNote X9 to maintain selected papers and eliminate duplicates.
Data extraction and analysis
Following the selection of the articles, Microsoft Excel was used to input all reported side effects in the articles. The first author’s name was presented in a row to represent the side effects mentioned in columns. In addition to the type of side effects, the total number of participants was recorded, as were their gender and age.
Three main analysis sections were used to summarise our results as follows: most reported, commonly reported, and the type of side effect. The most reported side effects were analysed based on three or more articles. The number of reported cases was statistically analysed using the ANOVA test to determine any significant differences between the results, which were consolidated into one table. The second analysis method presented the most commonly reported side effect, having been observed in over 1000 cases. In addition, moderate was defined as less than 1000 and more than 50 cases. The final task was to note the least common side effect, which accounted for less than 1% of the total number of cases. Finally, local, systemic, and allergic side effects were analysed to identify which types are widely distributed among AstraZeneca-vaccinated individuals within the Saudi Arabian population. A one-way and two-way ANOVA test was used to determine significance between side effects. In addition, a one tail T-test was performed to assess significance between genders. A P-value < 0.05 was considered scientifically significant.
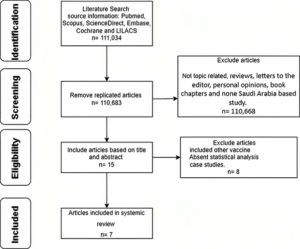
During the initial search, 1,060,588 papers were identified in the six electronic databases (PubMed, Scopus, Google Scholar, Embase databases, Cochrane library and LILACS) using advanced search methods, with 15 articles remaining after duplicate studies were eliminated. After reviewing the titles and abstracts, seven articles were found to match the eligibility criteria (Figure 1).
Figure 1. Study the literature search flow diagram. After removing duplicate studies, there were 15 papers left in six electronic databases (PubMed, Scopus, Google Scholar, Embase databases, Cochrane library, and LILACS). Despite this, seven publications met the requirements once the titles and abstracts were examined.
Most reported side effects in articles
In the seven articles, a total of 4838 participants reported side effects that were caused by the AstraZeneca vaccine. The participants were 53.76% male and 46.24% female, with a mean age of 35.6 and an age range of between 16 and 83 years of age. The P-value for gender significance was 0.4, meaning that there was no significant difference.
Side effects varied between articles. Thus, shared side effects reported by three or more articles are summarised in Table.
Table:
The side effects of the AstraZeneca COVID-19 vaccine that were reported in three or more articles.
Side effect |
Side effect per participant |
Percentage per study |
Total |
Ref. |
|---|---|---|---|---|
Fatigue |
1354/1569 |
86% |
1620 |
(3) |
236/385 |
61% |
(2) |
||
30/57 |
53% |
(17) |
||
Pain or redness at |
1347/1569 |
86% |
2190 |
(3) |
217/385 |
56% |
(2) |
||
the site of |
106/181 |
59% |
(16) |
|
injection |
35/57 |
61% |
(17) |
|
485/1592 |
30.5% |
(4) |
||
Gastrointestinal |
22/528 |
4% |
814 |
(1) |
symptoms |
25/526 |
5% |
(5) |
|
300/1569 |
19% |
(3) |
||
71/385 |
18% |
(2) |
||
8/181 |
4% |
(16) |
||
9/57 |
16% |
(17) |
||
379/1592 |
24% |
(4) |
||
Fever |
222/528 |
42% |
2439 |
(1) |
221/526 |
42% |
(5) |
||
1163/1569 |
74% |
(3) |
||
181/385 |
47% |
(2) |
||
134/181 |
74% |
(16) |
||
20/57 |
35% |
(17) |
||
498/1592 |
31% |
(4) |
||
Headache |
211/528 |
40% |
(1) |
|
210/526 |
40% |
(5) |
||
948/1569 |
60% |
1655 |
(3) |
|
159/385 |
41% |
(2) |
||
102/181 |
56% |
(16) |
||
25/56 |
44% |
(17) |
||
Cardiac disorder |
40/528 |
7.5% |
550 |
(1) |
(palpitation, chest |
25/526 |
5% |
(5) |
|
pain) |
478/1569 |
30% |
(3) |
|
7/1592 |
0.4% |
(4) |
||
Muscle pain |
263/528 |
50% |
1644 |
(1) |
262/526 |
50% |
(5) |
||
1100/1569 |
70% |
(3) |
||
19/57 |
33% |
(17) |
||
Breathlessness |
24/528 |
4.5% |
102 |
(1) |
23/526 |
4.3% |
(5) |
||
53/1596 |
3% |
(3) |
||
2/1592 |
0.1% |
(4) |
||
Sore throat/dry |
22/528 |
4% |
47 |
(1) |
mouth |
22/526 |
4% |
(5) |
|
3/1569 |
0.2% |
(3) |
Gastrointestinal symptoms; abdominal pain, diarrhoea and vomiting
Analysis of the 35 different side effects identified by the presented study results showed that nine were reported in three or more papers (Table). The following side effects were reported in those studies: fever (2439), pain or redness at the injection site (2190), headache (1655), muscle pain (1644), fatigue (1620), gastrointestinal symptoms (814), cardiac disorders (palpitation, chest pain) (550), breathlessness (102), and sore throat/dry mouth (47).
Most common, moderately common and less common side effects
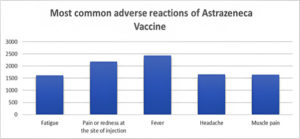
Quantitatively, the most common side effect of the AstraZeneca vaccine was presented to determine which adverse reaction was highly distributed among the Saudi Arabian population (Figure 2).
Figure 2. The most common side effects of the AstraZeneca vaccine were reported among the Saudi Arabia population.
The results showed that the most common complaints of the study participants were local effects on the site of injection, and some moderate symptoms. The most common side effects were: fever (2439), pain at the injection site (2190), headache (1655), muscle pain (1644), and fatigue (1620). The one-way ANOVA test’s P-value was 0.1912, meaning there are no significant differences between the most common side effects.
The moderately common adverse effects of AstraZeneca vaccine were as follows: chills (831), gastrointestinal symptoms (814), dizziness (650), cardiac disorder (550), insomnia (430), swelling (393), skin rash (348), itching (134), breathlessness (102), numbness (90), and eye muscle pain (68).
According to a two-tailed T-test, the P-value was 1.74, meaning there are no significant differences between moderately and most common side effects.
Furthermore, few individuals experienced rare side effects at a rate of less than 1%. The least common side effects reported were sore throat/dry mouth (47), swollen lymph nodes (44), swollen lips (32), loss of taste and smell (10), abnormal menstrual cycle (7), central nervous system-related symptoms (3), psychological disorder (3), hemodynamic vasovagal attack (2), and blood disorders (1).
Using two-way ANOVA test between less common, moderate, and most common side effects, the P-value was significant with a value of 0.03, which means there is a considerable difference between the rare side effects and the more common side effects.
Local, systemic and allergic reactions to AstraZeneca vaccine
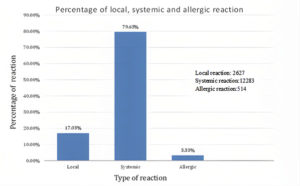
Side effects were divided into three descriptive side effects, namely local, systemic and allergic (Figure 3). Local effects are restricted at the site of vaccination, and include pain, swelling, or redness at the injection site. Conversely, a systemic effect is system-related; it affects either the entire body or an entire organ. These effects include fever, malaise, muscle pain, headache or gastrointestinal tract issues. The ingredients in the vaccine can also cause allergic reactions, which can lead to swollen lips, rashes and swollen lymph nodes.
Figure 3. A percentage of the post-vaccination adverse effects after immunization with Oxford AstraZeneca Vaccine.
The adverse systemic effects of the AstraZeneca vaccine were most commonly reported, accounting for approximately 80% of the total issues. This is followed by the local adverse effects, with a percentage of 17.03%. Lastly, the least common type of post-vaccination reaction was allergic reactions with 3.33%. The P-value using one-way ANOVA was 0.000006, meaning the systemic effect was highly significant as compared to the local and allergic side effects
Several vaccines were rapidly developed in response to the COVID-19 pandemic, including Pfizer and AstraZeneca. Saudi Arabia was one of the first countries to begin a COVID-19 vaccination campaign.23 However, the safety profile of these vaccines was not intensively evaluated, as symptoms varied between individuals within the Saudi population.2 Despite the efficacy of vaccination in terms of creating immunization against COVID-19 and reducing the number of hospitalised patients, the AstraZeneca vaccine was also found to cause intensive side-effects, such as neurological events.24 Therefore, the aim of this narrative review was to determine the most common side effect of AstraZeneca vaccination among the Saudi Arabian population. The results demonstrated that the AstraZeneca vaccine causes multiple side effects. Specifically, the most common complaints reported were systemic side effects.
The most common side effects of AstraZeneca vaccines in the Kingdom of Saudi Arabia have not been extensively examined. All articles found using the inclusion criteria were primarily cross-sectional studies, with various symptoms reported by each article. It was found that nine symptoms were commonly reported by the collected journals (Table). Symptoms such as local pain and redness of injection site, fever, fatigue and muscle pain were previously recognised by the majority of COVID-19 vaccines, including Pfizer, Moderna, AstraZeneca, Sinovac, and Sputnik.25-27 However, in Saudi Arabia, cardiac related symptoms and GIT-related symptoms appears to be widely looked.17 Symptoms that are both cardiac and GIT-related account for less than 20% of symptoms, with rare events being severe.25,28 The health status of severe cases needs to be investigated to identify the cause of severe reactions.
The most common side effect was fever after vaccination, accounting for approximately 50% of participants, followed by pain and redness at the site of injection, which accounted for 45%. Previous studies performed in Ethiopia, Czech Republic, Germany, and the UK found that the most common side effect was pain and redness at the site of injection, accounting for more than 65% and reaching up to 88%.9,13,26 Furthermore, headache, muscle pain and fatigue were also found to be highly common within the Saudi Arabian population. These systemic symptoms were found to be common after AstraZeneca vaccination, though only at mild to moderate severity.29
This narrative review found that local and systemic side effects account for approximately 97% of AstraZeneca vaccine’s adverse effects. Similar findings were found in a systematic review in the United Kingdom.30,31 Furthermore, a previous study that analysed phase III clinical trials of COVID-19 vaccines demonstrated that AstraZeneca-vaccinated patients are more likely to encounter systemic side effects as compared to Pfizer-BioNTech-vaccinated patients.29 These results support the findings of this review within the Saudi population. Conversely, only 17% of the participants were found to experience local side effects. This percentage contradicts another study performed by two European union states, which showed that 73% of AstraZeneca vaccination adverse events are local. However, this study had less than 100 participants.9 Finally, allergic reactions were very minimal, accounting for approximately 3%. A similar finding was reported in a previous study in Iraq, where only 3 participants faced allergic reaction 5 to 15 minutes after vaccination.15
In conclusion, The Oxford–AstraZeneca COVID-19 vaccine is similar to other vaccines in terms of the presence of some side effects. This review showed the most common side effects of the AstraZeneca vaccine in Saudi Arabia; systemic reactions were dominant as compared to localised and allergic reactions. In addition, the most common side effects were fever, pain at the site of injection, headache, muscle pain, and fatigue.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
JA,MA and RA conceptualized the study.NA, AAlf, AAlm, OA and AAbu collected the data and performed analysis. MA and AAbu reviewed and edited the manusrcript. JA and RA performed supervision. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported by Imam Mohammad Ibn Saud University, Research No. RG-21-11-06.
ETHICS STATEMENT
This study was approved by the Institutional Review Board (IRB) at Imam Mohammad ibn Saud Islamic University, with a project number 199-2022, dated February 15, 2022.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Alghamdi A, Ibrahim A, Alraey M, Alkazemi A, Alghamdi I, Alwarafi G. Side effects following COVID-19 vaccination: A cross-sectional survey with age-related outcomes in Saudi Arabia. J Adv Pharm Edu Res. 2021;11(3):119-125.
Crossref - Alhazmi A, Alamer E, Daws D, et al. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674.
Crossref - Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among saudivaccinees. Front Medic. 2021;8:1796.
Crossref - AlBahrani S, Albarrak A, Alghamdi OA, et al. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID-19 vaccine in Saudi Arabia. Int J Infec Dis. 2021;110:359-62.5.
Crossref - Young M, Crook H, Scott J, Edison P. Covid-19: virology, variants, and vaccines. BMJ Med. 2022;1(1):e000040.
Crossref - Alghamdi AA, Alkazemi A, Alissa A, Alghamdi I, Alwarafi G, Waggas HA. Adverse Events following AstraZeneca COVID-19 Vaccine in Saudi Arabia: A Cross-Sectional Study among Healthcare and Non healthcare Workers. Intervirology. 2022;65(2):104-109.
Crossref - Khan WH, Hashmi Z, Goel A, et al. COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions. Front Cell Infect Microbiol. 2021;11:690621.
Crossref - Assiri A, Al-Tawfiq JA, Alkhalifa M, et al. Launching COVID-19 vaccination in Saudi Arabia: lessons learned, and the way forward. Trav Medic Infect Dis. 2021;43:102119.
Crossref - Riad A, Pokorna A, Mekhemar M, et al. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines. 2021;9(6):673.
Crossref - Aleem A, Samad AB, Vaqar S. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. PMID: 34033342.
- Holm MR, Poland GA. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus-vectored COVID-19 vaccines for optimal efficacy. Vaccine. 2021;39(3):457-459.
Crossref - Ahsan W, Syed NK, Alsraeya AA, et al. Post-vaccination survey for monitoring the side effects associated with COVID-19 vaccines among healthcare professionals of Jazan province, Saudi Arabia. Saudi Med J. 2021;42(12):1341-1353.
Crossref - Solomon Y, Eshete T, Mekasha B, Assefa W. COVID-19 Vaccine: Side Effects After the First Dose of the Oxford Astra-Zeneca Vaccine Among Health Professionals in Low-Income Country: Ethiopia. J Multidiscipl Healthcare. 2021;14:2577.
Crossref - Tregoning JS, Brown ES, Cheeseman HM, et al. Vaccines for COVID-19. Clin ExpImmunol. 2020;202(2):162-192.
Crossref - Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab Syndr: Clinic Res Rev. 2021;15(5):102207.
Crossref - Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring-United States, December 14, 2020-January 13, 2021. Morbid. Mortal Weekly Report. 2021;70(8):283.
Crossref - Alghamdi A, Ibrahim A, Almutairi R, Joseph M, Alghamdi G, Alhamza A. A cross-sectional survey of side effects after COVID-19 vaccination in Saudi Arabia: Male versus female outcomes. J Adv Pharm Edu Res. 2021;11(2):51-56.
Crossref - Abedin M, Islam MA, Rahman FN, et al. Willingness to vaccinate against COVID-19 among Bangladeshi adults: Understanding the strategies to optimize vaccination coverage. PLoS One. 2021;16(4):e0250495.
Crossref - Jahan N, Rahman FI, Saha P, et al. Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study. Infect Dis Rep. 2021;13(4):888-901.
Crossref - Chen W. Promise and challenges in the development of COVID-19 vaccines. Human Vaccines & Immunotherapeutics. 2020;16(11):2604-2608.
Crossref - Mahallawi WH, Mumena WA. Reactogenicity and Immunogenicity of the Pfizer and AstraZeneca COVID-19 Vaccines. Front Immunol. 2021;12:794642.
Crossref - Al-Shaqaq A, Al-Demerdash M, Al-Abadi A, et al. Safety and Antibody Response to BNT162b2 and ChAdOx1 nCoV-19 Vaccines in Kidney Transplant Recipients. J Environ Sci Pub Health. 2021;5:411-423.
Crossref - Algaissi AA, Alharbi NK, Hassanain M, Hashem AM. Preparedness and response to COVID-19 in Saudi Arabia: Building on MERS experience. J Infec Pub Health. 2020;13(6):834-838.
Crossref - Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.
Crossref - McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. Vaccines. 2021;6(1):1-14.
Crossref - Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomized, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979-1993.
Crossref - Grana C, Ghosn L, Evrenoglou T, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022; 12(12):CD015477.
Crossref - Akhtar Z, Trent M, Moa A, Tan TC, Frobert O, MacIntyre CR. The impact of COVID-19 and COVID vaccination on cardiovascular outcomes. Eur Heart J Suppl. 2023;14:25(Suppl A):A42-A49.
Crossref - Funk CD, Laferriere C, Ardakani A. Target product profile analysis of COVID-19 vaccines in phase III clinical trials and beyond: an early 2021 perspective. Viruses. 2021;13(3):418.
Crossref - Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChA-dOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396(10249):467-78.
Crossref - Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258-2276.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.