ISSN: 0973-7510
E-ISSN: 2581-690X
Nosocomial infections mainly are due to inefficient cleaning in association with the uncontrollable prescription of antimicrobials resulting in the emergence of multi-drug resistant pathogens in the hospital environment. Objectives:The study aims to evaluate the impact of the implementation of culture-guided antibiotic policy with strict infection control strategies on the occurrence of nosocomial infections and the resistance pattern ofthe isolated clinical and environmental pathogens. The study was done in 2 periods. Firstly, (August 2016 – April 2017), routine disinfection procedures and the applied antibiotic policy were evaluated. Secondly, according to the results a new antibiotic policy depending on the culture sensitivity results were implemented starting from June 2017 to February 2018 in association with strict infection control practices. As a result of this intervention, A change in the type of the isolated microorganisms was observed.Antibiotic resistance was decreased. Mortality rate was reduced from 14.1% to 9.5% of neonates with nosocomial infections, the number of the prescribed antibiotics didn’t exceed 4 antibiotics decreasing the overall cost for neonates’ therapy during their hospital stay. Each hospital should have its own antibiotic policy with the application of strict infection control strategies for the control of nosocomial infection.
NICU, antibiotic policy, culture-based, environmental samples, infection control
Nosocomial infection is one of the common complications in hospitalized patients and is considered as an important cause of morbidity and mortality among different cases in the intensive care units especially in neonatal intensive care units (NICUs). As, it was found that neonatal mortality in the first 28 days of the child’s life represents one-third of the global child mortality.1
US Department of Health and Human Services for Disease Control and Prevention defined nosocomial infection as it is an infection that occurs during hospitalization which was not present at the time of admission2. It was reported that 3.1 million newborn deaths (40% of under-five deaths) each year globally occur in the neonatal period3. In developing countries, neonatal hospital-acquired infections were found to be higher than that in the developed countries by 3 to 20 times. These higher rates were attributed to the insufficient infection control measures (poor hand hygiene, insufficient number and distribution of washbasin, inadequate training of staff) applied during or after birth, crowded hospitals and limited resources resulting in acquiring neonatal sepsis, meningitis and pneumonia which may be fatal4. As a result, nosocomial infection is considered as a great challenge for the healthcare system because these infections increase the length of stay in hospital and cost. Newborns (especially low birth weight and premature neonates) defense mechanisms are insufficient for their protection including their inefficient body structural barriers, absence of the protective endogenous normal flora and immaturity of immune system. All these factors make neonates at risk of acquiring infections during their exposure to various therapeutic interventions such as intubation, total parenteral nutrition, ventilation and using central venous and urinary catheters5.
In developed countries, antibiotic prescribing policies depending on a very limited number of antibiotics are applied to decrease the possibility of the emergence of antibiotic-resistant bacteria. On the other hand, the choice of antibiotics in developing countries depends on many factors such as promotion presented by pharmaceutical companies, costand lack of prescriber knowledge6 that lead to the exposure of neonates to unnecessary broad-spectrum antibiotics resulting in the development of resistant microorganisms, as well as the increase in the risk of candidemia, necrotizing enterocolitis (NEC), hospital-acquired infections, and death in preterm infants7, 8.
Our study aims to study the prevalence of nosocomial infection, risk factors and the resistance pattern of the isolated strains from clinical and the environmental samples in NICU in Minia governorate, Egypt before and after using culture- based antibiotic policy with the application of the recommended infection control guidelines as recommended.
Study design
Our study was done in the main neonatal intensive care unit in our area which is included in Minia general hospital. First, an evaluation for the applied infection control measures and antibiotic policy was done in the period from August 2016 until April 2017 (9 months). Second, A culture-based antibiotic policy with daily and weekly follow-up for the implementation of the recommended infection control guidelines was applied starting from June 2017 to February 2018 (9 months).
Exclusion criteria
We excluded any neonate showing signs of infection at the time of admission and any neonate died or discharged before 48 hrs.
Data collection
Clinical data of the patients were collected including gestational age, birth weight, and method of delivery, pre- mature rupture of membranes (PROM), insertion of an umbilical catheter and maternal fever.
Patients
During the period from August 2016 to April 2017, the antibiotic policy applied in the NICU was evaluated. The policy includes ampicillin and cefotaxime as a first line therapy of early onset neonatal sepsis (EONS), and vancomycin and imipenem for late onset neonatal sepsis and piperacillin-tazobactam and linezolid as a second line therapy. A total of 780 neonates were admitted to the NICU. From which, 318 patients were excluded. Out of the 318 excluded neonates, 27 neonates were discharged in the first 48hrs, 15 neonates died and 276 neonates had the signs of infection at the time of admission and 462 showed no signs of infection were hospitalized for a period of more than 48hrs. Out of these 462 cases, 140 neonates had signs of sepsis. Furthermore, 100 environmental samples were collected from different sources.
Starting from June 2017 to February 2018 (9 months), A culture-based antibiotic policy was implemented. During this period, 844 neonates were admitted to the NICU. From which, 227 patients were excluded. As 26 neonates were discharged in the first 48hrs, 18 neonates were died and 183 neonates had the signs of infection at the time of admission while 617 showed no signs of infection were hospitalized for a period more than 48hrs. Out of these 617 cases, 180 neonates had signs of sepsis. Also, 100 environmental samples were collected from different sources.
Neonates considered to have sepsis in our study were those develop at least 3 out of the following conditions according to Egyptian Neonatal Network (EGNN)9: the presence of some risk factors such as prematurity and chorioamnionitis, presence of two or more signs of sepsis such as poor reflexes, lethargy, respiratory distress, bradycardia, apnea, convulsions, abdominal distension, and bleeding, abnormal total leucocyte count, abnormal total neutrophil (PMN) count, increased immature PMN count, increased immature to total PMN ratio, the ratio of immature to mature PMN is equal or more than0.3, platelets count is lower than 150 X 103/mm3, and pronounced degenerative changes in PMNs and positive CRP with or without a laboratory confirmed culture of the hospital acquired pathogens.
Samples collection, isolation and identification of microorganisms
Blood samples were obtained by the aid of the hospital laboratory. Blood cultures were positive when 2 different samples were collected under aseptic conditions from the same patient showed bacterial growth. Microorganisms were isolated and identified using the traditional microbiological methods10.
Antimicrobial susceptibility testing
Antibiotic susceptibility tests were performed using Kirby-Bauer disc diffusion method on Mueller-Hinton agar according (Oxoid, UK) to the standards of Clinical and Laboratory Standards Institute (CLSI). Antibiotic discs used are: Ampicillin (10µg), Ampicillin – Sulbactam (10⁄10 µg), Amoxicillin/Clavulanic acid (20⁄10 µg), Cefotaxime (30µg), Cefepime (30µg), Imipenem (10µg), Meropenem (10µg), Amikacin (30µg), Gentamicin (10µg), Azithromycin (15µg), Ciprofloxacin (5µg), Linezolid (30µg), Piperacillin-tazobactam (100⁄10 µg) and Aztreonam (30µg) (Oxoid, UK). Vancomycin resistance and methicillin resistant staphylococci was determined by agar dilution method using vancomycin (Mylan, Egypt) and cefoxitin (Apotex, Egypt)11.
Evaluation of the infection control measures
A checklist was prepared to follow-up the infection control measures applied in the NICU during the first and the second period (August 2016 to April 2017 and June 2017 to February 2018), During the first period, the daily routine infection control measures applied were evaluated while in the second period, a follow-up for the implementation to the recommended infection control guidelines was done and evaluated. The checklist included the measures followed in the disinfection and the cleaning of walls, hand washbasin, floor tables for the preparation of neonatal fluids, the counter, thermometer, balances, oxygen mask and incubators. In addition, hand wash practices of the medical staff and the applied visitor policy were evaluated in the two periods.
Determination of neonatal clinical days
Days of stay for the admitted neonates were recorded from the day of their admission until the day of their discharge (dead cases were excluded).
Mortality rate
Mortality rate was determined by determining the number of dead cases to the total number of admitted neonates showing signs of nosocomial infections or sepsis.
Statistical analysis
Statistical analyses were done using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Student t test, c2 test or Fisher’s exact test was used to compare proportions. All statistical analyses were two-sided, P< 0.05 indicates significant difference.
Demographic data and clinical presentation of neonates under investigation
This study was done in the period from August 2016 to February 2018. A total of 1624 neonates were admitted to the neonatal intensive care unit (NICU) of Minia General Hospital. Gestational age of the neonates with nosocomial infection was in the range of28-36 weeks while those showing no signs of nosocomial infection were with gestational age of more than 36 weeks. Most of the neonates were males. Most of neonates showing signs of nosocomial infection had a length of stay in the NICU more than 7 days(Table, 1).
Table 1 showed that 462/780 neonates during the period of August 2016 to April 2017 and 617/844 neonates during the period of June 2017 to February 2018 were admitted to NICU with different primary diagnosis of diseases that listed in table, 1 showing no signs of infection at the time of admission. Also, it was found that low birth weight neonates had more chance to develop nosocomial infections (Table, 1).
Table (1):
The characteristics of neonates admitted to NICU before and after the initiation of culture based antibiotic policy.
| Category | Infected neonates | Uninfected neonates | ||
|---|---|---|---|---|
| Before antibiotic policy N=114 (A) No. (%)* |
after antibiotic policy N=139 (B) No. (%)** |
before antibiotic policy N=348 (C) No. (%)* |
after antibiotic policy N=478 (D) No. (%)** |
|
| Age distribution: <28 weeks 28-36 weeks +36 weeks |
1(0.8) |
0(0) |
3(0.78) |
4(0.8) |
| Sex Female Male |
44(38.5)a 70(61.4) a |
66(47.4)b 73(52.5) b |
110(31.6)a 238(68.39) a |
189(39.5)b 289(60.4) b |
| Birth weight – ELBW<1000g – VLBW 1000-1500g – LBW 1500- 2500g – Normal 2500g |
1(0.8) 29(25.4) 66(57.8) a 37(32.4) |
0(0) 36(25.8) 75(53.9) b 28(20.1) |
2(0.5) 40(11.49) 114(32.7) a 192(55.1) |
4(0.8)
|
| Length of stay 3-7 days 7+ days |
33(28.9) 81(71) |
52(37.4) b 87(62.5) |
295(84.7) 53(15.2) |
417(87.2) b 61(12.7) |
| Primary diagnosis – Respiratory distress |
67(58.7) a |
80(57.5) b |
202(58) a |
145(30.3) b |
| – Low birth weight | 96(84.2) a | 111(79.8) b | 43(12.3) a | 64(13.3) b |
| – Neonatal jaundice | 31(27.1) a | 33(23.7) b | 177(50.8) a | 208(43.5) b |
| – Neonatal convulsions | 13(11.4) | 17(12.2) | 37(10.6) | 49(10.2) |
| – Congenital heart disease | 8(7) | 5(3.5) | 13(3.7) | 7(1.4) |
| – Bleeding tendency or hemorrhage | 4(3.5) | 1(0.7) | 14(4) | 18(3.7) |
| – Hypoxic ischemic encephalopathy | 3(2.6) | 2(1.4) | 6(1.7) | 3(0.6) |
| – Meconium aspiration syndrome | 3(2.6) | 5(3.5) | 3(0.8) | 4(0.8) |
| – Multiple congenital anomalies | 2(1.7) | 3(2.1) | 4(1.1) | 3(0.6) |
| – Apnea | 0(0) | 2(1.4) | 3(0.8) | 1(0.2) |
| – Hirschsprung disease | 0(0) | 2(1.4) | 3(0.8) | 2(0.4) |
N: The total number of the infected neonates.
*percents were correlated to the total number of infected neonates in each period.
aSignificant difference (P- value< 0.05) among infected (A) and uninfected (C) neonates before policy.
bsignificant difference (P- value< 0.05) among infected (B) and uninfected (D) neonates after policy.
Checking the level of compliance of the applied infection control measures to the national infection control guidelines and determining the applied antibiotic policy before and after the intervention:
Before the intervention (August 2016 to April 2017)
We found that the level of compliance ranged from 30 – 45% according to the checklist results. As, we found that cleaning procedures were performed by the housekeeping staff using water and soap or a detergent followed by a randomly diluted chlorine. For thermometers, disinfection was done by using 70% alcohol. Correct hand washing practices were observed only for 20% of the health workers. Furthermore, no clear policy for visitors was observed.
By revising the prescribed antibiotics during this period, we found that a combination of ampicillin and cefotaxime for EONS and a combination of vancomycin and imipenem for LONS were used as an empirical treatment while a combination of piperacillin-tazobactam and linezolid has been used in the severe infections without performing culture and sensitivity tests.
After the intervention (June 2017 to February 2018)
Infection control guidelines were checked daily. Daily and weekly follow-up to the housekeeping routine in NICU were performed to ensure a high degree of compliance to the instructions of national infection control guidelines. Adjustment of chlorine concentration ensured good disinfection. In addition, a clear visitors’ policy was applied, in which visitors showing active infections should not be allowed to enter NICU and restricting or limiting the number of visitors was applied. The health work staff was trained for the correct hand washing practices using alcohol 70% or betadine shampoo on entry into the unit and between neonates handling.
Identification of the isolated microorganisms from blood and environmental samples
Microorganisms isolated from blood samples collected from neonates before and after the intervention:
Before the intervention (August 2016 to April 2017)
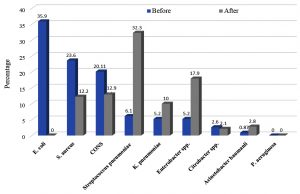
Out of 462 NICU neonates, 140 (30.3%) neonates (after 72 hrs) showed one or more signs of nosocomial infection or sepsis. A total of 114/140 (81.4%) samples were positive for microbial growth. Gram-positive cocci and Gram-negative rods were isolated at the same rate (57/114, 50 % each) but E. coli was the most common (35.9%), followed by S. aureus (23.6%), coagulase negative staphylococci (CONS) (20.11%).
After the intervention (June 2017 to February 2018)
A culture-based antibiotic policy was implemented depending on the prevalence of microorganisms isolated during the first period of study and, their resistance pattern. Choosing antibiotics was done in a manner that can decrease the misuse of the highly effective antibiotics and to decrease treatment cost, length of hospital stays and changing the resistance pattern of the circulating microorganisms. So, imipenem or azithromycin were recommended as an empirical antibiotic in hospital acquired infection in LONS until the results of culture sensitivity. It was found that a significant change (P< 0.05) in the microbial community represented by a change in the number of isolates before and after the intervention occurred. As the microbial community was changed to show that Gram positive cocci represented 57.5% while Gram negative rods represented 42.4%, respectively and S. pneumoniae became the most common (32.3%), followed by Enterobacter spp.(17.9%), CONS (12.9%) and S. aureus (12.2%) (Fig.1).
Fig. 1. Distribution of microorganisms isolated from blood samples before and after the implementation of culture-based antibiotic policy and restricted infection control guidelines
Microorganisms isolated from environmental samples collected from different sources before and after the intervention:
Before the intervention (August 2016 to April 2017)
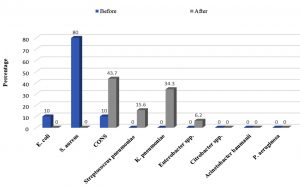
Out of one hundred environmental samples, 50 (50%) samples were positive for microbial growth. The most common environmental isolate was S. aureus (80%), followed by E. coli and CONS (10% each).
During the period of June 2017 to February 2018
Out of 100 environmental samples, 32 (32%) were positive for microbial growth and S. aureus was the most common pathogen (80%)(Fig. 2).
Fig. 2. Distribution of microorganisms isolated from environmental samples before and after the implementation of culture-based antibiotic policy and restricted infection control guidelines
The decrease in number of culture positive environmental samples may be attributed to the change in the daily routine of disinfection and the daily and routine follow-up to the implementation of the infection control guidelines.
In addition, It was observed that most of the environmental isolates were isolated from the floor and the counter before the intervention while most of the environmental isolates were isolated from the floor and the table used for medicine preparation after the intervention (Table 2)
Table (2):
Distribution of the isolated environmental pathogens according to the source of the samples.
| Sample source | Positive cultures before antibiotic policy (N=50) No. (%)a |
Positive cultures after antibiotic policy (N=32)No. (%)b |
|---|---|---|
| Walls | 0(0) | 0(0) |
| Hand sanitizer basin | 0(0) | 0(0) |
| The floor |
Total No.= 33 (66%)
|
10(31.2 )* CONS |
|
||
| Medicine preparation table | 0( 0 ) | Total No.= 8 (25%)** 2( 6.2 ) Enterobacter |
| 6(18.7 )K. pneumoniae | ||
| The counter |
Total No.= 7 (14%)*
|
0(0) |
|
||
| Thermometers | 5(10 ) S. aureus | 0(0) |
| The corners and the bed of incubators before sterilization | 0(0) | Total No.= 5 (15.6)* 3(9.3) Streptococcus pneumoniae |
| 2(6.2 ) K. pneumoniae | ||
| The corners and the bed of incubators after sterilization | 0(0) | 1(3.1 )Streptococcus pneumoniae |
| The balance | 0 (0) | 0(0) |
| The tubes of ventilators before sterilization | 3( 6 ) E. coli | 3(9.3 )K. pneumoniae |
| The tubes of ventilators after sterilization | 1(2 ) E. coli | 0( 0 ) |
| The oxygen masks before sterilization | 1(2) E. coli | Total No.= 3 (9.3)** 2(6.2) CONS |
| 1(3.1) Streptococcus pneumoniae | ||
| The oxygen masks after sterilization | 0(0) | 2(6.2)* CONS |
a percents were correlated to the number of positive cultures before the application of the antibiotic policy.
b percents were correlated to the number of positive cultures after the application of the antibiotic policy.
Significance of results before to antibiotic policy to those after antibiotic policy:
* significant value P-value< 0.05, ** highly significant P-value < 0.001.
Resistance pattern before and after the implementation of culture-based antibiotic policy and infection control measures follow-up
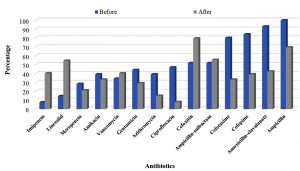
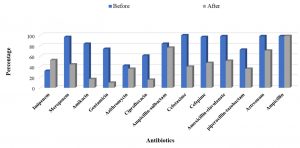
Figure 4 showed that there was a change in the resistance pattern of the isolated strains after the application of the culture- based antibiotic policy. As the table shows that there is an improvement in the susceptibility of the isolated Gram-positive bacteria to many antibiotics such as ciprofloxacin, cefotaxime, cefepime, amoxicillin/clavulanate, ampicillin, amikacin and gentamicin. On the other hand, an increase in the resistance of Gram positive bacteria to imipenem, linezolid, vancomycin, ampicillin/sulbactam was observed which may be attributed to the disappearance of some microorganisms such as S. aureus and the emergence of new strains such as Strep. pneumoniae (Supplementary Data. Table, 2& 4). For Gram negative isolates, significant increase in the susceptibility of the isolates to the tested antibiotics was observed except in case of imipenem and ampicillin which may be due to the emergence resistant isolates of K. pneumoniae and the decrease in incidence of the isolated E. coli (Supplementary Data. Table, 1 & 3).
Neonatal clinical days
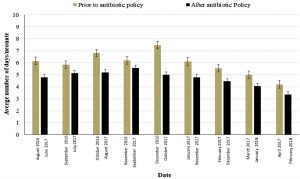
By comparing results of our study before and after the application of the culture- based antibiotic policy, we found that the number of the neonatal clinical days was decreased after the application of the new policy and the surveillance of infection control strategies (Fig. 5). As a result, for the decrease in neonatal clinical days, an increase in the number of the neonates admitted to the NICU by 12% was observed. This increase indicates the improvement in health care of the admitted neonates, a great value for the community by decreasing the length of stay in the NICU and saving places for neonates that in need for special care in the NICU.
Fig. 5. Average number of neonates’ clinical days before and after the implementation of the culture- guided antibiotic policy and strict infection control strategies
Mortality rate and general outcomes of this study
Generally, after the application of the culture- based antibiotic policy, the mortality rate was decreased from 14.1% to 9.5% of the total number of neonates suffering from nosocomial infection. The number of the prescribed antibiotics used during the period of hospital stay didn’t exceed 4 antibiotics. Also, the number of neonates treated with the first line of antibiotics was significantly increased and the consumption of cefotaxime was decreased resulting in the decease of the overall cost for neonates’therapy during their hospital stay. On contrary, there was an observable increase in the resistance of isolates to imipenem, vancomycin and linezolid. This resistance may be attributed to the lack of variable alternatives for antibiotics which pay the healthcare for the selection of these antibiotics from the culture sensitivity reports. Furthermore, the inability of prescribing azithromycin as an empirical antibiotic treatment because it is available as oral formulation only in our country and it is unsuitable for neonates.
Nosocomial infections especially in NICUs are a major cause of long-term morbidity and mortality. So, there is a need for good and active surveillance system to ensure the compliance of the implemented infection control strategies with the national infection control guidelines and to evaluate the applied antibiotic policy.
It is impossible to make the environment surrounding neonates to be completely sterile. As there are many factors that make neonates at risk to be infected including healthcare staff caring for them, their feeding, the use of many equipment and medical devices for their care. In addition to their immature immune system which makes them to be more susceptible for infections.
In our country, many studies reported that the incidence of nosocomial infections (NI) among neonates were 21.4%12 and 38.5%5 which are close to our results (25.3%) while a higher percent was reported by El-Fiky, et al.13. In another study done by Basiri, et al.14, the incidence of nosocomial infection was 5.7% while low incidence of nosocomial infections was reported in developed countries ranged from 3.5 to 11.1% in the USA and from 1.6% to 13.2% in Europe15-19.In our study, we found that most of the admitted neonates with gestational age in the range of 28-36 weeks and low birth weight. The majority of neonates under investigation were males. In addition, most of neonates showing nosocomial infections had a length of stay in the NICU that exceeded 7 days. These findings were supported by several studies20-23 that indicate the increase in the risk of nosocomial infection with the decrease in the gestational age and birth weight and the increase in the duration of the hospital stay. As Mugauri and Mashumba24 reported that neonates who had > 7 days stay, premature and low birth weight developed the signs of neonatal sepsis and klebseilla spp. were the most common. They found that the poor adhesion to the national infection control guidelines was the main cause of sepsis.
In this study, we found that infection control practices were not applied as recommended by the national infection control guidelines. Poor infection control practices facilitate the transfer of pathogenic organisms from the hospital environment to patients, health care team to patients and among patients occupying the same room. Other factors that facilitate the spread of organisms are the state of immune system which may be affected by illness or the use of drugs. In addition, antibiotic use which leads to the selection of resistant strains. It was found that 65% of nursing staff that is in contact with infected persons, their white coats, shirts, ties of doctors carried pathogenic flora25,26. There are many factors affect the transmission of pathogens such as infectious dose, route of administration and host susceptibility.
Many studies showed that the intervention in the applied disinfection strategies improves the effectiveness of housekeeping and cleaning practices. Datta, et al.27 reported that there was a reduction in methicillin resistant staphylococci (MRSA) acquisition by 62% and vancomycin resistant enterococci (VRE) by 22% for patients admitted to rooms which were occupied by patients colonized by these organisms. Denton, et al.28 showed that the replacement of detergent with 1000 ppm of hypochlorite decreased environmental contamination with Acinetobacter baummanii and ended outbreaks caused by these organisms.
Pessoa-Silva, et al.29 demonstrated that hands contamination with pathogenic strains and normal flora occurs at high rates especially in neonatal care and gloves do not fully protect workers. In addition, Pittet, et al.30 found that the amount of pathogens increased by time in ungloved hands which indicate the importance of decontaminating hands. Also, it was found that using alcohol gel before the contact with patient, decreased infection rates during 34 months.
After the daily and weekly follow-up to housekeeping routine in NICU in the second period, a decrease in the number of culture positive environmental samples was observed and CONS were the most common (43.7%).Hayden, et al.31 found that housekeeping education, monitoring of the implemented infection control guidelines and improved cleaning decreased the contamination of the hospital environment from 10% to 3-4%. Also, applying good hand washing practices decreased hand contamination rate from 55% to 10 – 11% and decreased the transfer of VRE.
Grabsch, et al.32 substituted the used detergent (quasi experimental) by hypochlorite (1000 ppm) in the routine disinfection of all rooms. Cleaning supervisors were shared to ensure the complete compliance of housekeeping protocol to the implemented protocol. A formal training program for cleaning staff was assessed to increase awareness by the infection control guidelines. They modified the health care workers personal protective equipment (HCW PPE) protocols for managing spread of infection as followed: wearing disposable protective sleeveless plastic aprons, application of routine alcohol-based hand rub (ABHR) instead of using gloves (except when performing surgical procedures). Their results showed significant decrease in new VRE colonization by 25% in high-risk patients, and VRE bacteremia across the entire hospital by 83.1%.
In our study, the most common infection sites were blood stream followed by respiratory tract which may be attributed to the exposure of neonates to invasive medical procedures such as umbilical catheterization, endotracheal intubation or Intravenous cannula which agreed with many studies33-35. The difference in results between different studies can be attributed to the difference in places at which studies were performed. The basis at which nosocomial infection was diagnosed is the incidence rate of blood culture positive results due to the inability to obtain adequate volume of blood samples36.
In developing countries, many studies showed that klebseilla spp. were the most common pathogens while in developed countries, it was found that Gram positive cocci especially coagulase negative Staphylococci (CONS) were the most common pathogen isolated from blood cultures16, 37-39. In the present study, Gram positive cocci and Gram negative rods (GNR) were isolated at the same rate (57/114, 50 % each) (before applying the new policy) but E. coli was the most prevalent pathogen (35.9%) but Gram positive cocci and Gram negative rods were found to represent 57.5% and 42.4% of the isolated strains, respectively in the second period. Many studies showed that Gram positive cocci were isolated at a higher incidence than Gram negative rods40, 41 while in a study done by Nambiar and Singh35, they reported that GNRs were isolated at a rate of 42.7% and Gram positive cocci were isolated at a rate of 33.5%.
In a study performed by Stoll, et al.16, at national institute of child health (NICHD), they found that 70% of the isolated organisms belonged to the Gram positive organisms (CONS, S. aureus, Enterococcus spp., streptococci group B) while GNR represented 20% of isolates that differs from our results. In a study done by Bokulich, et al.42 streptococci and staphylococci were found to be the most common pathogens. They found that the intensive cleaning of the NCIU reduced their prevalence while enterobacteriaceae members were found to be isolated at low rates. In addition, these organisms were the main causes of the infectious outbreaks occurred in the period of 2011-2012.
In our study, Mortality rate before the implementation of the new antibiotic policy was 14.1% while after the implementation of the new policy, training staff on how to apply the infection control protocols and hand washing practices, the mortality rate decreased to 9.5%. Many different researches were carried out in the developing countries showed that the mortality rates among neonates admitted to NICU ranged from 11.9 to 14.7% which is close to our results and higher than that reported in the developed countries (ranged from 6.1 to 7.1%)19, 43-45. The decrease in mortality rate in our study may be attributed to the implementation of the new antibiotic policy and the recommended infection control guidelines.
In a study done by Wu, et al.46, the use of a culture guided antibiotic stewardship program by trained staff resulted in a decrease in the misuse of antimicrobials and their costs in Japan, USA, Taiwan. As a result, antibiotic resistance decreased among Gram positive and Gram-negative rods and the predominant strains were E. coli and Klebseilla pneumoniae. Also, they reported an increase in the resistance of Gram-positive bacteria to cefazoline and Gram-negative rods to ampicillin/sulbactam. In addition, the duration of stay decreased to less than 2 weeks and the yearly mortality rate was reduced from 1.3% in 2011 to 1.1% in 2012 and 1% in 2013.
Our results showed an improvement in the susceptibility of the isolated Gram positive to many antibiotics such as ciprofloxacin, cefotaxime, cefepime, Amoxicillin/clavulanate, ampicillin, amikacin and gentamicin while an increase in the resistance of Gram positive to imipenem, linezolid, vancomycin, ampicillin/sulbactam was observed which may be attributed to the disappearance of some organisms such as S. aureus and the emergence of new strains such as S. pneumoniae. For Gram negative isolates, significant increase in the susceptibility of the isolates to the tested antibiotics was shown except in case of imipenem and ampicillin which may be due to the emergence of resistant isolates of K. pneumoniae and the decrease in incidence of the isolated E. coli.
In our study, we observed that despite of knowing the terminal disinfection protocol after the discharge of patients, this protocol was not practiced resulting in the infection of the new admitted neonates by the same bacteria. Also, we found that all staff did not receive formal or informal training on the infection control policy. Poor compliance of the implemented infection control protocols with national infection control guidelines and the absence of clear visitor’s policy. All these factors facilitate the transfer of resistant pathogens among the admitted neonates and from the healthcare and the hospital environment to neonates. As a result, failure of the antimicrobial therapy, increase in the mortality rate and the need to the use of multiple antibiotics were great challenges facing the health care team. So, there was a need to apply strict infection control practices to gain great benefits from the applied antibiotic policy. The same conclusion was obtained by Jinka, et al.47 who showed that the controlled antibiotic use is accompanied by decrease in infections caused by multi-drug resistant pathogens in NICUs. Furthermore, the success of antibiotic policy may be affected by poor infection control practices. So, application of strict infection control practices is essential to increase benefits gained by an antibiotic policy.
Considering the change of the distribution of microbial community by time, further regular studies should be performed to ensure these results and to help in the choice of the proper antimicrobials for the empiric treatment of hospital acquired infections in neonates.
As a result of this study, many benefits were gained which were:
- Increasing the rate of the participation of infection control health workers, physicians and nurses in the different training workshops on the infection control measures.
- Increasing the awarenes of the application of infection control measures and the role of microbiological monitoring in hospitals.
- Educating the medical staff about the antibiotic policy concepts and their importance in controlling the spread of antimicrobial resistance.
- Discussing the inappropriate prescription of antibiotics and its effects in the presence of doctors.
- Directing doctors to prescribe unrestricted or less expensive antibiotics instead of the restricted or the more expensive ones if both were recommended by the culture sensitivity results.
The implementation of antibiotic policy depending on the culture results with good infection control practices was of great importance in reducing the mortality rate, the resistance to the lifesaving antibiotics, length of stay in hospital and the treatment costs. So, it is recommended for each local hospital to have its own antibiotic policy.
Additional file: Additional Table S1 – S4.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Dal-Bo K, de Silva RM, Sakae TM. Nosocomial infections in a neonatal intensive care unit in South Brazil. Rev Bras Ter Intensiva. 2012;24(4):381-385.
Crossref - Sastre JBL, Cotallo DC, Colomer BF. Neonatal sepsis of nosocomial origin: an epidemiological study from the “Grupo de Hospitales Castrillo”. J Perinat Med. 2002;30(2):149-157.
Crossref - WHO. The World Bank, and The United Nations, Levels and Trends in Child Mortality. WHO. 2011.
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet (London, England). 2010;375(9730):1969-1987.
Crossref - Mohammed D, El Seifi OS. Bacterial nosocomial infections in neonatal intensive care unit, Zagazig University Hospital, Egypt. Egyptian Pediatric Association Gazette. 2014;62(3-4):72-79.
Crossref - Isturiz RE, Carbon C. Antibiotic use in developing countries. Infect Control Hosp Epidemiol. 2000;21(6):394-397.
Crossref - Almuneef MA, Baltimore RS, Farrel PA, Reagan-Cirincione P, Dembry LM. Molecular typing demonstrating transmission of gram-negative rods in a neonatal intensive care unit in the absence of a recognized epidemic. Clin Infect Dis. 2001;32(2):220-227.
Crossref - Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720-725.
Crossref - EGNN. Egyptian Neonatal Network. http://wwwegynewbornnet. 2010.
- Benson H. Microbiological application. Laboratory manual in General Microbiology. Wan C. Publishers. Dubuque, Melbourn. Oxford; 1994.
- CLSI. Performance standards for antimicrobial susceptibility testing; twenty- fifth informational supplement (M100-S25). Clinical and Laboratory Standards Institute. 2015.
- Abdel-Wahab F, Ghoneim M, Khashaba M, El-Gilany AH, Abdel-Hady D. Nosocomial infection surveillance in an Egyptian neonatal intensive care unit. J Hosp Infect. 2013;83(3):196-199.
Crossref - El-Fiky O, El-Mahdy M, Shebl A, Abdel-Maksoud Y, Abd ElRhahman S. Epidemiological study of nosocomial Infection of neonates admitted to Benha university neonatal Intensive care. Benha M J. 2003(20).
- Basiri B, Sabzehei MK, Shokouhi M, Moradi A. Evaluating the Incidence and Risk Factors of Nosocomial Infection in Neonates Hospitalized in the Neonatal Intensive Care Unit of Fatemieh Hospital in Hamadan, Iran, 2012 – 2013. Arch Pediatr Infect Dis. 2015;3(2):e23327.
Crossref - Sohn AH, Garrett DO, Sinkowitz-Cochran RL, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139(6):821-827.
Crossref - Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285-291.
Crossref - Raymond J, Aujard Y, Group ES. Nosocomial infections in pediatric patients a European, multicenter prospective study. Infect Control Hosp Epidemiol. 2000;21(4):260-263.
Crossref - Urrea M, Iriondo M, Thio M, et al. A prospective incidence study of nosocomial infections in a neonatal care unit. Am J Infect Control. 2003;31(8):505-507.
Crossref - Orsi GB, d’Ettorre G, Panero A, Chiarini F, Vullo V, Venditti M. Hospital-acquired infection surveillance in a neonatal intensive care unit. Am J Infect Control. 2009;37(3):201-203.
Crossref - Olsen AL, Reinholdt J, Jensen AM, Andersen LP, Jensen ET. Nosocomial infection in a Danish Neonatal Intensive Care Unit: a prospective study. Acta Paediatrica. 2009;98(8):1294-1299.
Crossref - Holikar S, Bhaisare K, Deshmukh L. Risk factors for nosocomial sepsis in NICU. Int J Recent Trends Sci Technol. 2012;4(3):141-145.
- Auriti C, Ronchetti MP, Pezzotti P, et al. Determinants of nosocomial infection in 6 neonatal intensive care units: an Italian multicenter prospective cohort study. Infect Control Hosp Epidemiol. 2010;31(9):926-933.
Crossref - Yang L, Peng M, Li H, Pang Y. Pathogen distribution and risk factors of nosocomial infections in neonates in the neonatal intensive care unit. Zhongguo dang dai er ke za zhi= Chinese Journal of Contemporary Pediatrics. 2013;15(2):112-116.
- Mugauri H, Mashumba A. Abstract P-206: Hospital-Acquired Neonatal Sepsis Outbreak In An Intensive Care Unit, Parirenyatwa Group Of Hospitals, Zimbabwe, 2016. Pediatr Crit Care Med. 2018;19(6S):110.
Crossref - Page K, Wilson M, Parkin IP. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J Mater Chem. 2009;19(23):3819-3831.
Crossref - Lopez P-J, Ron O, Parthasarathy P, Soothill J, Spitz L. Bacterial counts from hospital doctors’ ties are higher than those from shirts. Am J Infect Control. 2009;37(1):79-80.
Crossref - Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med. 2011;171(6):491-494.
Crossref - Denton M, Wilcox MH, Parnell P, et al. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. Journal of Hospital Infection. 2004;56(2):106-110.
Crossref - Pessoa-Silva CL, Dharan S, Hugonnet S, et al. Dynamics of bacterial hand contamination during routine neonatal care. Infect Control Hosp Epidemiol. 2004;25(3):192-197.
Crossref - Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999;159(8):821-826.
Crossref - Hayden MK, Blom DW, Lyle EA, Moore CG, Weinstein RA. Risk of hand or glove contamination after contact with patients colonized with vancomycin-resistant enterococcus or the colonized patients’ environment. Infect Control Hosp Epidemiol. 2008;29(2):149-154.
Crossref - Grabsch EA, Mahony AA, Cameron DRM, et al. Significant reduction in vancomycin-resistant enterococcus colonization and bacteraemia after introduction of a bleach-based cleaning–disinfection programme. J Hosp Infect. 2012;82(4):234-242.
Crossref - Drews MB, Ludwig AC, Leititis JU, Daschner FD. Low birth weight and nosocomial infection of neonates in a neonatal intensive care unit. J Hosp Infect. 1995;30(1):65-72.
Crossref - Gaynes RP, Edwards JR, Jarvis WR, et al. Nosocomial infections among neonates in high-risk nurseries in the United States. Pediatrics. 1996;98(3):357-361.
- Nambiar S, Singh N. Change in epidemiology of health care-associated infections in a neonatal intensive care unit. Pediatr. Infect Dis J.. 2002;21(9):839-842.
Crossref - Jeong IS, Jeong JS, Choi EO. Nosocomial infection in a newborn intensive care unit (NICU), South Korea. BMC Infectious Diseases. 2006;6(1):103.
Crossref - Zaidi AKM, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. The Lancet. 2005;365(9465):1175-1188.
Crossref - Couto RC, Carvalho EAA, Pedrosa TMG, Pedroso ER, Neto MC, Biscione FM. A 10-year prospective surveillance of nosocomial infections in neonatal intensive care units. Am J Infect Control. 2007;35(3):183-189.
Crossref - Srivastava S, Shetty N. Healthcare-associated infections in neonatal units: lessons from contrasting worlds. J Hosp Infect. 2007;65(4):292-306.
Crossref - Richards MJ, Edwards JR, Culver DH, Gaynes RP, System NNIS. Nosocomial infections in pediatric intensive care units in the United States. Pediatrics. 1999;103(4):e39-e39.
Crossref - Mireya UA, Marti PO, Xavier KV, Cristina LO, Miguel MM, Magda CM. Nosocomial infections in paediatric and neonatal intensive care units. J Infect Dis. 2007;54(3):212-220.
Crossref - Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature Methods. 2013;10(1):57.
Crossref - Nagata E, Brito ASJ, Matsuo T. Nosocomial infections in a neonatal intensive care unit: incidence and risk factors. Am J Infect Control. 2002;30(1):26-31.
Crossref - Brito DVD, de Brito CS, Resende DS, Moreira do OJ, Abdallah VOS, Gontijo Filho PP. Nosocomial infections in a Brazilian neonatal intensive care unit: a 4-year surveillance study. Rev Soc Bras Med Trop. 2010;43(6):633-637.
Crossref - Auriti C, Maccallini A, Di Liso G, Di Ciommo V, Ronchetti M, Orzalesi M. Risk factors for nosocomial infections in a neonatal intensive-care unit. J Hosp Infect. 2003;53(1):25-30.
Crossref - Wu C-T, Chen C-L, Lee H-Y, et al. Decreased antimicrobial resistance and defined daily doses after implementation of a clinical culture-guided antimicrobial stewardship program in a local hospital. J Microbiol Immunol Infect. 2017;50(6):846-856.
Crossref - Jinka DR, Gandra S, Alvarez-Uria G, Torre N, Tadepalli D, Nayakanti RPR. Impact of antibiotic policy on antibiotic consumption in a neonatal intensive care unit in India. Indian pediatrics. 2017;54(9):739-741.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.