ISSN: 0973-7510
E-ISSN: 2581-690X
Dysbiosis of intestinal microbiota may cause irregular digestive function, and intestinal wall inflammation. Over the past few years, probiotics generate bioactive metabolites, named postbiotics, have been discovered its crucial roles in modulation of intestinal microbiota. Single-strain postbiotics have positive effect on health of host, but the functions of multi-strain postbiotics remain unclear. This study proposed a useful application of multi-strain postbiotics and thereby establish the developing foundation of multi-strain postbiotics. Initially, various probiotics and postbiotics were screened for anti-inflammatory activity through inducing the transforming growth factor beta (TGF-β)and interleukin-10 (IL-10) in peripheral blood mononuclear cells (PBMCs). Then, we detailed the synergistic effects of 4Mix postbiotics (named as Probiotic Extracts of 4 strains- number 1, PE0401) consisted of metabolites generated from Lactobacillus salivarius AP-32, Lactobacillus acidophilus TYCA06, Lactobacillus plantarum LPL28, Bifidobacterium longum subsp. infantis BLI-02 on anti-inflammatory activity, anti-oxidative capacity, regulation of tight junction proteins. The results displayed that anti-inflammatory activity of 4Mix postbiotics PE0401 was stronger than other mixed postbiotic combinations. The anti-oxidative capacity, which correlated to anti-inflammation, also significantly increased as shown in DPPH and FRAP assays. The epithelial tight junction proteins expressed in mRNA levels (ZO-1, ZO-2, Occludin, JAM-A, and Claudin) were highly potent modulated by PE0401. In addition, PE0401 selectively promoted the growth of intestinal bacterial strains including Lactobacillus, Bifidobacterium strains and other beneficial bacteria. Therefore, this study provides a fascinating insight into the strategy to the treatment of the intestinal disorders. PE0401 may deliver as health functional food ingredient.
Postbiotic, Chronic Inflammatory Bowel Disease (IBD), Anti-inflammatory, Anti-oxidation, Tight Junction Protein, Growth Promotion of Beneficial Bacteria
Chronic inflammatory bowel disease (IBD) were usually caused by the repetition of symptoms of intestinal wall inflammation. Patients with Crohn’s disease or ulcerative colitis are prone to present complications such as diarrhea, abdominal pain, weight loss, malnutrition, growth retardation in children, osteoporosis, higher risk of developing cancer.1 Tight junction proteins on intestinal wall including Occludins, Junctional adhesion molecule A protein (JAM-A), Claudin and Zonula occludens (ZO) contribute to hold the integrity of gut barriers and maintain healthy intestinal function. Chronic inflammation of Crohn’s disease would lead to damage and erosion of tight junctions on intestinal tract.2 The elevated anti-inflammatory cytokines such as transforming growth factor beta (TGF-β) and Interleukin 10 (IL-10) may benefit to maintain intestinal barrier function and repair epithelial wound.3
The postbiotic is defined as the metabolites secreted by probiotic strains,4 which has been reported functions of anti-inflammation, anti-oxidation, growth promotion, antidiarrheal activity and digestion.4,5 Probiotic strains secrete amino acids, vitamins, bacteriocins, peptidoglycan-derived muropeptide, short chain fatty acids (SCFAs) and promote anti-inflammatory and anti-oxidant effect in human gut.6,7 Additionally, researchers discovered that Bacillus strains generating metabolic enzymes including amylase, protease, catalase and superoxide dismutase facilitate the growth of Lactobacillus.8 However, previous studies haven’t tested the functions of multi-strain postbiotic mixture.
Therefore, in this study, we aimed to (i) screen potential multi-strain probiotics presented anti-inflammatory activities, (ii) test the synergistic anti-inflammatory effect of multi-strain postbiotic mixture, which mixed from several individual probiotic metabolites (Lactobacillus salivarius AP-32, Lactobacillus acidophilus TYCA06, Lactobacillus plantarum LPL28, Bifidobacterium longum subsp. infantis BLI-02), (iii) measure the anti-oxidative effect of multi-strain postbiotic mixture, (iv) verify whether postbiotic mixture promote intestinal health via regulating tight junction protein genes in epithelial cells, and (v) observe whether postbiotic mixture facilitate the growth of other intestinal beneficial bacterial strains.
Following the in vitro results, the four strains mixed postbiotics (named Probiotic Extracts of 4 strains- number 1, PE0401) showed the synergistic effect on promoting growth of beneficial bacterial strains and anti-inflammatory activity for alleviating chronic inflammatory bowel disease (IBD) and other inflammatory disorders.
Preparation of Probiotic Bacterial Strains, Postbiotics and Culture Medium
Lactobacillus salivarius AP-32 (BCRC-910437, CCTCC-M2011127), L. acidophilus TYCA06 (BCRC-910813, CGMCC-15210), L. plantarum LPL28 (BCRC 910536, CGMCC-17954), Bifidobacterium longum subsp. infantis BLI-02 (BCRC 910812, CGMCC-15212) were deposited at the Bioresource Collection and Research Center (Hsinchu, Taiwan), China Center for Culture Collection (Wuhan, China) and China General Microbiological Culture Collection Center (Beijing, China). The probiotic strains of AP-32 and TYCA06 were isolated from healthy human intestines, whereas LPL28 was from miso and BLI-02 was from healthy human breast (Table).
The probiotics and postbiotics of L. gasseri AI-88, L. salivarius AP-32, L. acidophilus TYCA06, L. plantarum LPL28, B. longum subsp. infantis BLI-02, L. johnsonii MH-68, L. reuteri GL-104, L. helveticus RE-78, L. paracasei ET-66, L. rhamnosus CT-53, L. plantarum TSP05, Lactococcus lactis subsp. lactis LY-66, Streptococcus thermophilus SY-66, B. animalis subsp. lactis BB-115, B. animalis L-39, B. animalis subsp. lactis CP-9, L. paracasei MP137, L. rhamnosus MP108, L. rhamnosus L-85, L. plantarum L-323, S. thermophilus L-243, and L. paracasei L-180 were acquired from Glac Biotech Co. Ltd., Tainan, Taiwan. B. animalis subsp. lactis BB-12, S. thermophilus UASt-09, L. acidophilus LA-5, and L. rhamnosus LGG were gained from Chr. Hansen, Denmark. B. longum BB536 was purchased from Morinaga, Japan. The L. gasseri LG21 was purchased from Meiji, Japan. The L. fermentum CECT5716 was obtained from Biosearch Life, Spain (Table).
The strains were identified using 16s rRNA sequencing and the API 50CHL kit (BioMerieux, France). The four strains mixed postbiotics (named as Probiotic Extracts of 4 strains- number 1, PE-0401) was developed as followed: four probiotic strains including Lactobacillus salivarius AP-32, Lactobacillus acidophilus TYCA06, Lactobacillus plantarum LPL28, Bifidobacterium longum subsp. infantis BLI-02 were cultured with 1:1:1:1 ratio and growing in culture medium under anaerobic conditions at 37°C for 16 hrs. Then, centrifuging the fermented broth at 18000 rpm for 20 min, and collecting supernatant part of fermentation solution as the PE0401. The abbreviation of the four strains mixed postbiotics, PE0401, will be used for the following sections.
The compositions of culture medium were listed below:
Soybean protein isolate (Arshine, China) 10%, glucose (Union chemical, Taiwan) 5%, peptone (STBIO MEDIA, Taiwan) 3%, skim milk powder (Warrnambool chees& Butter factory, Australia) 2%, yeast extract (Angel yeast, China) 1%, monosodium glutamate (MSG)( PT Cheil Jedang Indonesia) 1%, potassium hydrogen phosphate (Budenheim USA) 0.05%, Tween 80 ( Musim mas, singapore) 0.05%, sodium citrate (qinqdao fuso refining& processing, China) 0.01%, Manganese sulfate (Jost Chemical, USA) 0.01% and filed with deionized water to 100%
Preparation of Peripheral Blood Mononuclear Cells
The human peripheral blood was gained from blood samples of healthy donors (Tainan Blood Center, Taiwan Blood Services Foundation). The peripheral blood mononuclear cells (PBMCs) were isolated from low-density fraction (around 42.5%-50% interface) by centrifugation (3000rpm, 10 min) through a Ficoll-Hypaque gradient (Pharmacia, Sweden). The RPMI-1640 medium containing 10% fetal bovine serum (FBS) was used to culture PBMCs. The experimental procedure was according to previous study.6
Anti-inflammatory Cytokine Assay
Loading 100 μl isolated PBMCs into 96 well plate (No. 167008, MicroWell™ 96-Well, Thermo Fisher, USA) at 4×105 cells/100μl/well. Then co-culturing PBMCs with 20 μl of the postbiotics or probiotics (4×106 CFU) at ratio of 1:10 (postbiotics: PBMCs) in the 5% carbon dioxide (CO2) at 37°C for 48hrs. The control group was treated with 20 μl of PHA (Phytohemagglutinin, 0.2 μg/ml), and the medium control group was treated with 20 μl of cell culture medium. After 48 hrs of incubation, collecting the supernatant of co-culture medium by centrifuging the 96 well plate at 3000 rpm for 10 min at 4°C. Then, using ELISA assay (eBioscience) to measure IL-10 and TGF-β of the collected supernatant. Finally, cytokines levels were measured by μQuant microplate spectrophotometer (BioTek, USA) at absorbance value of 450 nm. The experimental protocol was according to previous research.6
Cells Culturing
The Caco-2 cells (HTB-37™) are epithelial cells isolated from colorectal adenocarcinoma and was obtained from American Type Culture Collection (USA). The Eagle’s Minimum Essential Medium (ATCC No. 30-2003) with final concentration of 20% fetal bovine serum (ThermoFisher, USA) was used to culture Caco-2 cells. The incubation condition was under 5% carbon dioxide (CO2) at 37°C. The following tests of anti-oxidative assay and tight junction proteins in mRNA levels were based on Caco-2 culturing platform.
Free Radical Scavenging Assay
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) is a stable nitrogen-centered radical (Sigma-Aldrich, USA). When the antioxidant compounds bind to DPPH, it causes a decreased level in OD517, which determined the free radical scavenging activity. The DPPH assay is performed as following description: 1:1 mixed 0.2mM DPPH in methanol solution with 0.5% postbiotics or 10 ug/ml of vitamin C (positive control) or medium (blank group), respectively. The mixture was incubated for 30 min at room temperature in the dark before centrifuging at 12000rpm for 2 min at 4°C. The sample was measured at absorbance of 517nm with μQuant microplate spectrophotometer (BioTek, USA). The calculation formula is as follows:
Free radical scavenging ability = ODblank – ODsample) /ODblank X 100
Where ODBlank is the absorbance value of the cell medium, and ODsample is the absorbance value of the tested samples.
Measurement of Antioxidative Activity by FRAP Assay
Ferric-reducing ability of power assay (FRAP assay; Sigma‑Aldrich, St. Louis, MO, USA) is a common method used to test the reducing power of antioxidants. In an acidic environment (under pH 3.6), the trivalent iron (Fe3+) in the FRAP reagent will be reduced to divalent iron (Fe2+) by binding with antioxidants, and caused the change of color. The 0.5% postbiotics, 10 ug/ml vitamin C (positive control group), and medium control (blank group) were added to Fe3+-TPTZ solution, and detected the OD593 value, respectively. When the Fe3+-TPTZ complex is reduced to Fe2+-TPTZ, the solution color will turn from yellow to blue. The deeper blue color indicated the stronger antioxidant capacity. The measured value is compared with the standard calibration curve.
Real-time PCR Technique Detected Tight Junction Proteins in mRNA Levels
Seeding and culturing 3 x 105 Caco-2 cells on a 6-well cell culture plate for 6 days (change the medium every 3 days). Co-culturing postbiotics treated Caco-2 cells for 5 hrs. Washing cell culture medium with PBS twice then extracting mRNA by adding 500 μl Trizol (Invitrogen™, Waltham, Massachusetts, USA) to each well. Using High-Capacity RNA-to-cDNA™ Kit (Thermo, Waltham, Massachusetts, USA) to invert extracted mRNA into cDNA. The PowerTrack™ SYBR Green Master Mix (Thermo, Waltham, Massachusetts, USA) and specific primers were used to perform probe-based qPCR assay on the QuantStudio 6 Pro Real-Time PCR platform (Thermo, Waltham, Massachusetts, USA). The experimental protocol and specific primer sequences were according to the Figure 1 of previous study.9
Detecting Growth Rate of Probiotic Strains
The 4Mix postbiotic PE0401 and seven prebiotics were prepared in 4.5 ml of culture medium with a content of 0.25 wt% and 0.5 wt%, respectively. Seven prebiotics were listed as below: human milk oligosaccharides (HMO; DuPont, USA), Maltodextrin (UNION FOOD, Taiwan), FOS (fructooligosaccharides; GreenGo Biomedical international, Taiwan) and GOS (galactooligosaccharides; GreenGo Biomedical international, Taiwan), sorbitol (SHENG YUANG FOOD INDUSTRIAL CO., LTD, Taiwan), erythritol (Buildmore Enterprise Co., Ltd, Taiwan), and inulin (BIOMED HERBAL RESEARCH BIOMEDICAL GROUP, Taiwan). Next, preparing 0.5 ml bacterial liquid of Lactobacillus rhamnosus MP108 (1×109 CFU/ml), Bifidobacterium animalis subsp. lactis CP-9 (1×109 CFU/ml) and other probiotic strains listed in Table were added in each test medium, respectively. All the test samples were incubated at 37°C for 6 hrs. After that, 0.02ml of the co-culture medium (prebiotics or 4Mix postbiotics obtained in each probiotic medium) was measured with a spectrophotometer (MQX200, BioTek) at 600 nm. The relative growth fold of probiotic strain was presented by following formula:
Formula: A = (B – C) / (C – D)
Symbol Definition
A indicated relative growth rate; B indicated OD600 absorbance value of each group; C indicated OD600 absorbance value of medium control; D indicated OD600 absorbance value of blank (ddH2O).
Statistics
The statistical differences between the experimental groups and the control group was analyzed by Student’s t test (SPSS 12, IBM, USA). p < 0.05 (*), p< 0.01 (**), and p<0.001 (***) indicated statistical significance. All experiments were performed in triplicate and presented as mean±SD.
Screening Potential Probiotics (TYCA06, LPL28, BLI-02, AP-32, L-39, and L-323) by Measuring Cytokines of TGF-β and IL-10
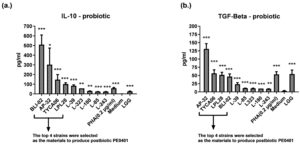
Firstly, we screened candidate strains with anti-inflammatory activity TGF-β. Several viable Lactobacillus and Bifidobacterium strains were co-cultured with PBMCs and detected changes of IL-10 and TGF-β by ELISA method. Results demonstrated that TYCA06, AP-32, BLI-02 and LPL28 effectively induced PBMCs to secrete IL-10 and TGF-β both for modulating anti-inflammatory activity (Figure 1). Whereas, the LGG strain could only induce a small amount of IL-10 and TGF-β.
Figure 1. Ranking probiotic candidates with anti-inflammatory assay
The anti-inflammatory activity of single-strain probiotic was evaluated by measuring the levels of IL-10 (a.) and TGF-β(b.) in PBMCs. The control group was treated with phytohaemagglutinin (PHA) (0.2 µg/ml), and the blank group was treated with medium only.
While comparing to medium control, the IL-10 levels induced by BLI-02, AP-32, TYCA06, LPL28, L-39, L-323, L-180, L-85, L-243, PHA, medium control and LGG were 509±99 pg/ml (***), 378±41 pg/ml (*), 147±51 pg/ml (***), 101±13 pg/ml (***), 91±18 pg/ml (***), 49±13 pg/ml (**), 37±12 pg/ml (**), 30±11 pg/ml (***), 27±5 pg/ml (***), 58±7 pg/ml (***), 0.1±0.6 pg/ml, 25±3 pg/ml (***), respectively (Figure 1a). While comparing to medium control, the TGF-β levels induced by AP-32, TYCA06, LPL28, BLI-02, L-39, L-85, L-323, L-180, L-243, PHA, medium control and LGG were 130±16 pg/ml (***), 56±10 pg/ml (***), 51±7 pg/ml (***), 46±7 pg/ml (***), 23±4 pg/ml (***), 19±13 pg/ml (***), 17±11 pg/ml (***), 15±8 pg/ml (***), 12±5 pg/ml ( **), 53±8 pg/ml (***), 3±0.9 pg/ml, 54±1 pg/ml (***), respectively (Figure 1, b). Six strains including TYCA06, LPL28, BLI-02, AP-32, L-39, and L-323, which revealed excellent anti-inflammatory effect, were selected to develop several mixed postbiotic combinations in the following test.
The 4Mix Postbiotics (PE0401) Revealed the Best Anti-inflammatory Activity among Different Postbiotic Combinations
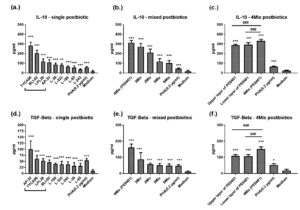
Next, we tested the anti-inflammatory effect of single postbiotics. The postbiotic TYCA06, BLI-02, LPL28, AP-32 induced PBMCs produced IL-10 to 283±34 pg/ml (***), 205±30 pg/ml (***), 118±27 pg/ml (***), 109±26 pg/ml (***), respectively (Figure 2a). AP-32, TYCA06, LPL28, BLI-02 significantly elevated TGF-β level to 100±33 pg/ml (***), 61±14 pg/ml (***), 52±8 pg/ml (***), 46±8 pg/ml (***), respectively (Figure 2d).
Among different postbiotic combinations, the 4Mix postbiotics (TYCA06, LPL28, BLI-02 and AP-32; PE0401) incited higher levels of TGF-β and IL-10 than other mixed postbiotic combinations (Figure 2b and d). The IL-10 levels incited by 4Mix postbiotics (PE0401), 3Mix postbiotics (AP-32, TYCA06, LPL28), 2 Mix postbiotics (AP-32, TYCA06), 5 Mix postbiotics (AP-32, TYCA06, LPL28, BLI-02, L-39), 6 Mix postbiotics (AP-32, TYCA06, LPL28, BLI-02, L-39, L-323), PHA and medium control were 313±21 pg/ml (***), 271±31 pg/ml (***), 210±36 pg/ml (***),119±29 pg/ml (***),103±25 pg/ml (***), 45±5 pg/ml (***), 17±10 pg/ml, respectively (Figure 2b). The TGF-β levels incited by 4Mix postbiotics (PE0401), 3Mix postbiotics, 2 Mix postbiotics, 5 Mix postbiotics, 6 Mix postbiotics, PHA and medium control were 161±21 pg/ml (***), 87±40 pg/ml (***), 57±6 pg/ml (***), 50±12 pg/ml (***), 48±10 pg/ml (***), 47±7 pg/ml (***), 10±4 pg/ml, respectively (Figure 2d).
Furthermore, to investigate which part of PE0401 solution presented the better anti-inflammatory activity, we once again separated the upper layer of PE0401 and the lower layer of PE0401 by re-centrifugation at 10000rpm for 20 min. The results showed that the original PE0401solution demonstrated better induction of IL-10 and TGF-β than the upper layer and the lower layer of the PE0401 (Figure 2c and f).
Figure 2. The anti-inflammatory effect of postbiotic combinations
The single probiotic (a. and d.), mixed probiotics (b. and e.), 4Mix probiotics (e. and f.) induced the levels of IL-10 or TGF-β in PBMCs. The control group was treated with phytohaemagglutinin (PHA) (0.2 µg/ml), and the blank group was treated with medium only.
The 4Mix Postbiotics (PE0401) Showed the Excellent Anti-oxidative Activity
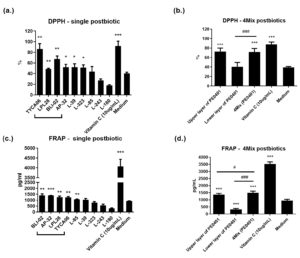
Moreover, we measured the anti-oxidative ability of single postbiotic and the 4Mix postbiotics (PE0401) (Figure 3). By comparing to the medium control, single postbiotic of TYCA06, LPL28, BLI-02, AP-32 and vitamin C significantly elevated free radical scavenging ability to 86±10% (**), 48±1% (**), 67±5% (**), 52±5% (*) and 91±9 % (***) in DPPH test, respectively (Figure 3a). Single postbiotics of BLI-02, AP-32, LPL28, TYCA06 and vitamin C also presented significant increase as 1397±131 pg/ml (** p<0.01), 1330±119 pg/ml (***), 1247±102 pg/ml (**), 1241±67 pg/ml (**) and 4070±366 pg/ml (***), respectively (Figure 3c).
Figure 3. The anti-oxidative activity of single postbiotic and 4Mix (PE0401)
The radical scavenging activity of single probiotic or 4Mix postbiotics (a and b) was assessed by DPPH assay, and the ferric-reducing ability was evaluated by FRAP assay (c and d). The positive control was treated with vitamin C (0.5%) and the blank group was treated with medium only.
The 4Mix postbiotics (PE0401) revealed significant increase in DPPH and FRAP assays (Figure 3b and d). In order to find out whether the anti-oxidative ability the original PE0401 solution comes from the precipitable part of death cell remnants or from the small molecules in the liquid part. The upper layer (the supernatant part of the centrifuged PE0401 solution) and the lower layer of PE0401 (the pellet part of the centrifuged PE0401 solution) were collected by re-centrifuging at 10000rpm for 20 min. The upper layer of the centrifuged PE0401 and the original PE0401 solution significantly rise anti-oxidative levels to 72±7 % (***) and 71±7% (***), respectively, by comparing to the medium control (39±1%). The original PE0401 solution presented higher DPPH level than the lower layer of centrifuged PE0401 (40±9%;###) (Figure 3b). FRAP assay showed similar result with DPPH test (Figure 3d). The upper layer of the PE0401 and the original PE0401 significantly rise anti-oxidative levels to 1365±93 pg/ml (***) and 1495±119 pg/ml (***), respectively, by comparing to the medium control (940±101 pg/ml). The original PE0401 solution presented significant higher DPPH level than the upper layer (###) and the lower layer of PE0401 (329±58 pg/ml;#) (Figure 3d).
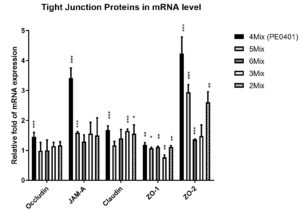
The 4Mix postbiotics (PE0401) significantly increased the mRNA levels of epithelial tight junction proteins (Occludin, JAM-A, Claudin, ZO-1 and ZO-2).
Next, to investigate whether PE0401 ameliorated gut inflammation via elevating intestinal tight junction proteins expression in mRNA and promoting the integrity of intestinal mucosa layer [3,4]. The 4Mix postbiotics (PE0401) significantly upregulated the relative fold in mRNA expressions of tight junction proteins, which were Occludin (1.45±0.15, ***), JAM-A (3.42±0.33, ***), Claudin (1.68±0.14, ***), ZO-1 (1.19±0.07,**) and ZO-2 (4.23±0.56,***), respectively (Figure 4). Other combinations of 5Mix postbiotics, 6Mix postbiotics, 3Mix postbiotics and 2Mix postbiotics also significantly elevated relative fold of ZO-1 mRNA expression, which were 1.07±0.04 (*), 1.11±0.03 (**), 0.77±0.08 (**) and 1.12 ±0.04 (**), respectively. Additionally, the 5Mix postbiotics also efficiently increased the relative fold of JAM-A and ZO-2 mRNA expressions, which were 1.59±0.04 (***) and 2.94±0.26 (***), respectively.
Figure 4. The postbiotic combinations regulated gut epithelial junction via elevating tight junction proteins in mRNA levels.
Gene expression of Occludin, JAM-A, Claudin, ZO-1 and ZO-2. The mRNA expressions in the medium control were set at 1.
The 4Mix Postbiotics (PE0401) Facilitate Growth of Probiotic Strains
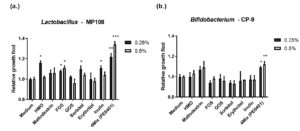
Finally, the 4Mix postbiotics (PE0401) was tested to improve intestinal health via facilitating gut beneficial bacteria including several Lactobacillus and Bifidobacterium strains (Figure 5 and Table). By comparing to the medium control, 0.25% and 0.5% of 4Mix postbiotics (PE0401) would significantly facilitate the growth fold of Lactobacillus rhamnosus MP108 to 1.22±0.03 (**) and 1.34±0.03 (***), respectively (Figure 5a); likewise, the growth fold of Bifidobacterium animalis subsp. lactis CP-9 were 1.09±0.01 (*) and 1.12±0.03 (**), respectively (Figure 5b).
Figure 5. The 4Mix postbiotics (PE0401) and prebiotic facilitated the relative growth fold of beneficial strains.
Different concentrations (0.25 and 0.5 %) of 4Mix postbiotics, prebiotic and medium control promoted the relative growth fold of (a.) Lactobacillus rhamnosus MP108 and (b.) Bifidobacterium animalis subsp. lactis CP-9.
Besides, prebiotics are mostly non-digestible food-grade fibers, sugar alcohols and oligosaccharides, which selectively improved the growth certain genera of gut microorganisms (mostly Lactobacilli and Bifidobacteria).10 Here, seven prebiotics, which included HMO, maltodextrin, FOS, GOS, sorbitol, erythritol, and inulin, presented significant growth promoted ability to beneficial bacteria. The 0.25% HMO, 0.25% FOS, 0.5% FOS, 0.25% sorbitol and 0.25% inulin significantly promoted the growth fold of L. rhamnosus MP108 to 1.16±0.02(*p<0.05), 1.08±0.01(*), 1.11±0.02(*), 1.10±0.03(*), and 1.11±0.02(*), respectively (Figure 5a). However, only PE0401 promoted the growth rate of B. animalis subsp. lactis CP-9, whereas seven prebiotics didn’t.
Table:
The 4Mix postbiotics (PE0401) promoted the relative growth fold of different probiotic strains.
Probiotic strains |
Source |
Relative growth fold |
|---|---|---|
Lactobacillus gasseri AI-88 |
Human breast milk; Glac, Taiwan |
1.62 ± 0.11*** |
L. gasseri LG21 |
Meiji, Japan |
1.15 ± 0.01** |
L. fermentum CECT5716 |
Biosearch Life, Spain |
1.46 ± 0.05*** |
L. johnsonii MH-68 |
Human intestine; Glac, Taiwan |
1.31 ± 0.07** |
L. reuteri GL-104 |
Human intestine; Glac, Taiwan |
1.39 ± 0.02*** |
L. helveticus RE-78 |
Kimchi; Glac, Taiwan |
1.42 ± 0.09** |
L. paracasei MP137 |
Human intestine; Glac, Taiwan |
1.17 ± 0.05** |
L. paracasei ET-66 |
Human breast milk; Glac, Taiwan |
1.27 ± 0.04*** |
L. rhamnosus CT-53 |
Human vagina; Glac, Taiwan |
1.28 ± 0.11** |
L. rhamnosus MP108 |
Human intestine; Glac, Taiwan |
1.34 ± 0.03*** |
L. rhamnosus LGG |
Chr. Hansen, Denmark |
1.15 ± 0.03* |
L. plantarum LPL28 |
Miso; Glac, Taiwan |
1.26 ± 0.05** |
L. plantarum TSP05 |
Kimchi; Glac, Taiwan |
1.21 ± 0.25* |
L. acidophilus TYCA06 |
Human intestine; Glac, Taiwan |
1.64 ± 0.04** |
L. acidophilus LA-5 |
Chr. Hansen, Denmark |
1.77 ± 0.01** |
Lactococcus lactis subsp. lactis LY-66 |
Human intestine; Glac, Taiwan |
1.16 ± 0.04* |
Streptococcus thermophilus SY-66 |
Fermented milk; Glac, Taiwan |
1.33 ± 0.04*** |
S. thermophilus UASt-09 |
Chr. Hansen, Denmark |
1.20 ± 0.03** |
Bifidobacterium animalis subsp. lactis BB-115 |
Human intestine; Glac, Taiwan |
1.13 ± 0.03** |
B. animalis subsp. lactis CP-9 |
Human breast milk; Glac, Taiwan |
1.12 ± 0.03** |
B. animalis subsp. lactis BB-12 |
Chr. Hansen, Denmark |
1.27 ± 0.07** |
B. longum subsp. infantis BLI-02 |
Human breast milk; Glac, Taiwan |
1.29 ± 0.04** |
B. breve Bv-889 |
Human breast milk; Glac, Taiwan |
1.21 ± 0.04* |
B. longum BB536 |
Morinaga, Japan |
1.49 ± 0.05*** |
Notes: Compared to the control group, *, P < 0.05 indicates significant differences; **, P < 0.01 indicates very significant differences ***, P < 0.001 indicates extremely significant differences.
Inflammation is an immunovascular response for human body to deal with harmful stimuli including pathogens, irritants, toxins and injuries. When irritants enter and damage cells, inflammatory response functions to release chemicals that enhance vascular permeability for facilitating migration of leukocytes to injured site. Through the inflammatory activities, the necrotic cells will be cleared up and tissue repair will be initiated.11 However, prolonged inflammation correlated with Crohn’s disease,1 ulcerative colitis,1 oxidative stress,12 aging,13 atherosclerosis,14 osteoarthritis,15 chronic pain,16 and cancer.17
Cytokines are peptides (5–20 kDa) produced by various cells, which play important roles in communicating between cells, mediating autocrine, paracrine and endocrine signaling, especially involved in inflammatory and anti-inflammatory responses.18,19 TGF-β and IL-10 belong to anti-inflammatory cytokines, which prohibit inflammatory response through regulating pro-inflammatory cytokines.20 The secretion of IL-10 increases the expression of specific endogenous receptors, and suppresses inflammatory cytokines. An animal model confirmed IL-10 protein regulated neuropathic and inflammatory pain.21 In addition, TGF-β, a cytokine induces Treg cells, was found to suppress macrophage and Th1 cell activity which produced pro-inflammatory cytokines.22
At present study, results showed individual probiotic strains of TYCA06, BLI-02, LPL28, and AP-32 effectively increased the TGF-β and IL-10 levels in PBMCs (Figure 1). In vitro test presented that the postbiotics of TYCA06, BLI-02, LPL28, and AP-32 significantly improved the TGF-β and IL-10 levels (Figure 2a and d). The 4Mix postbiotics (PE0401) demonstrated excellent anti-inflammatory effect among other mixed postbiotics (Figure 2b and e), in which the PE0401 revealed better anti-inflammatory function than upper layer and lower layer of re-centrifuged PE0401 (Figure 2, c and f). Besides, accumulated free radicals in human body would further lead to various inflammatory-associated diseases.23 Here, PE0401 presented anti-oxidative effect (Figure 3a and c), in which the upper layer of re-centrifuged PE0401 may play the major function in free radicals scavenging (Figure 3b and d). It’s presumed that four viable probiotic strains secreted anti-oxidative components to the upper layer of re-centrifuged PE0401. However, the major components of the 4Mix postbiotics involved in anti-inflammation and anti-oxidation should be analyzed in the future.
The inflamed colonic mucosa usually altered tight junction protein structure and impaired barrier function.24 A leaky intestinal barrier caused permeation of endotoxins, bacteria, and luminal antigens into the blood stream, which contributed to chronic intestinal inflammation and diarrhea.25 Previous study discovered that tight junction proteins of JAM-A, Occludin, and ZO-2( in mRNA levels) would significantly increase by treating probiotic strains of B. animalis subsp. lactis CP-9, L. salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and L. reuteri GL-104.9 Here, we discovered PE0401 also improved the expressions of Occludin, JAM-A, Claudin, ZO-1 and ZO-2 in mRNA levels (Figure 4).
Prebiotic products such as Human milk oligosaccharide (HMO) have been identified its benefit on promoting the growth in some probiotic strains.10,26 Postbiotics also functioned in facilitating growth of viable probiotic strains at previous study,27 and inhibiting the growth rate of pathogenic bacteria via short chain fatty acids (SCFAs).27,28 The present study discovered that PE0401promoted the growth rate of several beneficial microbial strains of Lactobacillus, Bifidobacterium and Streptococcus thermophilus (Table 1), notably L. rhamnosus MP108 and B. animalis subsp. lactis CP-9 (Figure 5). Our results suggested that the probiotic growth promotion rate of PE0401 was superior to other prebiotic products (Figure 5). PE0401 facilitated the growth of MP108 and CP-9, whereas seven selected prebiotics only promoted the growth of MP108. The functional components of the PE0401 should be measured by LC-MS and HPLC method in the future.29 In conclusion, PE0401 synergistically promotes growth of beneficial bacterial strains and anti-inflammatory activity, which will be a potential functional food for alleviating chronic inflammatory bowel disease (IBD) and other inflammatory dysfunctions.
ACKNOWLEDGMENTS
The authors would like to thank Lab members of the research and design department of Glac Biotech Co., Ltd. (Tainan, Taiwan) for their assistance in collecting and analysing experimental data.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
YWK and HHH designed the project. YFH, CHH, JHL, CHL, CCL, THY, YWC applied methodology, performed data analysis and project administration. WYL and CWC visualized the data. HHH performed supervision. WYL and Ych wrote the original draft. YWK, CWC and YCH reviewed the manuscript. YCH edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Vilela EG, da Gama Torres HO, Martins FP, et al. Evaluation of inflammatory activity in Crohn’s disease and ulcerative colitis. World J Gastroenterol. 2012;18(9):872-881.
Crossref - Schulzke JD, Ploeger S, Amasheh M, et al. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009;1165:294-300.
Crossref - Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte–epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151(4):616-632.
Crossref - Aguilar-Toala JE, Garcia-Varela R, Garcia HS, et al. Postbiotics: An evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105-114.
Crossref - Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53(6):821-828.
Crossref - Hsieh PS, An Y, Tsai YC, et al. Potential of probiotic strains to modulate the inflammatory responses of epithelial and immune cells in vitro. New Microbiol. 2013;36(2):167-179. PMID: 23686123
- Ayyanna R, Ankaiah D, Arul V. Anti-inflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats. Front Microbiol. 2018;9:3063.
Crossref - Yu T, Kong J, Zhang L, Gu X, Wang M, Guo T. New crosstalk between probiotics Lactobacillus plantarum and Bacillus subtilis. Sci Rep. 2019;9(1):1-9.
Crossref - Hsieh PS, Ho HH, Tsao SP, et al. Multi-strain probiotic supplement attenuates streptozotocin-induced type-2 diabetes by reducing inflammation and β-cell death in rats. Plos One. 2021;16(6):e0251646.
Crossref - Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics-a review. J Food Sci Technol. 2015;52(12):7577-7587.
Crossref - Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147(2):227-235.
Crossref - Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797.
Crossref - Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23-28.
Crossref - Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Eng J Med. 2005;352(16):1685-1695.
Crossref - Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471-478.
Crossref - Zhang JM, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45(2):27.
Crossref - Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-867.
Crossref - “Cytokine” in John Lackie. A Dictionary of Biomedicine. Oxford University Press. 2010. ISBN 9780199549351
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation. 2014;2014.
Crossref - Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Current opinion in pharmacology, 2009;9(4):447-453.
- Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45(2-3):389-395.
Crossref - Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β). Growth Factors. 1993;8(1):1-9.
Crossref - Arulselvan P, Fard MT, Tan WS, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016:5276130.
Crossref - Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116(2):301-309.
Crossref - Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol. 2016;120(6):692-701.
Crossref - Thongaram T, Hoeflinger JL, Chow J, Miller MJ. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J Dairy Sci. 2017;100(10):7825-7833.
Crossref - Ho HH, Kuo YW, Chen JF, et al. The Postbiotics, Totipro PE0401, and Probiotic Mixture, PF1001, Modulate the Gut Microbiota and Ameliorate Diarrhea in Weaning Piglets. Biomed J Sci Tech Res. 2020;28(1):21194-21205.
Crossref - Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microbial Cell Factories. 2020;19(1):1-22.
Crossref - Moradi M, Molaei R, Guimaraes JT. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme Microb Technol. 2021;143:109722.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.