ISSN: 0973-7510
E-ISSN: 2581-690X
Potassium mobilizing bacteria (KMB) strains have been isolated from waste mica mines in the Giridih district of Jharkhand, India, using Alexandrov media. These isolates were evaluated for their potential to dissolve water soluble-K from waste mica (muscovite and biotite). Identity was confirmed based on sequencing of 16S rDNA region of those isolates showing promising water soluble-K dissolving capacity. Strains were found to be different isolates of Bacillus cereus, two unconfirmed Bacillus species (strain- 6SB1 and GG6), and one each of B. velezensis and Paraburkholderia kururiensis. Finally, the four most efficient KMB were selected based on their K-mobilizing capability. The K5B (B. cereus) isolate showed the highest K-solubilising capacity in both muscovite and biotite enriched medium. Soil incubation study was conducted using soils of Giridih (Alfisol) with three gradient concentrations of both waste mica tailings and K-solubilising capacity of four KMB isolates (B. cereus, strain- K5B, K6, K15; and Bacillus sp. GG6- K12) were measured at 4, 7, 14 and 21 days intervals. The K release dynamics in incubated soils indicated that potassium was released from both types of micas to significantly higher water-soluble K (WS-K) and exchangeable K (Ex-K) pools due to the inoculation of KMB isolates. Apart from potassium solubilization, B. cereus strain K5B and Bacillus sp. GG6 showed capabilities to produce indole acetic acid (IAA) and gibberellic acid (GA). These results suggested that a combination of KMB strain and powdered mica tailings could be a suitable alternative to commercial chemical fertilizers and maintain soil nutrient status for plant uptake.
Potassium Mobilizing Bacteria (KMB), Muscovite, Biotite, 16S rDNA, Biofertilizers
Mineral nutrition and fertilization are the two most important factors for crop production and productivity. Among the plant nutrients, nitrogen (N) is regarded as the most effective nutrient for crop productivity and quality. Although, the continuous and long-term application of N alone reduces the efficiency of crop production.1 This might be due to an unbalanced nutrient supply, which is accelerated by depletion and leaching of basic cation like potassium in soils.2 To meet the food grain production in recent years, primarily focus shifted towards the use of nitrogen and phosphorus; while the use of potassium is often neglected.
After nitrogen (N), Potassium (K) is the second most abundant nutrient in plant leaves and also the most abundant cation in the plant cell.3 It is one of the crucial macronutrients after nitrogen (N) and phosphorus (P) for plant growth and maintenance of grain quality. It plays a major role in the synthesis of protein, enzyme activation, starch production, cellulose, and vitamins.4 Additionally, it influences photosynthesis at multiple levels and participates in nutrient transport and uptake. Potassium also imparts resistance against different biotic and abiotic stresses, which helps in increasing crop production and provides immunity to plant diseases.5,6 The deficiency of potassium leads to poor root development, stunted growth, and decreased immunity resulting in a significant reduction in yield. There are several reports available regarding the deficiency of potassium in Indian soil due to the rapid development of the agricultural sector. However, in comparison to nitrogenous or phosphatic fertilizers application of potassic fertilizers are often neglected. According to a report by Meena et al.7 out of 371 districts (~11 million soil test data), potassium fertility status was found to be low in 21% of districts, medium in 51% of districts, and high in 28% of districts. Moreover, potassium is required by plants in large amounts, but marginal farmers in India couldn’t afford potassic fertilizers due to its high cost.8 This situation is further complicated by the absence of potassium-bearing minerals for the production of potassic fertilizers in the country, which results in a huge amount of import from other countries.6
Potassium in the soil is available to plants by three means; dissolved in soil water (water-soluble K), adsorbed by clay or organic matter (non-exchangeable K), and held within a different crystal structure like mica or feldspar. Potassium held in organic matter is easily leached out due to its high solubility. Water-soluble K, available directly to plants shares only 0.1 – 0.2% of total soil potassium. Both water-soluble and exchangeable fractions of potassium comprised only 1 – 2% and the remaining 96 – 99% remain as soil unavailable fractions.9,10 The availability of K to plants depends upon several factors like availability of other forms of potassium (solution, exchangeable and non-exchangeable) and weathering of minerals like feldspar and micas Sparks.11 Some soil microorganisms have been reported to have the capacity of mobilizing insoluble or fixed forms of potassium from minerals like micas, illite, and orthoclase.12 They are often regarded as potassium mobilizing microorganisms (KMM).
KMM including both bacteria and fungi influences the availability of soil minerals, thus playing a central role in ion cycling and maintaining soil fertility.7 They produce some organic acids which help to dissolve rock-bound potassium or chelate silicon ions to bring potassium into the solution, thus make available to plants.13 There are certain bacteria, which have been reported to release a certain amount of potassium from minerals like aluminosilicate.12 Several bacterial species like Bacillus mucilaginosus, B. edaphicus, B. circulans, Paenibacillus spp., Acidithiobacillus ferrooxidans, Pseudomonas, and Burkholderia have been reported as efficient potassium mobilizing bacteria (KMB). Fungal species like Aspergillus terreus and A. niger were isolated from potassium-rich soil showed a great potential of mobilizing insoluble potassium in a liquid medium in presence of insoluble sources of potassium like feldspar and potassium aluminium silicate.14 While several fungal species including arbuscular mycorrhizal fungi have been reported to solubilize potassium, the KMBs are known for their efficiency for solubilising unavailable K and applications as microbial fertilizers, mining, and metallurgy.15 Apart from potassium solubilization, they have also been reported to release plant growth regulating substances, antibiotic production, organic matter biodegradation, and nutrient recycling.16,17 Therefore, using these microorganisms as biofertilizers could be an alternative option to chemical fertilizers along with maintaining soil fertility, agricultural improvement, and environmental sustainability.
India is the top producer of mica distributed over a total area ~ 38882 km in Munger district of Bihar and Koderma and Giridih districts of Jharkhand. During the processing of mica production, about 75% of total mined mica is generated as waste contains a significant amount of potassium (8 – 12 % K2O), which could be a potential source of potassium for agricultural uses.18 These waste mica tailings are categorized as muscovite mica, can effectively be used as sources of potassium if modified or altered by some chemical or biological processes.12 To this end, the use of potassium mobilizing bacteria can be an effective solution. However, the success of each KMB depends upon the identification of the efficient bacterial strain. Native bacterial strain in high potassium enriched soil might have more efficient potassium solubilization capability.6 isolated twelve potassium mobilizing rhizobacteria from some Kharif crops of potassium enriched mica mines from the Koderma district of Jharkhand, India. Two strains, namely A. tumefaciens OPVS 11 and Rhizobium pusense OPVS6 showed the highest potassium mobilizing ability. However, to date, there is no report of KMB isolated from Giridih district of Jharkhand, India which is the second-largest producer of mica next to Koderma. There are several active mines in Giridih, Jharkhand, where mining activities such as crushing, grinding, washing, smelting result in the generation of a huge amount of mica tailings. These bare tailings are very prone to erosion and cause environmental toxicity. Therefore, the combined use of native KMB and waste mica tailings could be a potential solution to inorganic chemical potassic fertilizer and environmental contamination. Moreover, there are little information available on KMB in rice agroecosystem and their potential as potassium solubilizer.
In view of the above discussion, the specific research objectives of this study were (i) isolation and characterization of promising KMB isolates from mica contaminated agricultural fields of rice; (ii) evaluation of potassium mobilising capacity of selected KMB isolates using both qualitative and quantitative approaches; (iii) assessing the ability of KMB isolates in producing plant growth promoting substances and other macro-nutrients (P-solubilization and N-fixation).
Soil sampling
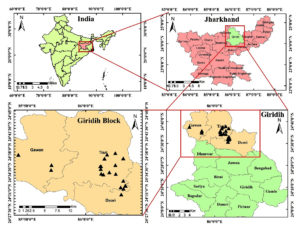
Rhizospheric soil samples were collected during the maturity phase of rice (Oryza sativa L.) from three blocks (Deori, Gawan, and Tisri) of Giridih district of Jharkhand, India in September 2016 (Figure 1). The majority of active mica mines in Giridih are distributed within these three blocks of the district. Samples were collected from rice fields near mica mines where leached out and surface run-off water from mines is stored naturally due to lower altitude. From each mine, three soil samples were collected randomly. Therefore, in total 63 soil samples (= sampling site) were collected from 21 mica mines. Each sampling site consisted of soil samples collected from three nearby rice fields following the W-pattern sampling method. Finally, ~1 Kg soil/site was collected following the subsampling method. Surface soil was removed before the collection of rhizospheric soil from a depth of ~10 cm.6 Samples were kept in sterile plastic bags, properly labelled, and brought back to the soil laboratory of the Indian Statistical Institute, Giridih within the same day, and stored at 4°C before processing. Samples of processed within seven days of collection.
Figure 1. Sampling locations (green circle) of mica mines (n= 21) distributed across three blocks (Deori, Gawan, and Tisri) of Giridih district of Jharkhand, India
Along with soil collection, waste mica tailings were also collected during sampling. These mica tailings were dumped near mines during the processing of mica. From each mine, ~1 kg mica tailings were collected, labelled properly, and stored in a plastic jar for further experiment. The pure form of both muscovite and biotite was obtained from the Geological Research Unit, Indian Statistical Institute, Kolkata, India. These forms were used for the potassium solubilization capacity test of the selected bacterial strain.
Potassium (K) content in waste mica
Both muscovite and biotite were grounded using a mixture grinder and passed through a 2mm sieve for further experimental use. Waste powder mica was immersed in sterile distilled water for 48 hours to eliminate water-soluble potassium. Total potassium content in waste mica was determined by following the hydrogen fluoride digestion method and measured by using a flame photometer (Systronics-130).19 The initial concentration of potassium in muscovite and biotite were found to be 26.98 mg/L and 27.98 mg/L respectively.
Isolation of potassium mobilizing bacteria (KMB)
Commercial Aleksandrow broth medium (CAB- HiMedia Laboratories, M-1997) was used for screening of KMB. The enrichment technique followed by a serial dilution technique (in 0.87 % normal sterile saline solution) was used for the isolation. In the enrichment process, 5 g of soil was inoculated into sterile CAB broth media and incubated at 28 ± 2°C under shaking conditions for seven days. After incubation, serially diluted (10-6) enriched KMB isolates were plated on CAB media with agar-agar (3%) followed by incubation at 28 ± 2°C for seven days.20,6 The pure culture of the colonies forming a clear halo zone was further established in Aleksandrow broth media.21 Screened KMB isolates were stained (gram-stain) for approximate detection of purity of each of KMB isolates, and KMB pure colonies (n = 30) were transferred to sterile slants on nutrient agar medium (HiMedia) and sterile glycerol stock medium for long term preservation at -20° C.6
Potassium mobilizing ability of screened KMB isolates (first layer screening)
The potassium solubilization capacity of KMB isolates were measured in both qualitative and quantitative approaches. In the case of the qualitative approach, pure KMB isolates were plated in modified CAB agar media as previously described and the zone of solubilization was measured using a digital calliper (Mitutoyo) after 7 days of incubation. Measurements were taken as described by Meena et al.6 For morphological characterization of pure KMB isolates, a standard phenotypic technique was followed as described by Holt et al.22
For quantitative assessment, 30 pure KMB isolates were selected which showed prominent halo zone formation during the qualitative assay. Those isolates were raised to a population of 10-8 CFU/ML/ml under overnight shaking (150 rpm, 28 ± 2°C, OD600 0.5) condition in 30 ml of commercial Aleksandrow broth (CAB) media. Uninoculated CAB served the purpose of control. These isolates (replications = 3) were incubated in a shaker incubator (150 rpm, 28 ± 2°C) for 7, 14, and 21 days as described by.6 Finally, temporal changes of available potassium content were measured using the flame-photometric method (Systronics-130). This experiment was repeated thrice for statistical analysis.
Quantification of soluble potassium and pH in modified Aleksandrow broth (second layer screening)
In the second layer of screening, 10 pure KMB isolates were selected based on their performance in the first layer of screening. These isolates were raised in manually prepared modified Aleksandrow broth media (MAB – per litter, 5.0 g glucose, 0.005 g MgSO4.7H2O, 0.1 g FeCl3, 2.0 g CaCO3, and 3 g of either powdered muscovite or biotite as a source of potassium).23 Incubation conditions and period of incubation were similar as described in the previous section (section- first layer screening). For quantification of soluble potassium, KMB inoculated broth (MAB) solutions (30 ml) were vortexed for 10 minutes followed by centrifugation at 5000 rpm for 30 minutes to separate the supernatant from grown bacterial cells and waste mica (both muscovite and biotite). Potassium concentration and pH of supernatant were measured by flame-photometry and pH meter, respectively. Finally, a comparison was made to uninoculated control. To prepare a standard curve, we followed the protocol as described by Meena et al.6 This experiment was repeated three times with three replications and the result was presented as mean ± SD with pooled data.
Physiological characterization of KMB isolates
For physiological characterization, the survival ability of KMB isolates was tested in a wide range of temperature, pH and saline conditions.24 For temperature tolerance, selected four KMB isolates (100 µl of 10-8 CFU/ML/ml) were grown in sterile 30 ml commercial Aleksandrow broth medium (CAB) and incubated at varying temperature ranges (20, 25, 30, 35, and 45°C) for 14 days with three replications. At 14 days after incubation (DAI), the turbidity of broth media was measured by optical density (OD) at 530 nm using a UV spectrophotometer. Relative growth of bacterial culture at varying temperature ranges was determined by using the OD data.23 Similarly, pH tolerance was measured by adjusting the pH level (4.5, 5.5, 6.5, 7.5, 8.5, and 9.5) of CAB using 0.1 N HCl and NaOH and incubated for 14 days at 30 ± 2°C. To assess the salt tolerance, KMB isolates were grown in CAB, supplemented with different concentrations of NaCl (1, 2, 4, 6, 8, and 10%), and incubated as described in the case of pH tolerance.
Plant growth-promoting attributes of KMB isolates
Indole acetic acid (IAA) production
KMB isolates (10-8 CFU/ml) were grown in CAB supplemented with L-Tryptophan (100 mg/L) in triplicates and incubated in a shaker incubator (150 rpm, 28 ± 2°C) for 7 days. After 7 DAI, cultures were centrifuged at 5000 rpm for 30 minutes to remove bacterial cells and excess insoluble elements of media. IAA production was measured by a spectrophotometric method using Salkowski reagent (50 ml, 35% Perchloric acid, and 1 ml 0.5 M FeCl3 solution).25 IAA production was measured as described by Saha et al.23
Gibberellic acid (GA) production
Gibberellic acid (GA) production was estimated by the 2, 4- Dinitrophenyl hydrazine (DNPH) method.26 Briefly, KMB isolates (10-8 CFU/ml) were grown in CAB (10 ml) for 10 days followed by centrifugation as described earlier (see section Indole acetic acid (IAA) production). An equal volume of Cell-free supernatant and ethyl acetate was mixed by vigorous vortex (10 minutes) and left for separation of ethyl acetate layer (repeated thrice). After complete evaporation of the ethyl acetate layer, the left-over supernatant was dissolved in an equal volume of absolute alcohol.27 1 ml of DNPH was added to this mixture (2ml) followed by subsequent hot (100°C for 5 min in the hot water bath) and cold incubation at room temperature. 5 ml of 10% KOH was added to this suspension and left of red wine colour development. The concentration of GA was measured using a spectrophotometer at 430 nm against the standard GA (HiMedia) as described by Banerjee et al.28
Hydrogen cyanide (HCN), Ammonia, and Siderophore production
To identify whether selected KMB isolates can produce HCN, they were streaked on a Kings’s B agar media supplemented with 0.4% (w/v) glycine. A Whatman (No.-1) filter paper soaked with alkaline picric acid (2% Na2CO3 in 0.5% picric acid) was placed on the lid of the Petri plate and incubated (28 ± 2°C) for 7 days. The Colour change of filter paper from red-brown to yellow indicated HCN production.29
Ammonia production ability was confirmed by a growing single colony of KMB isolates in 10 ml peptone broth (Peptone – 10 g/L; NaCl – 5 g/L) and incubated (28 ± 2°C) for 3 days. At 3DAI, Nessler’s reagent was added to the broth, and the change of colour from brown to yellow indicated ammonia production.30
Siderophore production; was determined by the CAS (Chrome Azurol S) assay.31,32 The pure bacterial colonies were stricken on CAS plates and incubated at 28 ± 2°C for 4 days. The orange-yellow halo around the growing colonies indicated siderophore production.
Phosphate mobilizing ability of KMB isolates
The phosphate solubilization ability was confirmed by raising (28 ± 2°C for 3 days) selected KMB isolates in Pikovskaya’s agar media-producing halo zone around a grown-up colony.33 The diameter of the halo zone provided a qualitative assessment. For, quantitative assessment, KMB isolates (10-8 CFU/ml) were grown in 30 ml of Pikovskaya’s broth in a shaker incubator (28 ± 2°C for 7 days, 150 rpm). Uninoculated Pikovskaya’s broth acted as the control. Soluble phosphorus of cell-free supernatant (centrifuge at 5000 rpm for 30 minutes) was measured by a modified Olsen method.34
Nitrogen-fixing ability of KMB isolates
Nitrogen-free Jensen agar media was used to grow (at 30°C for 3 days) the KMB isolates and colony growth was measured for qualitative assessment of nitrogen-fixing ability. For quantitative assessment, bacterial isolates were grown in Jensen broth media as previously discussed. The concentration of fixed nitrogen was measured by digestion and subsequent estimation by the modified Kjeldahl method.35
Soil incubation study
Soil incubation study was conducted at Agricultural experimental farm of ISI-Giridih, Jharkhand. 5 kg of sub-surface soil was autoclaved thrice for complete sterilization. Grounded waste micas (muscovite or biotite) were mixed thoroughly with 300 g sterilized soil at different concentrations (see Supplementary Table 1 for treatments detail). This mixture was kept in UV sterilized plastic container and brought to 60% water holding capacity, incubated with KMB (10-8 CFU/ml) isolates for 4, 7, 14, 21 days at 28 ± 2°C. Water-soluble and exchangeable potassium was measured at different DAI was measured following a standard protocol.36
Molecular characterization of KMB isolates
For confirmatory molecular identification, genomic DNA was isolated from a single colony of KMB isolates using a DNA isolation kit (NucleoSpin Microbial DNA, MACHEREY- NAGEL). Genomic DNA was stored at -20°C for further use. Amplification of 16S ribosomal DNA was carried out using bacterial universal primers sets, forward- 27f (5´-AGAGTTTGATCCTGGCTCAG-3´) and reverse- 1492r (5´-TACGGTTAC CTTGTTACGACTT-3´). For PCR amplification, 25 µl reaction mix contained 45 ng of genomic DNA, 5U/µl of Taq polymerase (Takara Bio Inc.), 2.5 µl 10 buffer, 2.5 mM dNTP Mixture, 1.5 mM MgCl2, and 10 pmol/µl of each primer. PCR reaction was performed using the following conditions: initial denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 94°C for 30 sec, annealing temperature at 54°C for 1min, extension at 72°C for 1min, and a final extension at 72°C for 7 min. To confirm amplification, PCR amplified product (5 µl) was visualized using 1% agarose gel electrophoresis and visualized in a gel documentation system (Bio-Rad). A 100 bp plus ladder (GeNetBio Corpo, Korea) was used to determine the product size. Once confirmed, amplified PCR products were gel purified using a PCR purification kit following the manufacturer’s protocol (Macherey-Nagel) and kept at -20°C for further analysis.6
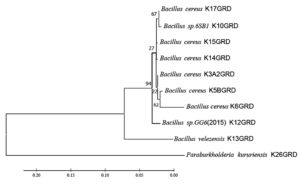
Gel purified samples were sent to AgriGenome Labs Pvt. (Hyderabad, India) for sequencing of 16S ribosomal DNA. The obtained sequence was analyzed using BioEdit (version-7.2.5) and then the BLAST tool was used for similarity search in NCBI (www.ncbi.nlm.nih.gov). The sequences were submitted to NCBI GenBank and finally, a phylogenetic tree was constructed using the Maximum-Likelihood method with 1000 bootstrap in Molecular Evolutionary Genetics Analysis Software (MEGA version-X).
Statistical analysis
All data obtained in the laboratory experiments were subjected to statistical analysis as ANOVA (analysis of variance), assessed by Least significant difference (LSD) with a probability p < 0.05, by using R statistical software (version- 4.0.2). The regression equation for the quadratic response for physiological characterization of KMB isolates was also conducted using the same software. For the soil incubation study, we have performed three-way ANOVA to identify the significance of potassium solubilization across doses, bacterial strains and time points.
Isolation and characterization of KMB isolates
During the initial isolation of KMB, a total of 95 bacterial colonies were found to grow in CAB media. Out of these, 30 bacterial isolates were found to produce a clear halo zone indicating the capacity to solubilize potassium.37 The diameter of the zone of solubilization ranged between 0.23 – 1.12 cm, varied significantly across KMB isolates (LSD = 0.16, p < 0.05). Out of 30 isolates, nine showed high (0.63 – 1.12 cm), nine moderate (0.47 – 0.61 cm) and rest 12 as low (0.18 – 0.44 cm) solubilization zone. Bacterial isolates K5B and K6 showed the highest diameter of potassium solubilization (Supplementary Figure 1). After the second layer of screening, 10 isolates were selected which showed the highest potassium mobilizing capacity. All the KMB isolates were found to produce slime with varying magnitude, while the K5B and K6 isolates showed the highest slime production (Table 1). Isolates named K12 and K15 showed moderate slime production ability, while the rest six KMB isolates produces less slime.6 In the case of colony morphology, seven isolates were found to produce white pigmentation, while the rest three produces creamy whitish pigmentation. All isolates showed smooth circular to round colony margin. In terms of colony elevation, K3A2, K5B, K12, K17, and K26 produced highly raised colonies, while the rest five KMB isolates slightly elevated colonies. Similar colony elevation morphology was observed by several authors.16,14 Six KMB isolates (K5B, K6, K12, K13, K15, and K26) appeared to be translucent, while the rest four were found to be opaque. All KMB isolates except K26 were found to be gram +ve, rods as described in previous studies on KMB.38 The K26 isolate belonged to the genus Paraburkholderia, which showed gram-ve reaction is also capable of potassium solubilization.39 Several studies reported the occurrences of different KMB isolates in a wide range of crops belonging to cereals, oilseeds, fruits, and ornamental crops as observed in our study.40 However, a study of KMB by Meena et al.6 observed that bacterial strains isolated from cereals like maize showed a greater zone of solubilization than isolates from oilseed crops. As all the KMB isolates in this study have been isolated from rice grown in a mica enriched zone might show promising responses as potassium solubilizers.
Table (1):
Colony characteristic of K-Mobilizing bacteria isolated from mica enriched rhizospheric soils of rice fields. Observations of colony morphology was taken at seven days of incubation grown in CAB agar plate.
| KMB Isolates | Source | Pigmentation | Gram reaction | Margin | Colony elevation | Optical density | Slime production | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Flat | Slightly raised | Highly raised | Translucent | Opaque | ||||||
| K3A2 | Rice field | White | + | Smooth | + | – | + | – | + | Low |
| K5B | Rice field | White | + | Smooth | + | – | + | + | – | High |
| K6 | Rice field | Creamy White | + | Smooth | – | + | – | + | – | High |
| K10 | Rice field | White | + | Smooth | – | + | – | – | + | Low |
| K12 | Rice field | White | + | Smooth | + | – | + | + | – | Medium |
| K13 | Rice field | White | + | Smooth | – | + | – | + | – | Low |
| K14 | Rice field | Creamy White | + | Smooth | + | + | – | – | + | Low |
| K15 | Rice field | White | + | Smooth | – | + | – | + | – | Medium |
| K17 | Rice field | White | + | Smooth | + | – | + | – | + | Low |
| K26 | Rice field | Creamy White | – | Smooth | + | – | + | + | – | Low |
Screening of KMB isolates
During first layer screening of quantitative assessment in CAB, potassium solubilization of KMB isolates (n=30) varied between 0.49 – 7.03 µg/ml; 1.07 to 9.66 µg/ml and 1.70 to 12.02 µg/ml at 7, 14 and 21 DAI respectively (Supplementary Figure 2). KMB isolate K5B showed the highest K-solubilization potential across all three time periods, while K6 showed the second-highest K-solubilization potential at 7 DAI (5.5 ± 0.45 µg/ml) and 14 DAI (7.28 ± 0.12 µg/ml). At 21 DAI, both K6 (10.76 ± 0.31 µg/ml) and K12 (10.74 ± 0.06 µg/ml) isolate showed higher potassium solubilization after K5B (Supplementary Figure 2). Finally,10 isolates were selected (K3A2, K5B, K6, K10, K12, K13, K14, K15, K17 and K26) based on cumulative solubilization potential after three observations for further assessment with both muscovite and biotite (MAB).
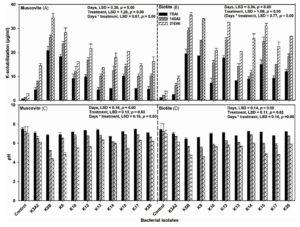
During the second layer of screening, a very minute amount of potassium content was observed in uninoculated control broth could be attributed to the structural disturbance in waste mica during shaking condition of incubation resulted in the release of potassium by hydrolysis.41,6 However, inoculation of KMB in MAB resulted in a significant release of potassium than control. The amount of potassium solubilization showed an increasing trend with the incubation period. All 10 KMB isolates were found to solubilize potassium from both muscovite (Mus) and biotite (Bio), but their ability of solubilization varied significantly both across time period (LSDMus = 0.38, LSDBio = 0.34) and isolates (LSDMus = 1.28, LSDBio = 1.08) (Figure 2A & 2B). In the case of waste muscovite, potassium solubilization ability varied from 4.41 to 20.80, 10.11 to 26.30 and 13.79 to 34.32 µg/ml at 7, 14 and 21 DAI (Figure 2A). In waste biotite, potassium solubilization ranged between 7.14 to 19.28, 11.46 to 29.0 and 20.18 to 35.35 µg/ml at three different time periods, respectively (Figure 2B). Overall, potassium solubilization of KMB isolates from waste biotite was found to be higher than waste muscovite, a similar trend was observed by Meena et al.6 Across all KMB isolates, K5B showed the highest potassium solubilization potential across time periods and the type of waste mica used as a potassium source. Apart from K5B; K6, K12 and K15 showed a considerable amount of potassium solubilization in both mica at 21 DAI (Figures. 2A and 2B). Therefore, an increase in potassium content in MAB than uninoculated control could be due to the production of organic acids by KMB isolates.23 Organic acids produced by KMB isolates might have played a significant role in destabilizing the crystal surface complex structure of waste mica or by complexion metals in solution.42 After the second layer of screening, four KMB isolates (K5B, K6, K12 and K15) were selected for further biochemical, physiological characterization and soil incubation studies based on cumulative potassium solubilization potential.
Figure 2. Quantitative second layer screening (A & B) of selected KMB isolates in modified Aleksandrow broth media using waste mica (both muscovite and biotite) as source of potassium incubated for three different time periods (7, 14 and 21 DAI). Dynamics of pH changes with time period was measured across bacterial isolates (C & D). Each bar represent mean ± SD values of observed variables from three technical replicates. Patterns inside bar represent different days of incubation
Impact of KMB isolates on pH dynamics of MAB
The initial pH of MAB was 7.6; which didn’t change significantly with the incubation period. A slight decrease in pH of uninoculated MAB could be due to the production of H+ ion during mechanical disturbance of waste mica. A similar observation was also reported by Etesami et al.43 The pH of KMB inoculated MAB decreased significantly over uninoculated control across all isolates with increase in incubation period (LSDMus = 0.16, LSDBio = 0.14) (Figures 2C & 2D). Our findings corroborate with previous studies6 and Saha et al23, who also found a similar trend. In the case of muscovite and biotite, K5B showed a significant decrease in pH at 7, 14 and 21 DAI compared to other KMB isolates (Figures 2C and 2D). At 21 DAI, the pH of MAB inoculated with K5B decreased to 4.26 in waste muscovite and 4.69 in waste biotite; while in the case of K6, it decreased to 4.82 and 4.59 in muscovite and biotite respectively (Figure 2C). As described in previous findings, KMB isolates produce mono-, di- and tri-organic acids like oxalic, citric, gluconic, fumaric, acetic, citric, and tartaric acids during incubation with waste might result in a decrease in pH of the media.16,42,44,45 Production of these organic acids leads to solubilization of crystal waste mica, which increases the abundances of Si4+ and K+ ions resulting in lowering the pH of inoculated MAB.46
Physiological characterization
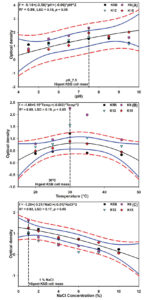
The highest bacterial growth of the isolate K6 was observed at pH 7.5, followed by K15, K12 and K5B. Bacterial cell growth showed an increasing trend with an increase in pH level up to 7.5, afterwards, a decreasing growth pattern was observed. Significantly lowest bacterial growth was observed at pH 4.5 (Figure 3A). This result showed at all KMB isolates performed best in the neutral pH range, but can withstand both acidic and alkaline conditions up to a certain extent and can be applied to both soil conditions.47,23 Relationship between pH range and bacterial cell growth showed a quadratic response that fits with following equation: f = -5.1405 + (-0.5894) × pH + (-0.0609) × pH², R² = 0.8655.
In the case of temperature tolerance, the highest bacterial cell growth was observed at 30°C in the case of all KMB isolates. Bacterial isolate K6 showed significantly higher cell growth than other isolates. In most of the cases, optimum bacterial growth was observed in the temperature range between 30 – 35°C. The lowest bacterial growth was observed at 20°C while decreasing growth pattern was observed at 40°C.23 The regression equation f = -1.6699 + 0.1539 × Temp. + (-0.0024) × Temp.², R² = 0.8951 showed the relationship between bacterial cell growth and temperature (Figure 3B). Cell growth of KMB isolates was also altered by the salt concentration of the media. The highest growth was observed at the lowest salt concentration of 1%, furthermore, a decreasing pattern of cell growth was observed with increasing concentrations of NaCl (Figure 3C). The isolate K6 showed the highest cell growth at 1% NaCl concentration, followed by K15, K12 and K5B. A quadratic response was found between salt concentrations and bacterial cell growth, can be inferred through following equation: f = -1.2885 + (-0.2123) × NaCl + (-0.0133) × NaCl², R² = 0.9259. However, these results showed that all four KMB isolates can survive in extreme pH, temperature and salinity range and can be employed in diverse environmental situations.48,49
Figure 3. Response of bacterial cell mass was observed under varying pH (A), temperature (B) and salt concentrations (C) range. Different colour and shaped points in plot represent four different KMB isolates, while black vertical dotted line signifies the optimum pH, temperature and salt concentrations for growth of KMB. Blue and red line represent 95% confidence and prediction band respectively
Plant growth promotion attributes of KMB isolates
All four KMB isolates showed their ability to produce different plant growth promotion substances, like indole-3-acetic acid (IAA), gibberellic acid (GA), hydrogen cyanide (HCN), ammonia and siderophores.50 The Highest IAA production (10.82 ± 0.6 µg/ml) was observed by KMB isolate K5B (Supplementary Figure 3A). No significant variation was observed in IAA production ability across KMB isolates (LSD = 0.36, p = NS). According to 23, Bacillus spp. showed the higher potential of IAA production, which corroborate with our findings, as all four isolates in our study also belong to the genus Bacillus spp. Significant variation in GA production was observed across KMB isolates (LSD= 0.59, p<0.05) (Supplementary Figure 3B). Highest GA production was observed in K12 (1.4 ± 0.12 µg/ml), followed by K15 (1.2 ± 0.11 µg/ml), K5B (1 ± 0.18 µg/ml) and K6 (0.49 ± 0.06 µg/ml) (Supplementary Figure 3). KMB isolates belonging to genera Pseudomonas, Azotobacter and Bacillus have been reported to produce GA.51,52
Hydrogen cyanide (HCN) production ability was observed among KMB isolates K5B, K6 and K12 indicated by turning of yellow picrate filter paper into red-brown (Supplementary Figure 4). In the case of ammonia production ability, all four isolates showed positive results by changing the yellow colour of peptone broth into brown (Supplementary Figure 4). Selected KMB isolates showed siderophore production ability by orange halo zone production around bacterial colonies in blue CAS agar plate. KMB isolates have been previously reported to produce all these growth-promoting substances.53-55 Production of HCN, ammonia and siderophores might play important role in the uptake of nutrients like iron (through siderophore production) and suppresses the growth of pathogenic fungi by the accumulation of ammonia.56,57,53 Therefore, in addition to potassium solubilization, all these isolates showed their potential in the production of plant growth promotion substances which might help in better plant growth and vigour.
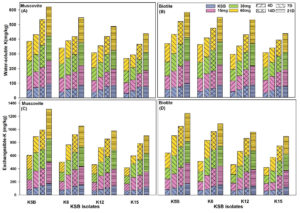
Figure 4. Potassium mobilization (both water soluble and exchangeable) potential of four KMB isolates from waste mica (both muscovite and biotite) inoculated in sterilized soil across treatments (for details, see Supplementary Table 1) and time points (7, 14 and 21 DAI) has been visualized using a stack bar. Each stack in bars represent the amount of mobilized potassium (WS-K or Ex-K) by a KMB isolate at certain dose of waste mica. Patterns in each bar represent days of incubations, while fill colour of each stack in a bar represent doses of waste mica
Apart from potassium solubilization, all four KMB isolates showed their potential in phosphate solubilization and nitrogen fixation. Previous studies also reported the beneficial effect of KMB on nutrient uptake and plant growth through different mechanisms like nitrogen fixation, the transformation of nutrient elements like phosphorus and iron when applied to seeds incorporated into the soil.58,59 In our study, the K12 isolate showed the largest (1.73 cm) clear halo zone in Pikovskaya’s agar plate followed by K15, K6 and K5B, respectively, indicating their potential of phosphate solubilization (Supplementary Figure 5A). Though K12 performed well during the qualitative assay, K15 showed the highest (87.03 mg/kg) phosphate solubilization potential during the quantitative assay (Supplementary Figure 5B). No significant variation was observed across KMB isolates in phosphate solubilization during both qualitative (LSD= 0.22, p = NS) and quantitative assays (LSD = 8.73, p = NS). K5B showed the highest colony diameter in Jansen agar media, indicating nitrogen-fixing potential (Supplementary Figure 5C). Other isolates also showed their nitrogen-fixing capacity, though no significant variation was observed in terms of colony diameter across KMB isolates. During the quantitative assay, K12 showed the highest nitrogen fixation of 0.4%, followed by K5B, K6 and K15, respectively (Supplementary Figure 5D). A recent study by Cherif-Silini et al. 60 showed both nitrogen fixation and phosphorus solubilization capability of KMB isolate belonging to Enterobacter hormaechei corroborate our findings. In our study, all KMB isolated belonged to genus Bacillus has been previously reported to show both N-fixation and P-solubilization ability supports our observations.61,62
Soil incubation study
A soil incubation study was performed to assess the potassium solubilization potential of KMB isolates when incubated in soil. The lowest potassium solubilization was observed in the case of control treatment where no waste mica was added across both water-soluble (WS-K) and exchangeable potassium (Ex-K) and mica types (Figure 4). The amount of potassium solubilization showed an increasing trend with increasing days and amount of waste mica (both muscovite and biotite) used. KMB isolate K5B showed the highest K-solubilization ability, followed by K6, K12 and K15 in both types of micas and potassium measured (WS-K & Ex-K) (Figure 4). In case of WS-K, significant variation was observed in case of muscovite across bacterial isolates (F3,192 = 125.8, p < 0.05), time points (F3, 192 = 221.9, p < 0.05), doses (F3, 192 = 377.7, p < 0.05) and also their interaction effect (F27, 192 = 1.85, p < 0.05). Similarly, significant variations of all these factors were observed in the case of biotite, but their interaction showed no significant variation (F27, 192 = 1.1, p = NS). Exchangeable potassium varied significantly in both mica across KMB isolates, time points and doses. However, their interaction effect showed significant variation in case of muscovite (F27, 192 = 5.5, p < 0.05), but not in biotite (F27, 192 = 1.34, p = NS). In microcosm study by 62 showed that inoculation of Bacillus pseudomycoides, a KMB strain isolated from tea soil increased the K-availability from 47 ± 7.1 mg/kg to 104.9 ± 7.1 mg/kg after 105 days of incubation. In another study by63 showed the inoculation of KMB strains belonging to Mesorhizobium sp., Paenibacillus sp. And Arthrobacter sp. isolated from rape rhizospheric soil not only increased the available potassium in the soil but also increased growth, vigour and biomass yield of ryegrass in potassium deficient soil. These results showed that our KMB isolates, specifically K5B isolates could potentially be used as biological fertilizer.
Phylogenetic analysis of KMB isolates
The 16S rDNA of 10 KMB isolates when compared to the know 16S bacterial sequence in NCBI formed two major clusters (Figure 5). In a single cluster, 9 out of 10 isolates belonged to genus Bacillus, while in another cluster one single KMB isolate was found belonging to genus Paraburkholderia. All KMB isolates showed 97 – 100% similarity with the available sequences of GenBank (Table 2). Among Bacillus, six isolates were found to be B. cereus, while two were unidentified species and the rest belonged to B. velezensis. Bacillus cereus is well-known species having K-solubilization ability.64 Both Bacillus and Pseudomonas are the two most widely studied genera having potassium solubilization property supports our observations.21 Though the genus Paraburkholderia is known for phosphate mobilizing ability, it has also been reported to solubilize potassium from soil and promote plant nutrition in potassium deficient soil.39
Table (2):
Molecular characterization of ten KMB isolates based on 16S rDNA have been submitted in NCBI GenBank. Closest species with similarity percentage of each isolate has been documented by using BLAST tool in NCBI.
KMB isolates |
Isolates denoted in NCBI |
Closest species with accession number |
Species similarity (%) |
GenBank accession number |
|---|---|---|---|---|
K3A2 |
Bacillus cereus K3A2GRD |
Bacillus cereus strain A3 |
100% |
MW785190 |
K5B |
Bacillus cereus K5BGRD |
Bacillus cereus strain YB1806 |
97.23% |
MW785191 |
K6 |
Bacillus cereus K6GRD |
Bacillus cereus strain L-05 |
97.78% |
MW785192 |
K10 |
Bacillus sp.6SB1 K10GRD |
Bacillus sp. 6SB1 |
100% |
MW785193 |
K12 |
Bacillus sp.GG6(2015) K12GRD |
Bacillus sp. GG6(2015) gene |
98.69% |
MW785194 |
K13 |
Bacillus velezensis K13GRD |
Bacillus velezensis strain LB122 |
99.78% |
MW785195 |
K14 |
Bacillus cereus K14GRD |
Bacillus cereus strain MD152 |
100% |
MW785196 |
K15 |
Bacillus cereus K15GRD |
Bacillus cereus strain YN01 |
100% |
MW785197 |
K17 |
Bacillus cereus K17GRD |
Bacillus cereus strain MD152 |
100% |
MW785198 |
K26 |
Paraburkholderia kururiensis K26GRD |
Paraburkholderia kururiensis strain P40 |
99.68% |
MW785200 |
The present study demonstrated that the application of KMB strains isolated from rice rhizospheric mica enriched soil can solubilize waste mica bound potassium into water-soluble and exchangeable forms, which are readily available to plants. After preliminary screening, 10 isolates showed potassium mobilizing potential among which four isolates (K5B, K6, K12 and K15) showed the most promising response. Among these four isolates, K5B identified as Bacillus cereus was found to show the highest potassium solubilization capacity during both quantitative and soil incubation assays. In all four isolates, the pH of broth was found to decrease with time indicating the production of organic acids (acidolysis) which could be a potential mechanism of solubilization of crystal bound potassium. All these four isolates showed their survival ability in wide temperature, pH and salinity range indicating that these isolates could be used across a wide range of climatic and edaphic situations. Apart from K-solubilization, these isolates showed their potential in the production of growth-promoting substances like IAA, GA, HCN, ammonia and siderophores and thus might play a significant role in plant growth promotion. Moreover, phosphate solubilization and nitrogen-fixing ability of KMB isolates might also increase the availability of nutrients to plants. Therefore, these KMB isolates showed their potential as biofertilizers to reduce the huge requirement of import of potassic fertilizers for ecologically benign and economically sustainable crop production. However, joint academic and industrial collaboration is required to develop large scale production of site-specific, climate-resilient active formulations of potassium mobilizing bacteria.
Additional file: Additional Table S1. Additional Figure S1-S5.
ACKNOWLEDGMENTS
The authors would like to thank Indian Statistical Institute for providing the essential laboratory and computer facilities for the research work, and are also thankful to Suvasri Dutta and Rupsha Nandi for their help in characterization of KMB isolates.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SM and SG performed field work. SG and SB performed laboratory analysis. SG, SM and SB performed data analysis. SG wrote the original draft. AM and PB reviewed and edited the manuscript and performed supervision. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported by Indian Statistical Institute, Giridih, Jharkhand, India, with grant number ISI/ TAC/2022-2023/5553G.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Natesan S, Ranganathan V. Content of various elements in different parts of the tea plant and in infusions of black tea from southern India. J Sci Food Agric. 1990;51(1):125-139
Crossref - Sparks DL. Dynamics of K in soils and their role in management of K nutrition. Potassium for Sustainable Crop Production. 2002;79-101.
- Sardans J, Penuelas J. Potassium: a neglected nutrient in global change. Glob Ecol Biogeogr. 2015;24(3):261-275.
Crossref - Etesami H, Emami S, Alikhani HA. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J Soil Sci Plant Nutr. 2017;17(4):897-911.
Crossref - Maqsood M, Shehzad MA, Wahid A, Butt AA. Improving Drought Tolerance in Maize (Zea mays) with Potassium Application in Furrow Irrigation Systems. Int J Agric Biol. 2013;15(6).
- Meena VS, Maurya BR, Verma JP, et al. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol Eng. 2015;81:340-347.
Crossref - Meena VS, Bahadur I, Maurya BR, et al. Potassium-solubilizing microorganism in evergreen agriculture: an overview. Potassium Solubilizing Microorganisms for Sustainable Agriculture. 2016;1-20.
Crossref - Hoa NM, Janssen BH, Oenema O, Dobermann A. Comparison of partial and complete soil K budgets under intensive rice cropping in the Mekong Delta, Vietnam. Agric Ecosyst Environ. 2006;116(1-2):121-131.
Crossref - Huo-Yan W, Jian-Min Z, Chang-Wen DU, Xiao-Qin C. Potassium fractions in soils as affected by monocalcium phosphate, ammonium sulfate, and potassium chloride application. Pedosphere. 2010;20(3):368-377.
Crossref - Britzke D, da Silva LS, Moterle DF, et al. A study of potassium dynamics and mineralogy in soils from subtropical Brazilian lowlands. J Soils Sediments. 2012;12:185-197.
Crossref - Sparks DL. Potassium dynamics in soils. Advances in Soil Science. 1987:1-63.
Crossref - Basak BB, Biswas DR. Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil. 2009;317:235-255.
Crossref - Bennett PC, Choi WJ, Rogers JR. Microbial Destruction of Feldspars. Mineral Mag. 1998;62A:149-150.
Crossref - Prajapati KB, Modi HA. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIBTech J Microbiol. 2012;1:8-14.
- Zhang C, Kong F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Applied Soil Ecology. 2014;82:18-25.
Crossref - Meena OP, Maurya BR, Meena VS. Influence of K-solubilizing bacteria on release of potassium from waste mica. Agric Sust Dev. 2013;1:53-56.
- Zhang A, Zhao G, Gao T, et al. Solubilization of insoluble potassium and phosphate by Paenibacillus kribensis CX-7: a soil microorganism with biological control potential. Afr J Microbiol Res. 2013;7(1):41-47.
Crossref - Nishanth D, Biswas DR. Kinetics of phosphorus and potassium release from rock phosphate and waste mica enriched compost and their effect on yield and nutrient uptake by wheat (Triticum aestivum). Bioresour Technol. 2008;99(9):3342-3353.
Crossref - Jackson JH. Selected writings of John Hughlings Jackson. Staples. 1958.
- Hu X, Chen J, Guo J. Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol. 2006;22:983-990.
Crossref - Sugumaran P, Janarthanam B. Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J Agric Sci. 2007;3:350-355.
- Holt JG, Krieg NR, Sneath PHA, Staley JT. Bergey’s manual of determinative bacteriology. Williams and Wilkins. Baltimore., 1994; MD 527.
- Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A. Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic Plains of India. Biocatal Agric Biotechnol. 2016;7:202-209.
Crossref - Atlas RM, Horowitz A, Krichevsky M, Bej AK. Response of microbial populations to environmental disturbance. Microb Ecol. 1991;22:249-256.
Crossref - Restu M, Bachtiar B, Larekeng SH. Gibberellin and IAA Production by Rhizobacteria from Various Private Forest. IOP Conference Series: Earth and Environmental Science. 2019:012018.
Crossref - Graham HD, Thomas LB. Rapid, simple colorimetric method for the determination of micro quantities of gibberellic acid. J Pharm Sci. 1961;50(1):44-48.
Crossref - Zeigler RS, Powell LE, Thurston HD. Gibberellin A4 production by Sphaceloma manihoticola, causal agent of cassava super elongation disease. Phytopathology. 1980;70:589-593.
Crossref - Banerjee A, Biswas JK, Pant D, et al. Enteric bacteria from the earthworm (Metaphire posthuma) promote plant growth and remediate toxic trace elements. J Environ Manage. 2019;250:109530.
Crossref - Miller RL, Higgins VJ. Association of cyanide with infection of birdsfoot trefoil by Stemphylium loti. Phytopathology. 1970;60:104-110.
Crossref - Cappuccino JG, Sherman N. Microbiology: A Laboratory Manual., 2011; (9th edition).
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47-56.
Crossref - Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils. 1991; 12:39-45.
Crossref - Pikovskaya RI. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948; 17:362-370.
- Olsen SR. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture. 1954.
- Kizilkaya R. Nitrogen fixation capacity of Azotobacter spp. strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of soils. J Environ Biol. 2009;30(1):73-82.
- Grewal JS, Kanwar JS. Forms of potassium in Punjab soils. J Indian Soc Soil Sci. 1966;14(1):63-67.
- Altomare C, Norvell WA, Bjorkman T, Harman G. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol. 1999;65(7):2926-2933.
Crossref - Archana DS, Nandish MS, Savalagi VP, Alagawadi AR. Screening of potassium solubilizing bacteria (KSB) for plant growth promotional activity. Bioinfolet-A Quarterly Journal of Life Sciences. 2012;9:627-630.
- Mahmud AA, Upadhyay SK, Srivastava AK, Bhojiya AA. Biofertilizers: A Nexus between soil fertility and crop productivity under abiotic stress. Current Research in Enviromental Sustainability. 2021;3:100063.
Crossref - Friedrich S, Platonova NP, Karavaiko GI, Stichel E, Glombitza F. Chemical and microbiological solubilization of silicates. Acta Biotechnol. 1991;11(3):187-196.
Crossref - Yu X, Liu X, Zhu T-H, Liu G-H, Mao C. Co-inoculation with phosphate-solubilzing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur J Soil Biol. 2012;50:112-117.
Crossref - Stillings LL, Drever JI, Brantley SL, Sun Y, Oxburgh R. Rates of feldspar dissolution at pH 3-7 with 0-8 mM oxalic acid. Chem Geol. 1996;132(1-4):79-89.
Crossref - Etesami H, Emami S, Alikhani HA, Potassium solubilizing bacteria (KSB):Mechanisms, promotion of plant growth, and future prospects A review. J SoilSci Plant Nutr., 2017; 17:897–911
- Mo B, Supanjani, Lian B. Interactions between Bacillus mucilaginosus and silicate minerals (weathered adamellite and feldspar): Weathering rate, products, and reaction mechanisms. Chinese Journal of Geochemistry. 2011;30:187-192.
Crossref - Maurya BR, Meena VS, Meena OP. Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos. 2014;27(1):181-187.
Crossref - Son H-J, Park G-T, Cha M-S, Heo M-S. Solubilization of insoluble inorganic phosphates by a novel salt-and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour Technol. 2006;97(2):204-210.
Crossref - Song SK, Huang PM. Dynamics of potassium release from potassium-bearing minerals as influenced by oxalic and citric acids. Soil Science Society of America Journal. 1988;52:383-390.
Crossref - Kulkarni S, Nautiyal CS. Characterization of high temperature-tolerant rhizobia isolated from Prosopis juliflora grown in alkaline soil. J Gen Appl Microbiol. 1999;45(5):213-220.
Crossref - Fischer SE, Fischer SI, Magris S, Mori GB. Isolation and characterization of bacteria from the rhizosphere of wheat. World J Microbiol Biotechnol. 2007;23:895-903.
Crossref - Desai SA. Isolation and characterization of gibberellic acid (GA3) producing rhizobacteria from sugarcane roots. Biosci Discov. 2017;8:488-494.
- Biswas JK, Banerjee A, Rai MK, et al. Exploring potential applications of a novel extracellular polymeric substance synthesizing bacterium (Bacillus licheniformis) isolated from gut contents of earthworm (Metaphire posthuma) in environmental remediation. Biodegradation. 2018;29:323-337.
Crossref - Kotasthane AS, Agrawal T, Zaidi NW, Singh US. Identification of siderophore producing and cyanogenic fluorescent Pseudomonas and a simple confrontation assay to identify potential bio-control agent for collar rot of chickpea. 3 Biotech. 2017;7:137.
Crossref - Gupta S, Pandey S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front Microbiol. 2019;1506.
Crossref - Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A. Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India.
J Basic Microbiol. 2016;56(1):44-58.
Crossref - Swamy MK, Akhtar MS, Sinniah UR. Response of PGPR and AM fungi toward growth and secondary metabolite production in medicinal and aromatic plants. Plant, Soil and Microbes. 2016:145-168.
Crossref - Richard PO, Adekanmbi AO, Ogunjobi AA. Screening of bacteria isolated from the rhizosphere of maize plant (Zea mays L.) for ammonia production and nitrogen fixation. Afr J Microbiol Res. 2018;12(34):829-834.
Crossref - Herridge DF, Peoples MB, Boddey RM. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil. 2008;311:1-18.
Crossref - Sindhu SS, Parmar P, Phour M, Sehrawat A. Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. Potassium solubilizing microorganisms for sustainable agriculture. 2016:171-185.
Crossref - Vafadar F, Amooaghaie R, Otroshy M. Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J Plant Interact. 2014;9(1):128-136.
Crossref - Cherif-Silini H, Silini A, Yahiaoui B, Ouzari I, Boudabous A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann Microbiol. 2016;66:1087-1097.
Crossref - Pramanik P, Goswami AJ, Ghosh S, Kalita C. An indigenous strain of potassium-solubilizing bacteria Bacillus pseudomycoides enhanced potassium uptake in tea plants by increasing potassium availability in the mica waste-treated soil of North-east India. J Appl Microbiol. 2019;126(1):215-222.
Crossref - Xiao Y, Wang X, Chen W, Huang Q. Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol J. 2017;34(10):873-880.
Crossref - Ali AM, Awad MYM, Hegab SA, et al. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J Plant Nutr. 2021;44(3):411-420.
Crossref - Zhou H, Zeng X, Liu F-F, Guan-Zhou Q, Yue-hua H. Screening, identification and desilication of a silicate bacterium. J Cent South Univ Technol. 2006;13:337-341.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.