Bioethanol might be one of the most potent solutions to overcome the demand for liquid transportation fuel. The demand for ethanol has been continually increasing on account of the growth of user industries and use of ethanol as a fuel. However, the production and availability of ethanol has largely lagged behind. The major problem with bioethanol production is the availability of raw material. Lignocellulosic biomass is the most promising feedstock considering its abundant availability and low cost. Ethanol production is much more challenging and difficult when lignocellulosic material is to be used as raw material. Unlike the starch-based materials, pretreatment and hydrolysis of lignocellulosic material produce a mixture of pentose and hexoses along with other inhibiting compounds, causing many problems in the fermentation process. Bioconversion of lignocellulosic biomass to ethanol requires three major unit operations including pretreatment, hydrolysis, and fermentation, which is comprehensively summarize in this review.
Lignocellulosic biomass, bioethanol, Pretreatment, Hydrolysis, Fermentation.
Over the last few decades, the negative impacts of fossil fuel on the environment and consequent global warming, progressive demand for energy, inevitable depletion of the world’s energy supply, and the unstable oil market have renewed the interest of society in searching for alternative fuels (Solomon et al., 2007). The alternative fuels are expected to satisfy several requirements including substantial reduction of greenhouse gas emission, worldwide availability of raw materials, and capability of being produced from renewable feed stocks. Many alternative fuel sources have been explored, and among them bioethanol has received most attention (Wheals et al., 1999). Production of fuel ethanol from biomass seems to be an interesting alternative to traditional fossil fuel, which can be blended with petrol or used as neat alcohol in dedicated engines (Hahn-Hagerdal et al., 2006). Ethanol is currently produced from sugars, starches and cellulosic material. The first two groups of raw material are currently the main resources for ethanol production, but concomitant growth in demand for human feed similar to energy could make them potentially less competitive and perhaps expensive feed stocks in the near future, leaving the cellulosic material as the only potential feedstock for production of ethanol (Taherzadeh and Karimi, 2007). Cellulosic material obtained from wood and agricultural residues, municipal solid wastes and energy crops represent the most abundant global source of biomass (Lin and Tanaka, 2006).The most significant challenge for biofuel production is to develop feasible and efficient conversion process, suitable for each specific biomass feedstock that are capable of powering everyday life without bringing harmful environmental changes. This paper aims to summarize the various methodsfor conversion of lignocllusoic biomass to bioethanol.
Pretreatment
Plant cell walls have a natural resistance to chemical, physical and biological degradation. Lignin, which is the second most abundant natural polymer and currently not directly used in ethanol production, is partially linked to polysaccharides in the cell wall (Zhu et al., 2008). Therefore, an efficient pretreatment process is needed for cellulose conversion process which promotes the disruption of the lignocellulosic matrix in order to facilitate acid or enzyme catalyzed hydrolysis (Silverstein et al., 2004).A successful pretreatment must avoid the degradation or loss of carbohydrate, avoid the formation of by-products inhibitory to the subsequent hydrolysis and fermentation process and finally must be cost-effective (Wyman, 1996). There are three main categories of pretreatment namely physical, chemical and biological pretreatment. In this section, promising cost-effective pretreatment methods are reviewed, followed by a brief discussion of the pros and corns of each technology.
Physical pretreatment
Physical pretreatments are methods without addition of chemicals or micro-organisms. They use external forces to reduce the lignocellulosicbiomass in to fine particles in order to increase the surface area of the material. According to the forces used, the physical pretreatment can be further divided into two sub-catalogs: mechanical (dry, wet, vibratory ball milling) and non- mechanical method (pyrolysis, steam explosion, irradiation and microwave treatment) (Kumar et al., 2009; Zheng et al., 2009).
Mechanical commination
Mechanical pretreatment use shearing force to reduce biomass particle size, change the lignocellulose structure, and reduce degree of polymerization and crystallinity of cellulose (Kumar et al., 2009). Depending on the final size of the material, the mechanical pretreatment consist of milling, grinding or chipping. Chipping leads to 10 to 30 mm particles, and milling and grinding leads to 0.2 to 2 mm particles in size (Sun and Cheng, 2002). Milling includes ball milling, two roll milling, hammer milling, compression milling, agitation bead milling, pan milling, fluid energy milling, and colloid milling etc., (Zhenget al., 2009). Improper application of mechanical pretreatment will lead to carbohydrate losses, and as result ethanol yield will be reduced (Bridgeman et al., 2007). In recent practices combination of mechanical pretreatment and chemical size-reduction is commonly employed to make it more cost-effective and successful.
Pyrolysis
Pyrolysis is also used as pretreatment of lignocellulosic biomass by treating it with temperature greater than 300oC, which results in rapid decomposition of biomass to gaseous products and residual char. The process can be enhanced with the presence of oxygen (Shafizadeh and Bradbury, 1979). Mild acid hydrolysis (1 N H2SO4, 97oC, 2.5 hr.) of the residues from pyrolysis pretreatment has resulted in 80-85% conversion of cellulose to reducing sugars with more than 50% glucose (Fan et al., 1987). When zinc chloride or sodium carbonate is added as catalyst, the decomposition of pure cellulose can occur at a lower temperature (Sun and Cheng, 2002). Pyrolysis pretreatment prior to enzymatic hydrolysis of news paper and cardboard was examined by Leustean (2009).
Steam explosion
Steam explosion is exposing biomass to steam under high pressure and temperature followed by a decompression at the end (Harun et al., 2011). It is biomass fractionation process in which high-pressure, high-temperature steam is introduced into a sealed chamber containing woody lignocellulosic material in the form of chips or agricultural residues. After 1-5 min, the pressure is release, causing the steam to expand within the lignocellulosic matrix, separating individual fibers with minimal loss of material (Mabee et al., 2006). Liquid hot water (LHW) pretreatment (co-current and counter-current) is a pretreatment similar to steam explosion, except that, in LHW pretreatment, instead of steam, biomass is merged into hot water with certain pressure and temperature (Mosier et al., 2005). Both these processes are able to cleave the acetyl groups and uronic acid from hemicellulose and consequently acidify the medium (water at high temperature also acts as acid). As a result, acidic condition will cause partially hydrolysis of hemicellulose and amorphous cellulose to oligosaccharides and to fermentable sugars (Weil et al., 1997). Since, it is chemical free process; no environmental cost in terms of chemical recycling is needed which results in reduction of operating cost. For soft wood, steam pretreatment with addition of an acid catalyst such as H2SO4 or SO2 is prerequisite to reach high sugar yields. Acid increases the recovery of hemicellulose sugars and it improves the enzymatic hydrolysis of solid fraction (Hahn-Hagerdal et al., 2006). Due to strong catalytic activity, along with removal of hemicellulose it also yields inhibitory substances (Bertilsson, 2007).
Irradiation
Radiation includes Gamma-rays, microwave, ultrasound, pulsed electrical filed, ultraviolet and electron-beam which can pre-treat the biomass by decreasing the crystallinity and degree of both polymerization (disruption of beta-1, 4-glycosidic bonds) and reactivity of cellulose. In addition, the high energy of these radiations will lead to the formation of free radicals, which leads to a further degradation of the lignocellulosic material (Kumar et al., 2009). Lu and Kumakura, (1995) proposed that combine treatment of peracetic acid with increasing dose of radiation up to 500 kGy (KiloGray) or above can significantly enhance enzymatic hydrolysis of wheat straw. Similar result was also observed by Yang et al., (2008) by studying the effect of Gamma -radiation (500 kGy) on wheat straw and achieved 13.4% yield. Among radiations, microwave pretreatment comprises of high temperature treatment usually 160-180oC which is sufficient to soften the main component of the cell wall and decreases the crystallinity of cellulose. Previous studies shows that microwave treatment change the crystalline structure of cellulose, degrade lignin and hemicellulose which result in increasing the enzymatic accessibility, while in contrary, elevated temperature may also cause some useful components in the feed stock to decompose. Therefore, microwave pretreatment has been studied in conjunction with chemical reagents for pretreatment at lower temperature (Zhu et al., 2005). Keshwani et al., (2007) investigated the effect of microwave on switch grass and achieved greatest sugar yield by using microwave exposure of switch grass immersed in 3% sodium hydroxide for 10 minutes at 250 watts. However, still this approach is limited to laboratory level due to cost and safety concern. Unfortunately, irradiation pretreatment are reported to consume high levels of energy and require long process time with expensive high quality equipment.
Chemical pretreatment
Chemical pretreatment, mainly employing chemical agents such as acid, alkali, salts, organic solvents as well as oxidizing agents for enhancing hydrolysis of lignocellulosic biomass by removing hemicellulose and lignin (Moiser et al., 2005). Different than physical methods, chemical pretreatments are mainly used for modifying the lignin in the biomass, removing hemicellulose, and to change cellulose polymerization as well as cellulose crystalline structure (Hahn-Hagerdal et al., 2006).
Acid pretreatment
Acid pretreatment is one of the oldest and most commonly used methods. There are many types of acid pretreatments including use of sulfuric acid, hydrochloric acid, phosphoric acid, peracetic acid, nitric acid etc., (Balat et al., 2008). Among all pretreatment methods, dilute acid pretreatment was one of the most studied and widely used (Agbogbo and Wenger, 2006). The main function of dilute acid pretreatment is to effectively remove the hemicellulose sheathing over cellulose, while at the same time loosening the structure of lignin and decrease the crystallinity of cellulose. The dissolved hemicellulose in the liquid phase is removed from the solid biomass residues and may be separately hydrolyzed to xylose and other 5C or 6C sugars, or eventually broken down to furfural (Moiser et al., 2005). Furfural can be recovered from distillation and is widely applicable as solvent in petrochemical refining (Paturau, 1987). Depending on the substrate and condition used, between 80 and 95% of the hemicellulose sugar can be recovered by dilute acid pretreatment (Jeffries and Jin, 2000). Previous study reveals that corn fiber can be enzymatically saccharified to fermentable sugar with a yield of 85-100% after pretreatment with dilute acid at moderate temperature (Saha et al., 2005).
Alkaline pretreatment
Alkaline pretreatment process utilizes lower temperature and pressure compare to other pretreatment technologies (Balat et al., 2008). However, unlike acid pretreatment, it is much more time consuming (Mosier et al., 2005) and some of the alkali is converted to irrecoverable salt or incorporated as salt into the biomass by the pretreatment reaction (Silverstein, 2004). During alkali pretreatment, biomass is soaked in the dilute alkali solution and treated for varying periods of time and temperature. The major effect of alkali pretreatment is the saponification of intermolecular ester bonds which crosslink lignin and carbohydrates, thus increasing porosity and internal surface of the biomass matrix as well as decreasing the degree of crystallinity of cellulose (Sun and Cheng, 2002), resulting in improved susceptibility of remaining polysaccharides to enzyme attach during hydrolysis. Furthermore, alkali will remove the acetyl and uronic acid groups from hemicellulose to enhance the accessibility of enzyme (Chang et al., 1998).NaOH, Na2CO3, Ca(OH)2 (lime), KOH, NH4OH, and aqueous ammonia were used to hydrolysate wheat straw, switch grass, corn cob, corn stover, corn husk and municipal solid wastes (Xu, 2012). Sharma et al., (2002) investigated the alkali pretreatment on sunflower stalk and reported that sodium hydroxide at 0.5% (w/v) along with autoclaving for 1.5 hour at 1.05kg/cm2 was the most effective processing condition as evaluated by the following-up enzymatic hydrolysis. Silverstein et al., (2007) studied the effect of different concentration of sodium hydroxide on cotton stalk and reported that 2% (w/v) concentration of sodium hydroxide at 121oC for 60 minutes was found to be an optimum process for delignification, while Binod et al., (2010) got 96% yield of sugar by using 4% NaOH and near about similar results were also reported by Kaur et al., (2012). Beside sodium hydroxide, calcium hydroxide (lime) is also an effective pretreatment agent which is the least expensive chemical with safe handling among all hydroxides. Furthermore, calcium can be recovered from the reaction system by introducing carbon dioxide (Karr and Holtzapple, 2000). The remaining lignin rich residues recovered from the alkaline wash can be used as feed stock for generating electricity and steam (Hamelincket al., 2005).
Ammonia fiber/freeze explosion
Ammonia can disrupt the crystalline structure of cellulose and deacetylate acetyl linkage, thus greatly increasing the efficiency of enzymatic hydrolysis (Gollapalli et al., 2002).Ammonia fiber/freeze explosion (AFEX) pretreatment involves liquid ammonia and steam explosion (Hamelinck et al., 2005). In this process, lignocellulosic material is placed in pressure vessel with liquid ammonia (NH3) at a loading of about 1-2 kg NH3/kg dry biomass. Pressures exceeding 12 atm are required for operation at ambient temperature (Silverstein, 2004). Removal of hemicellulose and lignin is not significant for AFEX pretreatment as for acid or alkali pretreatment, while the structure of lignin is modified or altered during the process and the hemicellulose is also depolymerized by interacting with ammonia (Wyman et al., 2005). Therefore, pretreated cellulose can be more easily and quickly hydrolyzed to glucose even when the enzyme loading is not high (Dale et al., 1996). Alizadeh et al., (2005) reported that the cellulose hydrolysis efficiency of AFEX- treated switch grass reached as much as 93% compared to that of untreated samples which only showed a cellulose conversion of 16%. One modification in such pretreatment is known as Ammonia Recycle Percolation (ARP) is commonly applied by passing aqueous ammonia (5-15%w/w) through biomass feedstock at elevated temperatures (160-180oC) and then separating the ammonia for recycle (Kim and Lee, 1996; Kim et al., 2003). Under such condition, aqueous ammonia swells the biomass, degrades lignin and interrupts the interactions between lignin and carbohydrates (Moiser et al., 2005). Besides, residual ammonia in the pretreated products has no inhibitory effect on downstream processes and it is compatible with microorganisms without extra conditioning. Furthermore, it is reported that ammonia can even have some advantageous influence on fermentation (Dale et al., 1985).
Cellulose solvent
Cellulose solvent is a chemical additive such as alkaline H2O2, ozone and glycerol which can disrupt the structure of cellulose within biomass feedstock and improving the enzyme digestibility during hydrolysis. However, these chemicals additives are too expensive to be used at large scale (Moiser et al., 2005).
Ozonolysis
Ozone can also be used to degrade lignin and hemicellulose in many lignocellulosic materials. The degradation was essentially limited to lignin and hemicellulose was slightly attached but cellulose was hardly affected. Silverstein et al., (2007) investigated the effect of ozone in comparison with sodium hydroxide and sulfuric acid on cotton stalk and found good results by using sodium hydroxide solution for pretreatment as compare to ozone and acid.
Supercritical fluids (hydro thermolysis)
Supercritical fluids pretreatment is a process in which water used as solvent. It is most environmental friendly process where no need for separate neutralization. Water is maintained in liquid state under certain pressure at elevated temperatures, and it can penetrate the cell wall of biomass feedstock, hydrate cellulose and remove hemicellulose by disrupting the linkages between these structural components (Wyman et al., 2005). Mok and Antal, (1992) reported that by mixing biomass material including switch grass with the hot compressed liquid water for up to 15 minute at temperature between 200 and 230oC, about half of the total biomass can be dissolved, within which 4-22% of cellulose, 35-60% of lignin and approximately 100% hemicellulose can be dissolved.
CO2 explosion
Similar to steam and ammonia explosion pretreatment, CO2 explosion is also used for the pretreatment of lignocellulosic biomass. Zheng et al., (1998) compared CO2 explosion with steam and ammonia explosion for pretreatment of recycled paper mixture, sugarcane bagasse and pulping waste of recycled paper and found that CO2 explosion was more cost effective than ammonia explosion and did not cause the formation of inhibitory compound that could occur in steam explosion.
Biological pretreatment
Pretreatment through biological entities for removal of lignin or hemicellulose from lignocellulosic biomass is referred as biological pretreatment. Compared to physical and chemical process, biological pretreatment is more complicated and time consuming.
Microbial degradation
Pretreatment of lignocellulosic biomass can be carried out by microbial degradation of lignin. Lignin is degraded by different classes of enzymes, which are produced by different microorganism, such as white-rot fungi like Pleurotusostreatus and Pycnopouscinnabarinus etc. These organism produces some combinations like, lignin peroxidase (LiP) and manganese peroxidase (MnP), fungi producing MnP and laccase, while some other producesLiP and laccase, and fungi which produce neither LiP nor MnP, but laccase and aryl alcohol oxidase or some other enzymes (Hatakka, 1994). Enzyme laccase (EC: 1.10.3.2), is belongs to the family of blue multicopper oxidase. It oxidizes a variety of aromatic hydrogen donors by catalyzing one electron oxidation of four reducing substrate molecules concomitant with the four electron reduction of molecular oxygen to water (Piontek et al., 2002). Another important enzyme is lignin peroxidase (EC: 1.11.1.14), oxidizes aromatic compounds by single electron abstraction. Crystallographic structure of lignin peroxidase from the white-rot fungus P. chrysosoporium, shows 343 amino acid residues, the heme, four carbohydrates, and two calcium ions. This lignin peroxidase shows the typical peroxidase fold and the heme has a closer environment as found in other peroxidase (Choinowski et al., 1999). Shi et al., (2009) investigated the pretreatment effect of Phanerochaetechrysosporuim on cotton stalk under submerged cultivation (SmC) and solid state cultivation(SSC) and found significant lignin degradation i.e. 19.38% and 35.53% for SmC and SSC respectively. One main challenge of this pretreatment is to preserving cellulose from fungal culture and purified without loss of sugars.
Ensiling
Silage is a traditional technology used to preserve large quantities of cellulosic material harvested for storage in a year-round system. Through the ensiling process, the rate of carbohydrate degradation is strictly controlled by creating a disadvantageous anaerobic environment in which microbes favor acetic and lactic acid fermentation. Therefore, pH is greatly reduced within the system (Ren, 2006). Ensiling is not only a storage method for crops or ruminant feeding; it also contributes to the saccharification of plant cell wall and mixed acid fermentation. During this process, the structures of cellulosic biomass are broken down and the degradability of the biomass matrix is greatly improved (Richard et al., 2001). Chen et al., (2007) investigated the potential of using ensiling as cost effective pretreatment for bioethanol production from agricultural residues such as cotton stalk and wheat straw. Unlike different methods of pretreatment, it is highly time consuming technology.
Molecular modification
Molecular modification is referred as an alteration of intrinsic characteristic of cellulosic feedstock thus making the biomass matrix more digestible (Ragauskas et al., 2006). It is recent molecular technology among different traditional pretreatment. One intriguing research area is the modification of cinnamoyl-CoA reductase (CCR) gene which is responsible for lignin biosynthesis. Upon appropriate expression of the modified CCR gene, the interaction between lignin and holocelluloses is weakened and twice the amount of monomeric sugar yield can be obtained during hydrolysis compared with that of the natural feedstock (Boudet et al., 2003). Another type of modification defines as molecular farming, which has been tested for biofuel production. During this process, plants are capable of producing polysaccharide hydrolyase enzyme and depolymerizing cellulose “in situ” (Rishi et al., 2001).
Hydrolysis
Hydrolysis is the method by which glycosidic bonds of lignocellulosic substrates are cleaved. It is used to facilitate the dissolution of chemical by reaction with water, and is especially effective on some organic compounds those are relatively resistant to solubilisation and degradation (Yang, 2008). The most commonly applied methods to hydrolyze the cellulosic biomass can be classified in two groups: acid and enzymatic hydrolysis.
Acid hydrolysis
The solubility of cellulose in acid has been detected already in 1815. The first industrial process however was developed in 1942 and run in Italy (Roehr, 2000). The acid hydrolysis can be performed by high acid concentration at a low temperature or that of low concentration at a high temperature (Dehkhoda, 2008). Research reveals that under controlled condition, acid hydrolysis of lignocellulosic biomass mainly produced xylose from xylan with the cellulosic and lignin fractions remaining unaltered. Xylan is more susceptible to hydrolysis by mild acid treatment due to its amorphous structure compared to cellulose, which need sever treatment condition for its crystalline nature (Rahmanet al., 2007). Since 5-carbon sugars degrade more rapidly than 6-carbon sugars, one way to decrease sugar degradation is to have a two-stage process. The first stage is conducted under mild process conditions to recover the 5-carbon sugars while the second stage is conducted under harsher conditions to recover the 6-carbon sugars (Demirbas, 2008). There are two basic type of acid hydrolysis processes commonly used: dilute acid and concentrated acid.
Dilute acid hydrolysis
Dilute acid hydrolysis is the oldest technology for converting cellulosic biomass to ethanol. In this process, the hemicellulose fraction is depolymerized at lower temperature than the cellulosic fraction (Chandel et al., 2007a). The dilute acid process involves a solution of about 1% sulfuric acid concentration in a continuous flow reactor at a high temperature (about 488K) (Graf and Koehler, 2000). Most dilute acid processes are limited to a sugar recovery efficiency of around 50%. The primary challenge for dilute acid hydrolysis processes is how to raise glucose yields higher than 70% in an economically viable industrial process while maintaining high cellulose hydrolysis rate and minimizing glucose decomposition (Xiang et al., 2004). Dilute acid hydrolysis occurs in two stages to take advantage of the differences between hemicellulose and cellulose. The first stage is performed at low temperature to maximize the yield from the hemicellulose; and the second, higher-temperature stage is optimized for hydrolysis of the cellulosic portion of the feedstock. The liquid hydrolysate are recovered from each stage, separated from solid material and lignin, neutralized (and detoxified) prior to fermentation (Farooqi and Sam, 2004). The big advantage of dilute acid hydrolysis process is its fast rate of reaction, which facilitates continuous processing. Disadvantage of this process is considered as low sugar yield (Badger, 2002). Romero et al., (2007), yielded 92% hemicellulose fractions from dilute acid hydrolysis (0.75N) of olive tree pruning.
Concentrated acid hydrolysis
Concentrated acid process provides complete and rapid conversion of cellulose to glucose and hemicellulose to 5-carbon sugar with little degradation, but the critical factor is needed to make the process economically viable by optimizing sugar recovery and cost effectively recover the acid for recycling. The concentrated acid process uses 70% sulfuric acid at 40oC to 50oC for 2 to 4 hour in a reactor. The low temperature and pressure will lead to minimize the sugar degradation. The hydrolyzed material is then washed to recover the sugar. In the next step, the cellulose fraction has to be depolymerized. The solid residue from the first stage is de-watered and soaked in 30-40% sulfuric acid for 50 minute at 100oC for further cellulose hydrolysis. The primary advantage of the concentrated acid process is the potential for high sugar recovery efficiency, but this process offers more potential for cost reductions than the dilute sulfuric acid process (Demirbas, 2005; Chandel et al., 2007a; Demirbas, 2007).Iranmahboob et al., (2002) performed the concentrated acid hydrolysis of mixed wood chips and was found to be maximum sugar recovery (78-82% of theoretical yields) achieved by using 26% sulfuric acid concentration for 2 hours of residence time. Liao et al., (2006) yielded glucose at a yield of 84% and hemicellulose at a yield of 80% from fibers of dairy manure by using 75% H2SO4and upon results he reported that acid concentration was the most important factor to alter the sugar components (cellulose and hemicellulose) in dairy manure; while Yang, (2008) noticed that two other individual factors, residence time and temperature also had significant influence on compositional changes of lignocellulosic material.
By-products of acid hydrolysis
Dilute-acid hydrolysis is a cheap and fast process to obtain sugar from lignocellulosic biomass; however, a significance drawback of dilute-acid hydrolysis is the generation of several by-products during the process. Some of them are toxic to fermenting microorganism (Palmqvist and Hahn-Hagerdal, 2000b). Inhibition by these compounds decreases yield and productivity as well as disturbing cell growth during fermentation. Cellulose, hemicellulose and lignin are broken down to mainly glucose, mannose or xylose, and phenolic compounds during acid hydrolysis, respectively. As soon as the monomers are produced, further decompositions occur during these process conditions yielding other unexpected compounds such as 5-hydroxymethyl furfural (HMF) from hexoses, and furfural from pentoses. HMF and furfural are also decomposed into mainly levulinic acid and formic acid. Moreover, aliphatic acids, mainly acetic acid are released from acetyl groups contained in hemicelluloses, while lignin is also decomposed and releases phenolic compounds (Purwadi, 2006).
Organic acid
A large number of aliphatic acid are present in dilute-acid hydrolysates originated from wood extractives, lignin degradation and sugar degradation. Acetic acid is major acid constituent in hydrolysate and is mainly produced from degradation of the acetyl group in the polysaccharide, whereas levulinic acid and formic acid are the products of sugar degradation (Luo et al., 2002).The undissociated weak acid is liposoluble and can diffuse across the plasma membrane in to cytosol (intracellular fluid) and thus decreasing the cytosolic pH, which results as intracellular dissociation. Two mechanisms have been proposed to explain the inhibitory effect of weak acid: uncoupling and intracellular anion accumulation (Russell, 1992). In order to maintain intracellular pH, proton must be transported across the membrane by the action of plasma membrane ATPase which results in an increase of ATP consumption, and thereby causes lower biomass yield while in anaerobic condition, ATP generation is achieved by the ethanol production pathway resulting in higher ethanol yield at the expense of biomass formation. According to uncoupling theory, the critical extracellular concentration of undissociated acid exceeds the transport capacity of the plasma membrane ATPase, and intracellular acidification occurs. Anionic accumulation theory proposed that, the anionic form of the acid is captured in the cell and undissociated acid will diffuse into the cell until equilibrium is reached, which results in an intracellular acidification occur (Palmqvist and Hahn-Hagerdal, 2000b).
Phenolic compounds
Phenolic compounds are mainly considered as product of lignin degradation formed after acid treatment. There are number of phenolic compounds recognized in lignocellulosichydrolysate, including 3-methoxy-4-hydroxybenzaldehyde, 4-hydroxyacetophenone, vanillic acid and 4-hydroxybenzoic acid (Klinke et al., 2004).Phenolic compounds have been suggested to exert a considerable inhibitory effect in the fermentation of lignocellulosichydrolysate; the low molecular weight phenolic compounds being most toxic, however, the mechanisms of the inhibiting effect have not been elucidated. It was proposed that, phenolic compounds partition into biological membranes and cause loss of integrity, thereby affecting their ability to serve as selective barriers and enzyme matrices (Heipieperet al., 1994; Palmqvist and Hahn-Hagerdal, 2000b).
Furans compound
Furfural and 5-hydroxymethyl furfural (HMF) are the byproducts of pentoses and hexoses respectively. HMF, on continue heating, yield levulinic acid and formic acid. Furfural has been reported to be a strong inhibitor for Saccharomycescerevisiae. The furfural concentration above 1 g/L was found to decrease significantly the CO2 evolution rate, the cell multiplication and the total viable cell number in the early phase of fermentation. Furfural is also metabolized by Saccharomycescerevisiae under aerobic, oxygen-limited and anaerobic conditions. During fermentation furfural reduction to furfuryl alcohol occurs with high yields.Furans effect on cellular growth by inhibiting the enzyme, alcohol dehydrogenase and cause lowering in membrane permeability resulting in longer lag phase in cell growth (Palmqvist et al., 1999; Taherzadehet al., 1999; Palmqvist and Hahn-Hagerdal, 2000b).
Detoxification
The inhibitors release during hydrolysis can be removed by applying proper detoxification process. Detoxification methods are broadly divided in to three categories namely biological, physical and chemical detoxification method.
Biological detoxification
Biological detoxification referrers as treatment of hydrolysate with enzymes peroxidase and laccase obtained from the lignolytic fungus Trametesversicolor (Jonssonet al., 1998). The filamentous soft-rot fungus Trichodermaressei has also been reported to degrade inhibitors in a hemicellulose hydrolysate obtained after steam pretreatment of willow, resulting in around three times increased ethanol productivity and four times increased ethanol yield (Palmqvistet al., 1997). In contrast to the treatment with purified laccase, it was reported that treatment with Trichodermaressei resulted in removal of acetic acid, furfural and benzoic acid derivative, which could not be seen with former (Palmqvist and Hahn-Hagerdal, 2000a).
Physical detoxification
Physical detoxification carried out either by evaporation or membrane separation. Evaporation under vacuum can eliminate volatile compounds such as acetic acid, furfural and vanillin from lignocellulosichydrolysate. However, this method retains the concentration of non-volatile toxic compounds (extractive and lignin degradation) in the hydrolysate (Chandel et al., 2007b). A previous study shows that, significance reduction of inhibitors was observed during detoxification of hemicellulose hydrolysate of willow by roto-evaporator (Palmqvist and Hahn-Hagerdal, 2000a). Another method is membrane separation method where adsorptive micro porous membranes, having surface functional group attached to their internal pores, which may eliminate the cell wall derived inhibitors from the lignocellulose acid hydrolysate. During clarification of inhibitors, the feed is being pumped through the membrane pores that bind to the solute predominantly by convection (Chandelet al., 2007b). Successful studies have been done by using membrane extraction method for removal of inhibitors form sulfuric acid derived hemicellulose hydrolysate obtained from corn stover (Grzenia et al., 2010).
Chemical detoxification
Chemical detoxification is most promising method among the three types and can be carried out by using different ways. The first important chemical method is alkali treatment; in this process pH is increased up to 9-10 with liming and readjustment to 5.5 with sulfuric acid,but the optimum concentration of lime varies and depends on type and concentration of acid in hydrolysate, while it was noticed that over liming has drastically affects the furans reduction while other inhibitors are comparatively less affected by it (Martinez et al., 2000). Van Zylet al., (1988) reported that Ca(OH)2 treatment gives better results for increasing fermentability than NaOH adjustment due to precipitation of toxic compounds. Over liming with a combination of high pH and temperature for a long time has been considered as a promising detoxification method for dilute sulfuric acid-pretreatment hydrolysate of lignocellulosic biomass (Martinz et al., 2001). Another method of chemical detoxification is activated charcoal treatment. It is cost effective and having good adsorptive nature without affecting level of sugar in hydrolysate (Canilha et al., 2008). Treatment with ion exchange resin is also an effective for lignin removal from hydrolysate. It has been reported that ion exchange resins diminish furans (63.4%) and total phenolics (75.8%) from sugarcane bagasse acid hydrolysate (Chandel et al., 2007b).
Enzyme hydrolysis
Enzyme hydrolysis is another method of degrading pretreated cellulose to mono sugars with the help of complex of enzyme known as cellulases. Bacteria and fungi both are able to yield cellulases suitable for digestion of the plant cell wall polysaccharides, although some of these microorganisms vary significantly in characteristics. Cellulomonasfimi and Thermomonosporafusca are the most extensive studied bacteria; while Trichoderma and Aspergillus are two fungal genera that are of great interest to researchers (de Vries and Visser, 2001).During enzymatic hydrolysis of lignocellulosic biomass, cellulases components, including β-glucosidase and endoglucanase have more binding affinity towards lignin than to the carbohydrates, resulting in lower efficiency of saccharification. Hence, to achieve maximum hydrolysis of cellulosic biomass, which is prerequisite for ethanol fermentation, an appropriate delignification treatment of biomass is required (Kaya et al., 2000; Gupta et al., 2009).
Biochemistry of cellulases
In the middle of the twentieth century began the discussion about the complexity of the natural cellulolytic enzymes and their different abilities to degrade cellulose. It was speculated that there are three types of enzyme activities involved in hydrolyzing cellulose: C1, which would convert crystalline cellulose to amorphous, Cx, which would hydrolyze amorphous cellulose to cellobiose, and β-glucosidase, which would hydrolyze the soluble cellobiose to glucose. In the following years, a number of groups began to identify and characterize the specific enzymes present in these components. The current opinion about cellulases diversity and action still agrees with the synergistic and coordinated attack of cellulose for a complex of enzymes, facilitating the degradation of the polymer. These enzymes are described in terms of three major classes of cellulases. The endoglucanases (EC 3.2.1.4, EG) act randomly on soluble and insoluble cellulose chain. The exoglucanases, which include cellobiohydrolases (EC 3.2.1.91, CBHs), acts processively to preferentially liberate cellobiose (and glucose in some cases) from the reducing and non-reducing ends of the cellulose chain. The β-glucosidase (EC 3.2.1.21) liberates D-glucose from cellobiose and exoglucosidases. Among the studied microorganism, fungi are most active against natural polymers, being capable of producing different amounts of each type of cellulases, which act synergistically (Himmel et al., 1999; Tolan and Foody, 1999; Lynd et al., 2002; Picartet al., 2007; Sohailet al., 2009).Almost all commercial cellulases obtained by submerged fermentation are produced by the fungi Trichoderma, Humicola, Aspergillus and Penicillium, while proteins from Trichoderma and Aspergillus involved in the transcriptional regulation of the genes encoding cellulases and hemicellulases have already been identified. The inducer molecules produces during degradation of the lignocellulosic material regulate positively the expression of these enzymes, e.g., cellobiose, D-xylose and L-arabinose. In general, cellulases are inhibited by its end products, cellobiose and glucose. Its action is also inhibited or inactivated by several classes of compounds, including strong oxidants or reducing agents, metal ions, salts, solvents, and surfactants (de Vries and Visser, 2001; Mach and Zeilinger, 2003; de Vries, 2003).
Cellobiose dehydrogenase
Cellobiose dehydrogenase (CDH) is produced extracellularly by number of wood and cellulose degrading fungi when grown on cellulose. It oxidizes the reducing end of cellobiose and cellooligosacchrides to their corresponding 1, 5-lactones, which are subsequently hydrolyzed to carboxylic acid in aqueous environment. CDH oxidizes very few other sugar, the most efficient substrates being β-1, 4-linked disaccharides with a β-glucose moiety at the reducing end. Complete function of CDH is not fully understood. It is not an essential component of the lignocellulosic-degrading enzyme complex but can enhance both cellulose and lignin degradation (Baminger et al., 2001).
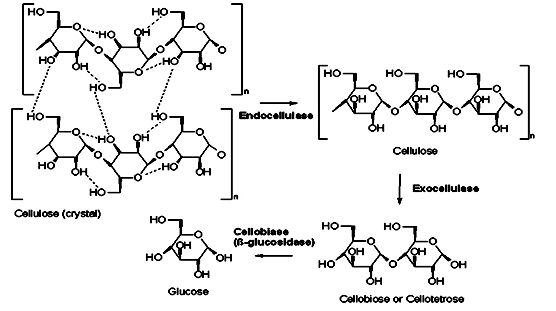
Figure: Reaction Route of Cellulose to Glucose (Source: Carcieri et al., 2010).
Types of fermentation based on enzymatic hydrolysis of biomass
Enzymatic hydrolysate can be fermented by two ways known as Separate hydrolysis and fermentation (SHF) and Simultaneous saccharification and fermentation (SSF).
Separate hydrolysis and fermentation (SHF)
Enzymatic hydrolysis performed separately from fermentation step is known as separate hydrolysis and fermentation (SHF). The main advantage of SHF is the ability to carry out each step under optimal condition, since hydrolysis and fermentation have different temperature optima. Another benefit of this process is recycling of yeast cells since sugar solution can be filtered prior to fermentation. A problem, however, is that the sugar decreases the efficiency of the enzyme due to product inhibition (Chandelet al., 2007a).
Simultaneous saccharification and fermentation (SSF)
The enzymatic hydrolysis and fermentation can also be performed in a combined step-the so-called simultaneous saccharification and fermentation (SSF). It gives higher bioethanol yields and requires lower amount of enzyme because end-product inhibition from cellobiose and glucose formed during enzymatic hydrolysis is relieved by the yeast fermentation (Balatet al., 2008). In SSF, the glucose produced is immediately converted to ethanol and therefore in this process sugar inhibition is avoided, since the fermenting organisms are mixed with the enzyme and the slurry. Disadvantages associated with SSF are mixing/cooling problem; the optimal temperature for fermentation is approximately 30oC, while for hydrolysis is about 50oC, thus SSF must be operated at intermediate temperature while another drawback is that the fermenting organisms cannot be recycled (Dehkhoda, 2008).
Direct microbial conversion (DMC)
Direct Microbial Conversion is a process of converting cellulosic biomass to ethanol. In this process both ethanol and all required enzyme are produced by a single microorganisms. However, DMC is not considered as leading process alternative because there are no robust organisms available that can produce cellulases or other cell wall degrading enzymes in conjunction with ethanol with high yield. Singh and Kumar, (1991) noticed that several strain of Fusariumoxysporum have potential to converting cellulose to ethanol only in one step process. The main disadvantage of F. oxysporum is its slow conversion rate of sugars to ethanol as compared to yeast (Chandelet al., 2007a).
Fermentation
Ethanol fermentation begins with the completion of glycolysis, which is also termed as EMP (Embden-Meyerhoff-Parnas) pathway (Dienet al., 2003). Glycolysis (EMP Pathway) is composed of three stages, namely activation of glucose, hexose splitting and energy extraction; the overall reaction formula for the EMP is summarized in following equation (Yang, 2008).
Equation:
Glucose + 2ATP + 4ADP + 2 Pi + 2NAD+ →
2 Pyruvate + 4ATP + 2ADP + 2NADH+H+
In most microorganisms, end product is lactic acid under anaerobic conditions, but in case of ethanologen microorganisms, pyruvate is first converted to acetaldehyde by reducing a molecule of CO2 out of pyruvate, and then acetaldehyde is reduced to ethanol along the redox reaction between NADH and NAD+. This metabolic pathway is less efficient, than the tri carboxylic acid cycle (TCA cycle), but can be performed in the absence of oxygen. However, ethanologen microbes can form ethanol in the presence of oxygen, when the glucose concentration is higher than the maximum, that can be consumed by TCA cycle (Kreb’s cycle), which is so called Crabtree effect. This is the basis of very high gravity ethanol fermentation, when ethanol is produced under aerobic conditions (Bvochoraet al., 2000).
According to overall reaction of the fermentation, the theoretical maximum yield is 0.51kg bioethanol and 0.49kg carbon dioxide per kg of xylose and glucose (Hamelincket al., 2005).
Equation:
3C5H10O5 → 5C2H5OH + 5CO2
C6H12O6 → 2C2H5OH + 2CO2
Fermentation techniques
Ethanol fermentation can be performed as a batch, fed batch or continuous process. The choice of most suitable processes will depend upon the kinetic property of microorganisms and type of lignocellulosichydrolysate in addition to process economic aspects (Chandelet al., 2007a).
Batch fermentation
Batch fermentation is a process where substrate and separately grown cells (inoculum) are charged into the bioreactor with nutrient and enzymes required. In batch fermentation, the microorganisms works in high substrate concentration initially and a high product concentration finally (Olsson and Hahn-Hagerdal, 1996). The batch process is a multi-vessel process; allow flexible operation and easy control over the process while characterized by low productivity with an intensive labor (Sharma, 1988).
Fed-batch fermentation
In fed batch fermentation, microorganism works at low substrate concentration with an increasing ethanol concentration during the course of fermentation process. It is regarded as combination of batch and continuous operation and found to be a very popular type of process in ethanol industry. Fed batch cultures often provide better yield and productivities than batch cultures for the production of different microbial metabolites. In this operation feed solution which contains substrate, yeast culture, important minerals and vitamins are fed at constant intervals while effluent is removed discontinuously. The startup of fed-batch operation is similar to batch process. Subsequently substrate fed into the bioreactor in a specified manner, after the growth limiting substrate (generally carbon source) which is given at the beginning of the process has consumed. The concentration of substrate must be kept constant in the reactor which the feeding is made, in this way the substrate inhibition can be kept at a minimum level in fed-batch process by adding substrate at the same rate at which it is consumed. Substrate concentration can be measured and feed controlled accordingly, so the level can be kept low. The substrate consumption rate can be calculated from measured factor such as carbon dioxide (Roehr, 2000).
Continuous fermentation
In a continuous process, nutrients are continuously supplied to the bioreactor and product stream is continuously withdrawn at the same rate as the supply, resulting in constant volume. In principle, continuous cultivation is efficient in terms of productivity per volume unit, but they are also sensitive to infections (Dehkhoda, 2008). This type of fermentation can be performed in different kind of bioreactors- stirred tank reactors (single or series) or plug flow reactors. Since cells are continuously being washed out of the bioreactor, there must be a cell growth that corresponds to the dilution rate, otherwise washout occurs. This problem can be circumvented by the use of cell retention (recirculation or immobilization), but there must be at least some production of new cells, otherwise the culture will age and lose its fermentative capacity (Brandberg, 2005).
Microorganisms
Microorganisms play a significant role in production of ethanol form renewable resources and thus, selection of suitable strain is essential for the individual process. An idial organism has capability of consuming both pentoses and hexose sugars, high tolerance against substrate;ethanol as well as inhibiting compound, high ethanol yield and minimum nutrient requirement is the essential features of an ideal microorganism (Van Zylet al., 2007). Although no microorganism has been found yet to meet all these requirements, development of a desirable strain is the focus of many studies. Thus far wide varieties of microorganisms including yeast, bacteria and fungi have been exploited offering different advantages and disadvantages by early researchers (Olsson and Hahn-Hagerdal, 1993).
Yeast (Saccharomycescerevisiae)
Saccharomyces cerevisiae is one of more than 1000 validated yeast species belonging to the fungi kingdom. It is unicellular eukaryotic organism, specialized in growing on sugars and can be isolated from fruits, plants and soil also (Rose and Harisson, 1993).It can tolerate ethanol concentration as high as 20% of fermentation medium (Lin and Tanaka, 2006). Yeast cell are facultative anaerobe, round to oval with diameter about 5-10 µm, most yeast are reproduced by budding, maximum number of buds are found on growing cells is around 25, and doubling time of the cells can be around 90 minutes in as favorable growth environment. Its robustness makes it a suitable organism for fermentation of lignocellulosichydrolysate. The main disadvantage of yeast in ethanol production process is lacking of mechanisms to take up pentose sugars as substrate, still it is the prime organisms for ethanol production (Balatet al., 2008).
Life cycle of Saccharomycescerevisiae
Saccharomycescerevisiae is a unicellular eukaryote which can reproduced both sexually (meiosis) and asexually by budding (mitosis). Yeast has two mating type, called “a” and “α”. When grown on rich medium, two haploid cells with opposite mating types merge to form a diploid cell. Meiosis and spore formation can therefore be induced by alternation of the culture condition. Haploid cells are capable of mating with other haploid cells of the opposite mating type (an “a” cell can only mate with a “α” cell, and vice versa) to produce a stable diploid cell. Diploid cells, usually upon facing stressful conditions such as nutrient depletion, can undergo meiosis to produce four haploid spores: two “a” spores and two “α” spores. The whole process takes around 24 hours to complete (Houston et al., 2004; Dehkhoda, 2008).
The cell division of yeast occurs by budding in which a daughter cell is initiated as an outgrowth from the mother cell, followed by nuclear division, cell-wall formation, and finally cell separation. Yeast cell grows in three main phases- lag phase, the exponential growth phase and the stationary phase (Asaduzzaman, 2007). Immediate after inoculation, the cells are entering in a brief lag phase where they are biochemically active but not dividing. The lag phase refers as initial growth phase, when number of cells remains relatively constant prior to rapid growth phase, also referred as adaptation time. Oxygen is rapidly absorbed during the lag phase. The yeast needs this oxygen to grow in order to produce important cell wall constituent. This phase is very important in building new healthy cell that will be able to complete fermentation. During this phase the individual cells are actively metabolizing, in preparation for cell division. The cells usually activate the metabolic pathways to make enough of the essential nutrients to begin active growth. The lag phase can be shortened by using a large inoculums or an inoculum’s culture that is already growing exponentially under similar condition.
As the yeast comes out of the lag phase, it starts to consume the sugars in solution, CO2 is produced, cell count will increase rapidly and ethanol will start to produce. The exponential phase occurs because yeast rapidly consumes sugar. Glucose is used first, then fructose and sucrose. Once the cell starts actively metabolizing, they begin DNA replication and shortly after, the cells divide. This is the period in which the cell growth most rapidly. The time it takes the culture to double is called generation time. This exponential phase depends on several factors: the organism itself, the growth medium and the temperature, are all important factors in determining the generation time. It is the time period during which the specific growth (µ) is constant and it is at a maximum (µ max) for given strain and the environmental conditions and then a zero growth period which is called stationary phase. At this point yeast growth slows down and finally become to zero which is called zero growth period (Tuite and Oliver, 1991).
Effect of oxygen
Saccharomycescerevisiae cannot stay a live more than 4 or 5 generation without oxygen, unless the ergestrol and twin (as fatty acid sources) be added to the medium. Complete oxidation of the sugar to carbon dioxide and water will give optimum cell production. Under conditions of high dissolved oxygen concentrations, fermentation of the sugars to ethanol are inhibited, this effect calls Pasture Effect. Respiration release more energy than fermentation and therefor is the preferred process. The Pasteur Effect is defined as an‘inhibition of the activity’ of the fermentation pathway by respiration; moreover, the Pasteur Effect is an inhibition of the fermentation pathway by an end product of aerobic glucose utilization.Many Saccharomyces species are sensitive to glucose and their respiration is repressed in the presence of a concentration of glucose greater than 1.0g/L under such condition biomass yield decreases and ethanol will be produced. This is known as a Crabtree effect or counter-pasture effect. The named given after the English biochemist Herbert Grace Crabtree, the Crabtree effect describes the phenomenon whereby the yeast, produces ethanol aerobically in the presence of high external glucose concentrations rather than producing biomass via the TCA cycle, the usual process occurring aerobically in most yeasts e.g. Kluyveromycesspp. Increasing concentrations of glucose accelerates glycolysis, which results in the production of appreciable amounts of ATP through substrate-level phosphorylation. This reduces the need of oxidative phosphorylation done by the TCA cycle via the electron transport chain and therefore decreases oxygen consumption. In a study of the Crabtree effect in various yeast strains, growing on a medium containing 30g/L glucose, seven of eight Saccharomyces species tested gave a positive Crabtree effect (Tuite and Oliver, 1991; Thomson et al., 2005).
Pentose fermenting microorganisms
The ability to ferment pentoses is not widespread among microorganism and most promising yeast identified so far, are Pichiastipites, Pachysolentannophilus, Candidashehatae able to ferment xylose naturally but these organisms are sensitive to ethanol and inhibitors, and require careful monitoring as compare to S. cerevisiae (Hahn-Hagerdalet al., 2007). While there areseveral filamentous fungi belonging to genera Fusarium, Rhizopus and Mucor are capable of assimilating hexoses and pentoses. Especially M. heimalisand M. indicus have been shown to be good ethanol produces with drawback of increasing viscosity by attaching to the growth medium (Millatiet al., 2005).
There are three main bacterial microorganisms discovered to ferment sugar into ethanol are Escherichiacoli, Klebsiellaoxytoca, and Zymomonasmobilis. The former two are able to ferment a variety of sugars to ethanol while the later gives high yields of ethanol but is specific to glucose and fructose sugars. Zymomonasmobilis is naturally able to produce ethanol with a high productivity but it has narrow substrate range and cannot consume mannose, galactose or xylose and also sensitive to inhibitors. There is another bacterium, Escherichiacoli that has a broad substrate range and is able to convert glucose, mannose, galactose, xylose and arabinose to ethanol, but ethanol yield is much more lesser than S. cerevisiae because of inhibitor and product sensitivity as well as different other product formation. Research have been done on producing maximum ethanol by using hexoses and pentoses from genetically engineered E. coli, K. oxytoca and Z. mobilis (Dienet al., 2003).
Table (1):
Growth characteristics of natural pentose-fermenting microorganisms.
Microorganism |
Glu |
Xyl |
Ara |
Man |
Cel |
Temp. range(oC) |
pH range |
|---|---|---|---|---|---|---|---|
Filamentous fungi |
|||||||
Fusariumoxysporum |
+ |
+ |
+ |
+ |
+ |
28-32 |
5-6 |
Neurosporacrassa |
+ |
+ |
– |
– |
+ |
28-37 |
5-6 |
Moniliasp. |
+ |
+ |
– |
– |
– |
26 |
5 |
Mucorsp. |
+ |
+ |
– |
– |
– |
30 |
5.4 |
Yeast |
|||||||
Saccharomyces cerevisiae |
+ |
– |
– |
+ |
– |
30-35 |
3-7 |
Klyuvermycesmarxians |
+ |
+ |
+ |
+ |
– |
30-35 |
3-7 |
Pachysolentannophilus |
+ |
+ |
+ |
– |
– |
28-32 |
2.5-7 |
Candida shehatae |
+ |
+ |
+ |
+ |
– |
28-32 |
3-7 |
Pichiastiptis |
+ |
+ |
+ |
+ |
– |
28-32 |
3-7 |
Mesophillic bacteria |
|||||||
Bacillus polymyxa |
+ |
+ |
+ |
+ |
– |
35-37 |
5.5-8 |
Aerobacterhydrophila |
+ |
+ |
+ |
+ |
– |
35-37 |
5.5-8 |
Klebsiella pneumonia |
+ |
+ |
+ |
+ |
– |
35-37 |
5-6 |
Clostridium acetobutylicum |
+ |
+ |
+ |
+ |
+ |
35-37 |
4-8 |
Thermophilic bacteria |
|||||||
Clostridium thermocellum |
+ |
+ |
+ |
– |
+ |
65 |
4-8 |
C. thermohydrosulfuricum |
+ |
+ |
+ |
– |
– |
65 |
4.7-8 |
C. thermosaccharolyticum |
+ |
+ |
+ |
+ |
– |
60 |
5-8 |
C. htermosulfurogenes |
+ |
+ |
+ |
+ |
– |
60 |
4.5-7.5 |
Thermoanerobacterethanolicus |
+ |
+ |
+ |
+ |
– |
69 |
4.4-9.5 |
Glu-glucose; Xyl-xylose; Ara-arabinose Man-mannosecel-cellulose
(Source: Abbi et al., 1996).
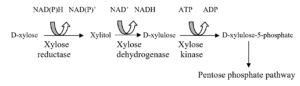
Biochemistry of xylose fermentation
Bacteria can directly convert xylose to xylulose with xylose isomerase. However, yeast that are capable of consuming pentose, first reduce xylose to xylitol with NADPH-dependent xylose reductase (aldose reductase EC 1.1.1.21), and subsequently oxidized to xylulose with NAD+-dependent xylitol dehydrogenase (D-xylose reductase, EC 1.1.1.9) to form D-xylose-5-phosphate. Ribolosephosphate-3-epimerase (5.1.3.1), transaldolase (2.2.1.2) and transketolase (2.2.1.1) sequentially convert alpha-xylose-5-phosphate into glyceraldehyde-3-phosphate and fructose-6-phosphate by non-oxidative rearrangement leading to ethanol formation by EMP pathway. Alternatively, phosphoketolase (4.1.2.9) can split D-xylose-5-phosphaste into glyceraldehyde-3-phosphate and acetylphosphate. Phosphoketolase is known to be important in lipid-producing yeast, especially when they are grown on D-xylose. In some D-xylose fermenting yeast, an oxidative pentose phosphate mechanism is present to metabolize D-xylose (Dienet al., 2003). However, wild-type strains of Saccharomycescerevisiae are unable to ferment D-xylose directly and can only act on xylulose to produce ethanol when exogenous xylose isomerase is introduced to the D-xylose containing system (Du Preez, 1994). Saccharomycescerevisiae are genetically modified for the purpose to ferment both pentoses and hexoses by incorporating xylose reductase and xylitol dehydrogenase sequences but consequent gene expression was not satisfactory and moreover, certain amount of xylitol is also produced along with the yield of ethanol, because of existence of redox cofactor imbalance- NADPH and NAD+, which are linked with xylose reductase and xylitol dehydrogenase, respectively (Freer et al., 1997, Jeffries and Jin, 2004).
Bioethanol is currently made by large scale fermentation of sugars that are extracted from energy crops. Production of ethanol from biomass seems to be an interesting alternative to traditional fossil fuel but the comprehensive process development and optimization of lignocellulosic biomass is still required to make the process economically and environmentally viable. The choice of the best technology for lignocellulose to bioethanol conversion should be decided on the basis of overall economic, environmental and energy potentials. The focus of this review is on current status on the available ethanol production technologies in terms of pretreatment hydrolysis and fermentation. Acid treatment gives a possibility to carry out rapid cellulose conversion at batch level, but it still leave some problems and questions unresolved which needs sincere efforts, including acid recovery and increase in sugar concentration after hydrolysis, minimization of sugar loss and precipitate production after detoxification. Isolation of potential laccase and cellulases producers and there scale up for biological delignification and efficient enzyme hydrolysis should be considered as best alternative at commercial scale. Ethanol is currently made by large scale fermentation using yeast, but application of various co-culture combinations and their optimization study for scale up of ethanol production process is necessary. Lastly, ethanol may not be the only ultimate product from any lignocellulosic biomass. It opens opportunities to alter the ultimate product such as biogas, biodegradable plastics, enzymes etc.
- Abbi, M., Kuhad, R.C., and Singh, A., Bioconversion of pentose sugar to ethanol by free and immobilized cells of Candida shehatae NCL-3501: fermentation behavior. Process Biochem., 1996; 31: 555-560.
- Agbogbo, F., and Wenger, K., Effect of pretreatment chemicals on xylose fermentation by Pichia stipites. Biotechnol Lett., 2006; 28: 2065-2069.
- Alizadeh, H., Teymouri, F., Gilbert, T. I., and Dale, B.E., Pretreatment of switch grass by Ammonia Fiber Explosion (AFEX). Appl Biochem Biotechnol, 2005; 121-124: 1133-1142.
- Asaduzzaman, MD., Standardization of yeast growth curves from several curves with different initial size. Master’s Thesis. Department of mathematical sciences, Chalmers University of technology and Goteborg University SE, Goteborg, Sweden 2007.
- Badger, P.C., Ethanol from cellulose: a general review. In: Janick J, Whipkey A, editors. Trends in new crops and new uses. Alexandria, VA: ASHS Press, 2002; 17-21.
- Balat, M., Balat, H., and Oz, C., Progress in bioethanol processing. Prog Energ Combust., 2008; 34: 551-573.
- Baminger, U., Subramaniam, S. S., Renganathan, V., and Haltrich, D., Characterization of cellobiose dehydrogenase from the plant pathogen Sclerotium (Athelia) rolfsii. Appl Environ Microbiol. 2001; 67(4): 1766–1774.
- Bertilsson, M., Simultaneous saccharification and fermentation of sprucc-a comparison of pretreatment condition and different enzyme preparations. Master Thesis. Department of Chemical Engineering, Lund University, Sweden 2007.
- Binod, P., Sindhu, R., Singhania, R.R., Vikram, S., Devi, L., Nagalaxmi, S., Kurien, N., Sukumaran, R.K., and Pandey, A., Bioethanol production from rice straw: an overview. Bioresour Technol., 2010; 101: 4767-4774.
- Boudet, A.M., Kajita, S., Grima-Pettenati, J., and Goffner, D., Lignin and lignocellulosic: a better control of synthesis for new and improved uses. Trends Plant Sci. 2003; 8(12): 576-581.
- Brandberg, T., Fermentation of undetoxified dilute acid lignocelluloses hydrolyzates for fuel ethanol production. PhD Thesis. Chalmers University of technology 2005.
- Bridgeman, T.G., Darvell, J.J., Jones, J.M., Williams, P.T., Fahmi, R., Bridgwater, A.V., Barraclough, T., Shield, I., Yates, N., Thain, S.C., and Donnison, I.S., Influence of particle size on the analytical and chemical properties of two energy crops. Fuel., 2007; 86(1-2): 60-72.
- Bvochora, J.M., Read, J.S., and Zvauya, R., Application of very high gravity technology to the co-fermentation of sweet stem sorghum juice and sorghum grain. Ind Crop Prod., 2000; 11: 11-17.
- Canilha, L., Carvalho, W., Felipe, M.G.A., and Silva, J.B.A., Xylitol production from wheat straw hemicellulose hydrolyzate: hydrolyzate detoxification and carbonsource used for inoculum preparation. Brazillian J Microbiol., 2008; 39: 333-336.
- Carcieri, S., Clardy, E., and Zahid, N.S., Analyzing the ability of modified yeast to ferment xylose to ethanol. Project report, Worcester Polytechnic Institute (WPI), 2010; April, 27.
- Chandel, A.K., Chen, E.C., Rudravaram, R., Narasu, M.L., Rao, L.V., and Ravindra, P., Economics and environmental impact of bioethanol production technologies: an appraisal. Biotechnol Mol Biol Rev. 2007a; 2: 14-32.
- Chandel, A.K., Kapoor, R.K., Singh, A., and Kuhad, R.C., Detoxification of sugarcane bagasse hydrolyzate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol. 2007b; 98: 1947-1950.
- Chang, V.S., Nagwani, M., and Holtzapple, M.T., Lime pretreatment of crop residues bagasse and wheat straw. Appl Biochem Biotechnol. 1998; 74(3): 135-159.
- Chen, Y., Sharma-Shivappa, R.R., and Chen, C., Ensiling agricultural residues for bioethanol production. Appl Biochem Biotechnol. 2007; 143(1): 80-92.
- Choinowski, T., Blodig, W., Winterhalter, K.H., and Piontek, K., The crystal structure of lignin peroxidase at 1.70 angstrom resolution reveals hydroxy group on the C-beta of tryptophan 171: a novel radical site formed during the redox cycle. J Mol Biol. 1999; 286: 809-827.
- Dale, B.E., Henk, L.L., and Shiang, M., Fermentation of lignocellulosic materials treated by ammonia freeze explosion. Dev Ind Microbiol., 1985; 26: 223-233.
- Dale, B.E., Leong, C.K., Pham, T.K., Esquivel, V.M., Rios, I., and Latimer, V.M., Hydrolysis of lignocellulosic at low enzyme levels: application of the AFEX process. Bioresour Technol., 1996; 56: 111-116.
- Dehkhoda, A., Concentrating lignocellulosic hydrolyzate by evaporation and its fermentation by repeated fed-batch using flocculating Saccharomyces cerevisiae. Master’s Thesis, University College of Boras, Sweden 2008.
- Demirbas, A., Bioethanol from cellulosic material: a renewable motor fuel from biomass. Energ Source.Part A, 2005; 27(8): 327-337.
- Demirbas, A., Progress and recent trends in biofuels. Prog Energy Combus Sci., 2007; 33: 1-18.
- Demirbas, A., The importance of bioethanol and biodiesel form biomass. Energ Source. Part B., 2008; 3: 177-185.
- de Vries, R.P., and Visser, J., Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev., 2001; 65(4): 497-522.
- de Vries, R.P., Regulation of Aspergillus genes encoding plant cell wall polysaccharide degrading enzymes, relevance for industrial production. Appl Micrbiol Biotechnol., 2003; 61: 10-20.
- Dien, B.S., Cotta, M.A., and Jeffries, T.W., Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol., 2003; 63: 258-266.
- Du Preez, J.C., Process parameters and environmental factors affecting D-xylose fermentation by yeasts. Enzyme Microb Technol., 1994; 16(11): 944-956.
- Fan, L.T., Gharpuray, M.M., and Lee, Y.H., In: Cellulose hydrolysis Biotechnology Monographs. Springer, Berlin, 1987; p. 57.
- Farooqi, R., and Sam, A.G., Ethanol as transportation fuel. Centre for Applied Business Research in Energy and the Environment (CABREE), climate Chang Initiative., 2004; University of Alberta, Canada. [ www.business.ualberta.ca/cabree]
- Freer, S.N., Skory, C.D., and Bothast, R.J., D-xylose metabolism in Rhodosporidium toruloides. Biotechnol Lett., 1997; 19(11): 1119-1122.
- Gollapalli, L.E., Dale, B.E., and Rivers, D.M., Predicting digestibility of ammonia fiber explosion (AFEX)-treated rice straw. Appl Biochem Biotechnol. 2002; 98-100: 23-35.
- Graf, A., and Koehler, T., Oregon cellulose-ethanol study: an evaluation of the potential for ethanol production in Oregon using cellulosic based feedstocks. Salem, Oregon, USA: Oregon Dept. of Energy; 2000; June 96 , [ www.ethanol-gec.org/information/briefing/20a]
- Grzenia, D.L., Schell, D.J., and Wickramsinghe, S.R., Detoxification of biomass hydrolyzates by reactive membrane extraction. J Membr Sci., 2010; 348: 6-12.
- Gupta, R., Sharma, K.K., and Kuhad, R.C., Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, a woody substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis-NCIM 3498. Bioresour Technol, 2009; 100: 1214-1220.
- Hahn-Hagerdal, B., Galbe, M., Gorwa-Grauslund, M.F., Liden, G., and Zacchi, G., Bioethanol- the fuel of tomorrow from the residues of today. Trends Biotechnol., 2006; 24(12): 549-56.
- Hamelinck, C.N., Van Hooijdonk, G, and Faaij, A.P.C., Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenerg. 2005; 28: 384-410.
- Harun, M.Y., Dayang Radiah, A.B., Zainal Abidin, Z, and Yunus, R., Effect of physical pretreatment on dilute acid hydrolysis of water hyacinth (Eichhornia crassipes). Bioresour Technol. 2011; 102(8): 5193-5199.
- Hatakka, A., Lignin-modifying enzymes from selected white-rot fungi- production and role in lignin degradation. FEMS Microbiol Rev., 1994 13: 125-135.
- Hector, R.E., Mertens, J.A., Bowman, M.J., Nichols, N.N., Cotta, M.A., and Hughes, S.R., Saccharomyces cerevisiae engineered for xylose metabolism requires gluconeogenesis and the oxidative branch of the pentose phosphate pathway for aerobic xylose assimilation. Yeast. 2011; 28: 645-660.
- Heipieper, H.J., Weber, F.J., Sikkema, J., Kewelo, H., and de Bont, J.A.M., Mechanisms of resistance of whole cell to toxic organic solvents. TIBTECH. 1994; 12: 409-415.
- Himmel, M.E., Ruth, M.F., and Wyman, C.E., Cellulases for commodity products from cellulosic biomass. Curr Opin Biotechnol., 1999; 10: 358-364.
- Houston, P., Simon, P.J., and Broach, J.R., The Saccharomyces cerevisiae recombination enhancer biases recombination during interchromosomal mating-type switching but not in interchromosomal homologous recombination. Genetics, 2004; 166(3): 1187-1197.
- Iranmahboob, J., Nadim, F., and Monemi, S., Optimizing acid hydrolysis: a critical step for production of ethanol form mixed wood chips. Biomass Bioenerg. 2002; 22: 401-404.
- Jeffries, T.W., and Jin, Y.S., Ethanol and thermotolerance in the bioconversion of xylose by yeasts. Adv Appl Microbiol. 2000; 47: 221-68.
- Jeffries, T.W., and Jin, Y.S., Metabolic engineering for improved fermentation of pentoses by yeasts. Appl Microbiol Biotechnol. 2004; 63: 495-509.
- Johansen, C.L., Coolen, L., and Hunik, J.H., Influence of morphology on product formation in Aspergillus awamori during submerged fermentation. Biotechnol Prog., 1998; 14: 233-240.
- Karr, W.E., and Holtzapple, M.T., Using lime pretreatment to facilitate the enzymatic hydrolysis of corn stover. Biomass Bioenerg. 2000; 18: 1099-1120.
- Kaur, U., Oberoi, H.S., Bhargav, V.K., Sharma-Shivappa, R.R., and Dhaliwal, S.S., Ethanol production from alkali and ozone treated cotton stalk using thermo tolerant Pichia kudriavzevii HOP-1. Ind crop prod. 37, 219-226.
- Kaya, F., Heitmann, J.A., and Thomas, W.J., Influence of lignin and its degradation products on enzymatic hydrolysis of xylan. J Biotechnol, 2000; 80: 241-247.
- Keshwani, D. R., Cheng, J. J., Burns, J.C., Li, L., and Chiang, V., (2007). Microwave pretreatment of switch grass to enhance enzymatic hydrolysis. ASABE Paper No. 077127. St. Joseph, Mich.: ASABE.
- Kim, S.B., and Lee, Y.Y., Fractionation of herbaceous biomass by ammonia-hydrogen peroxide percolation treatment. Appl Biochem Biotechnol, 1996; 57-58: 147-156.
- Kim, T.H., Kim, J.S., Sunwoo, C., and Lee, Y.Y., Pretreatment of corn stover by aqueous ammonia. Bioresour Technol. 2003; 90: 39-47.
- Klinke, H.B., Thomsen, A.B., and Ahring, B.K., Inhibition of ethanol producing yeast and bacteria by degrading products produced during pretreatment of biomass. Appl Microbiol Biotechnol., 2004; 66: 10-26.
- Kumar, P., Barrett, D.M., Delwiche, M.J., and Stroeve, P., Pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 2009; 48(8): 3713-3729.
- Leustean, I., Bioethanol from lignocellulosic material. J Agroalimentary Process Technol. 2009; 15: 94 -101.
- Liao, W., Liu, Y., Liu, C., Wen, Z., and Chen, S., Acid hydrolysis of fiber from dairy manure. Bioresour Technol. 2006; 97: 1687-1695.
- Lin, Y., and Tanaka, S., Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol. 2006; 69: 627-645.
- Lu, Z., and Kumakura, M., Enzymatic hydrolysis of wheat straw irradiated by electron-beam in presence of per acetic acid solution. Isot Environ Health Stud. 1995; 31(1): 151-160.
- Luo, C., Brink, D.L., and Blanch, H.W., Identification of potential fermentation inhibitors in conversion of hybrid poplar hydrolyzate to ethanol. Biomass Bioenerg. 2002; 22: 125-138.
- Lynd, L.R., Weimer, P.J., Van Zyl, W.H., and Pretorius, I.S., Microbial cellulases utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev., 2002; 66(3): 506-577.
- Mabee, W.E., Gregg, D.J., Arato, C., Berlin, A., Bura, R., and Gilkes, N., Updates on softwood-to-ethanol process development. Appl Bio-chem Biotchnol, 2006; 129-132: 55-70.
- Mach, R.L., and Zeilinger, S., Regulation of gene expression in industrial fungi: Trichoderma. Appl Microbiol Biotechnol. 2003; 60: 515-522.
- Martinez, A., Rodriguez, M.E., York, .S.W., Preston, J.F., and Ingram, L.O., Effect of Ca(OH)2 treatments (“overliming”) on the composition and toxicity and bagasse of hemicellulose hydrolyzates. Biotechnol Bioeng. 2000; 69: 526-536.
- Martinez, A., Rodriguez, M.E., Wells M.L., York, S.W., Preston, J.F., and Ingram, L.O., Detoxification of dilute acid hydrolyzate of lignocellulose with lime. Biotechnol Prog. 2001; 17: 287-293.
- Millati, R., Edebo, L., and Taherzadeh, M.J., Performance of Rizopus, Rhizomocur, and Mucor in ethanol production form glucose, xylose and wood hydrolyzates. Enz Microb Technol., 2005; 36: 294-300.
- Mok, W.S.L., and Antal Jr., M.J., Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water. Ind Eng Chem Res., 1992; 31: 1157-1161.
- Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., and Ladish, M., Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol. 2005; 96(6): 673-686.
- Olsson, L., and Hahn-Hagerdal. B., Fermentative performance of bacteria and yeast in lignocellulose hydrolyzate. Process Biochem. 1993; 28(8): 249257.
- Olsson, L., and Hahn-Hagerdal, B., Fermentation of lignocellulosic hydrolyzates for ethanol production. Enzyme Microb Technol., 1996; 18: 312-331.
- Palmqvist, E., Hahn-Hagerdal, B., Szengyel, Z., Zacchi, and G., Reczey, K., Simultaneous detoxification and enzyme production of hemicellulose hydrolyzates obtained after steam pretreatment. Enz Microb Technol. 1997; 20: 286-293.
- Palmqvist, E., Almeida, J.S., and Hahn-Hagerdal, B., Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiae in batch culture. Biotechnol Bioeng. 1999; 62: 447-454.
- Palmqvist, E., and Hahn-Hagerdal, B., Fermentation of lignocellulosic hydrolyzate. I: inhibition and detoxification. Bioresour Technol. 2000a; 74: 17-24.
- Palmqvist, E., and Hahn-Hagerdal, B., Fermentation of lignocellulosic hydrolyzate. II: inhibitors and mechanism of inhibition. Bioresour Technol., 2000b; 74: 25-33.
- Paturau, J.M., Alternative uses of sugarcane and its byproducts in agro industries. Food and Agricultural Organization of the United Nations (FAO) 1987.
- Picart, P., Diaz, P., and Poster, F.I.J., Cellulases from two Penicillum sp. Strains isolated from subtropical forest soil: production and characterization. Lett Appl Microbiol. 2007; 45: 108-113.
- Piontek, K., Antorini, M., and Choinowski, T., Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-angstrom resolution containing a full complement of coppers. J Biol Chem. 2002; 277: 37663-37669.
- Purwadi, R., Continuous ethanol production from dilute-acid hydrolyzate: detoxification and fermentation strategy. Ph.D. Thesis (Taherzadeh, M.J.). Chalmers University, Sweden 2006.
- Ragauskas, A.J., Williams, C.K., Davison, B.H., Britovsek, G., Cairney, J., Eckert, C.A., Frederick, W.J., Hallett, J.P., Leak, D.J., Liotta, C.L., Mielenz, J.R., Murphy, R., Templer, R., and Tschaplinski, T., The path forward for biofuels and biomaterials. Science., 2006; 311: 484-489.
- Rahman, S.H.A., Choudhury, J.P., Ahmad. A.L., and Kamaruddin, A.H., Optimization studies on acid hydrolysis of oil palm empty fruit bunch fiber for production of xylose. Bioresour Technol. 2007; 98: 554-9.
- Ren, H., Effect of cell wall degrading enzymes and chemicals on corn stover preservation and pretreatment during ensilage processing. Ph. D. diss. University Park, Pa.: Pennsylvania State University, Department of Agricultural and Biological Engineering 2006.
- Richard, T.M., Proulx, S., Moore, K.J., and Shouse, S., Ensilage technology for biomass pretreatment and storage. ASAE Meeting paper No. 016019. St. Joseph, Mich.: ASAE 2001.
- Rishi, A.S., Nelson, N.D., and Goyal, A., Molecular forming in plants: a current perspective. J Plant Biochem Biotechnol. 2001; 10(1): 1-12.
- Roehr, M., (2000). Biotechnology of Ethanol, Classic and future application. Publication: Wiley.
- Romero, I., Sanchez, S., Moya, M., Cstro, E., Ruiz, E., and Bravo, V., Fermentation of olive tree pruning acid-hydrolyzates by Pachysolen tannophilus. Biochem Eng J. 2007; 36: 108-115.
- Rose, A.H., and Harisson, J.S., The yeast. Second edition. Academic press. London UK 1993.
- Russell, J.B., Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J Appl Bacteriol., 1992; 73:363-370.
- Saha, B.C., Iten, L.B.,Cotta, M.A., and Wu, Y.V., Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol Prog. 2005; 21: 816-822.
- Shafizadeh, F., and Bradbury, A.G.W., Thermal degradation of cellulose in air and nitrogen at low temperatures. J Appl Poly Sci. 1979; 23: 1431-1442.
- Sharma, G., Developments in bioreactor for fuel ethanol production. Process Biochem. 1988; 23: 138-145.
- Sharma, S.K., Karla, K.L., and Grewal, H.S., Fermentation of enzymatically saccharified sunflower stalks for ethanol production and its scale up. Bioresour Technol. 2002; 85: 31-33.
- Shi, J., Sharma-Shivappa, R.R., Chinn, M.S., and Howell, N., Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalk for ethanol production. Bioresour Technol. 2009; 33: 88-96.
- Silverstein, R.A., A comparison of chemical pretreatment methods for converting cotton stalk to ethanol. Master’s Thesis. Biological and Agricultural Engineering, North Caroline State University 2004.
- Silverstein, R.A., Chen, Y., Sharma-Shivappa, R.R., Boyette, M.D., and Osborn, J. A., A comparison of chemical pre-treatment methods for improving saccharification of cotton stalks. Bioresour Technol. 2007; 98: 3000-3011.
- Singh, A., and Kumar, P.K., Fusarium oxysporum: status in bioethanol production. Crit Rev Biotechnol. 1991; 11(2): 129-47.
- Sohail, M., Siddiqi, R., Ahmad, A., and Khan, S.A., Cellulases production from Aspergillus niger MS82: effect of temperature and pH. N Biotechnol. 2009; 25: 437-441.
- Solomon, B.D., Barnes, J.R., and Halvorsen, K.E., Grains and cellulosic ethanol: History, economics, and energy policy. Biomass Bioenerg. 2007; 31: 416-425.
- Sun, Y., and Cheng, J., Hydrolysis of lignocellulosic material for ethanol production: a review. Bioresour Technol. 2002; 83: 1-11.
- Taherzadeh, M.J., Gustaffson, L., Niklasson, C., and Liden, G., Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J Biosci Bioeng. 1999; 87: 169-174.
- Taherzadeh, M.J., and Karimi, K., Acid-Based hydrolysis processes for ethanol from lignocellulosic materials: A Review. Bioreserces. 2007; 2: 472-499.
- Tolan, J.S., and Foody, B., Cellulases from submerged fermentation. Adv Biochem Eng Biotechnol, 1999; 65: 41-67.
- Tuite, M.F., and Oliver, G., Saccharomyces Handbook. Plenum press. New York, 1991.
- Van Zyl. C., Prior, B.A., and du Preez. J.C., Production of ethanol from sugar cane bagasse hemicellulose hydrolyzate by Pichia stipites. Appl Biochem Biotechnol.1988; 17: 357-369.
- Van Zyl, W.H., Lynd, L. R., Den Haan, R. and McBride, J. E., Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Adv Biochem Eng Biotechnol. 2007; 108: 205-235.
- Weil, J.R., Sarikaya, A., Rau, S. L., Goetz, J., Ladisch, C.M., Brewer, M., Hendrickson, R., and Ladisch, M.R., Pretreatment of yellow poplar sawdust by pressure cooking in water. Applied Biochem Biotechnol. 1997; 68(1-2): 21-40.
- Wheals, A.E., Basso, L.C., Alves, Denise, M.G., and Amorin, H.V., Fuel ethanol after 25 years. Trend Biotechnol. 1999; 17(12): 482-487.
- Wyman, C.E., Ethanol production from lignocellulosic biomass: Overview. In: Wyman CE, editor. Handbook on Bioethanol: production and utilization. Washington DC: Taylor and Francis., 1996; p. 1-16.
- Wyman, C.E., Dale, B.E., Elander, R.T., Holtzapple, M., Ladisch, M.R., and Lee, Y.Y., Coordinated development of leading biomass pretreatment technologies. Bioresour Technol., 2005; 69: 1959-1966.
- Xu, L., Biofuel production by using integrated anaerobic fermentation. PhD Thesis. University of Minnesota 2012.
- Xiang, Q., Lee, Y.Y., and Torget, R.W., Kinetics of glucose decomposition during dilute acid hydrolysis of lignocellulosic biomass. Appl Biochem Biotechnol., 2004; 113-116: 1127-1138.
- Yang, C., Shen, Z., Yu, G, and Wang, J., Effect and aftereffect of Gamma-radiation pretreatment on enzymatic hydrolysis of wheat straw. Bioresour Technol. 2008; 99(14): 6240-6245.
- Yang, Y., Ethanol production potential of acid pretreated switch grass varieties. Master’s Thesis. North Carolina State University 2008.
- Yu, Z., and Zhang, H., Ethanol fermentation of acid-hydrolysed cellulosic pyro lysate with Saccharomyces cerevisiae. Bioresour Technol. 2004; 93, 199-204.
- Zheng, Y., Lin, H.M., and Tsao, G.T., Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol Prog. 1998; 14: 890-896.
- Zheng, Y., Pan, Z., and Zhang, R., Overview of biomass pretreatment for cellulosic ethanol production. Int J Agric Biol Eng. 2009; 2(3): 102-6.
- Zhu,S., Wu, Y., Yu., Z., Liao, j., and Zhang, Y., Pretreatment by microwave/alkali of rice straw and its enzymatic hydrolysis. Process Biochem, 2005; 40: 3082-3086.
- Zhu, L., O’Dwyer, J.P., Chang, V.S., Granda, C.B., and Holtzapple, M.T., Structural features affecting biomass enzymatic digestibility. Bioressour Technol. 2008; 99(9): 3817-3828.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.