ISSN: 0973-7510
E-ISSN: 2581-690X
Corrugated cardboard boxes are one of the largest paper-based packaging forms used for shipping and handling of wide variety of products in different end-use industries due to low cost, low weight and recyclability. Due to its organic composition, they are highly susceptible to spoilage from heat-resistant microbial spores, leading to economic losses and health risks. In this study, the efficacy of lipopeptides produced from Bacillus amyloliquefaciens MTCC 10456 against thermotolerant Thermoascus crustaceus, Neosartorya hiratsukae and Bacillus subtilis, isolated from spoiled cardboard boxes, was investigated. Lipopeptides were isolated by salt-precipitation of fermentation broth and activity-guided Reverse Phase-High Performance Liquid Chromatography (RP-HPLC). Inhibitory fractions consisted of bacillomycin D and surfactin, which were identified using liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS/MS) analysis. Mixture of lipopeptides with nisin (3:2 w/w) asserted significant synergistic effect on the tested pathogens which reduced the minimum inhibitory concentrations (MIC) values and increased their inhibition spectra. Preservative coating containing lipopeptides and nisin was applied on the corrugated cardboard surfaces by mixing with starch-based additive by spread-coating method. It demonstrated biopreservative efficacy against the targeted microorganisms at during the observational period of 180 days. Reduction in microbial count of 4 log cycles was observed in 20 days and showed controlled release of coated peptides which indicate its suitability for packaging purposes. Findings from this study suggests an effective and scalable strategy to prevent microbial spoilage thereby extending the storage period of cardboard boxes.
Bacillus amyloliquefaciens, thermotolerant microorganisms, preservative coating, lipopeptides, corrugated cardboard boxes
Corrugated cardboard boxes are one of the most widely used secondary packaging materials due to their flexibility, low weight, low cost and environmental-friendly nature. Corrugated cardboard packaging is a sub-segment of the paper packaging market which accounts for 44% of the total market and was estimated at USD 69.91 billion in 20191. The demand for corrugated boxes is continually witnessing substantial growth (more than 4%) due to the increasing demand from retail e-commerce packaging industry. The major challenges faced by this market are environmental concerns due to desertification, toxins production, microbial spoilage of paper and machinery leading to huge losses1.
Paper-based packaging material is reused and recycled more than any other packaging materials such as metals and plastics, which makes it an economically viable and sustainable choice2. In paper-making process, starch or cellulose is the most common cost-effective additive, which also imparts mechanical strength and good barrier properties. However, the starch and cellulose derivatives show sensitivity to microbial attack. At suitable temperature (30-45°C) and pH (4-10) conditions during paper manufacturing process, it provides an ideal environment for the growth of microorganisms. Extreme temperatures during the paper drying process kill most of the contaminants but heat-resistant spores of bacteria and fungi, which leads to smell, discoloration, irregularity and overall decrease in display quality of the paper3. The thermotolerant spoilage microorganisms present on the cardboard surface can penetrate and damage the packaged goods and may cause health hazards to the consumers. Thus, in response to the increasing demand for safe and high-quality goods, it is necessary to develop new bio-based or naturally derived antimicrobial coating material without disturbing the barrier and strength properties for packaging paper4.
Members of Bacillus species are known to produce diverse molecules including secondary metabolites which exhibit a wide spectrum of antimicrobial activities such as bacteriocins, lipopeptides, polyketides and peptide antibiotics. Due to antimicrobial action of lipopeptides, they have potential for agricultural, pharmaceutical and environmental applications5. Bacillus spp. form resistant spores due to which they are ubiquitous in nature, exhibit high thermal tolerance and have shorter growth rates, and are thus considered to be good candidates as biological control agents. There is a growing interest in the commercialization of these bacteria-derived metabolites because of their biodegradability, availability and low toxicity, which are desirable factors for their potential applications in the industry6,7. Thus, antagonistic metabolites produced from Bacillus spp. can be considered as an alternative to existing antimicrobial agents used for preservation of paper-based packaging material. The use of such natural antimicrobial agents could be an efficient method in antimicrobial packaging, as they present lower risk to the consumers as compared to the synthetic antimicrobial agents and can be made available throughout the year4,8,9.
In this research work, the susceptibility of thermotolerant microorganisms, isolated from spoiled corrugated cardboard boxes to the lipopeptides from seaweed-associated B. amyloliquefaciens MTCC 10456 was evaluated. This is the first study to suggest that lipopeptides produced by B. amyloliquefaciens MTCC 10456 in combination with nisin, can be considered as potential candidates for the development of preservative coating for cardboard-based packaging systems for prolonged storage.
Identification of cardboard spoilage microflora
The corrugated cardboard boxes used for this study were procured from “Parksons packaging Ltd, Pune, India”. Microbial contamination of the cardboard boxes was estimated by international standard method based on agar flooding method10. 10 x 10 mm of cardboard samples were placed in 10 cm Petri dishes and molten Potato Dextrose agar (PDA, Himedia, Mumbai, India) was poured over it. Incubation was carried out for 7 days at 27°C. The individual bacterial and fungal colonies were counted and spread on NA and PDA plates, respectively, to obtain pure cultures. Each of the isolated bacterial and fungal colony was identified and characterized by 16S rDNA gene sequence analysis.
Fermentative production and extraction of lipopeptides
Bacillus amyloliquefaciens strain MTCC 10456 was procured from ‘Microbial Type Culture Collection and Gene Bank’ (MTCC), Chandigarh, India. It was preserved as stock culture in 20% (v/v) glycerol at -80°C after growing in Nutrient Broth (NB, Himedia, India) at 37°C for 24 h. The seed culture of B. amyloliquefaciens MTCC 10456 was initiated in 100 mL Tryptone soya broth (Himedia, Mumbai, India) at 37°C at 150 rpm for 18 h as seed culture. It was then inoculated into 900 mL of Tryptone soya broth and fermentation was carried out at 30°C for 72 h with shaking at 150 rpm. Centrifugation was carried out at 7000 rpm for 20 min to remove the biomass and the pH of the supernatant was set at 8.5. It was then gently added with ammonium sulphate ((NH4)2SO4) powder to achieve saturation level of 60% with continuous stirring to dissolve the salt completely and stored at 4-8°C for 12-14 h. Precipitated proteins were the collected by centrifugation at 8000 rpm for 20 min. The salt precipitated protein fraction was then desalted by passing through PD-10 desalting column (GE Healthcare, Chicago, United States) filled with 8.3 mL of Sephadex G-25 resin and eluted in 100 mM sodium phosphate buffer (pH 7.4). The desalted fraction was designated as “LP extract or LPE” and freeze-dried for storage.
Separation and identification of lipopeptides by mass spectrometry
Identification of lipopeptides present in the LPE was done by Reverse Phase-High-performance liquid chromatography (RP-HPLC) using an HPLC system (1200 series, Agilent, Santa Clara, United States). 10 mg of LPE was injected into the column (C18 Eclipse plus, 4.6 mm internal diameter x 150 mm length and particle size 5 μm) from Agilent, Santa Clara, United States. A gradient solvent system of 0.1% trifluoroacetic acid (Sigma-Aldrich, St. Louis, United States) in water: A and 0.1% trifluoroacetic acid in acetonitrile: B (Sigma-Aldrich, St. Louis, United States) was used for the program: 0 to 20 min: 0-40% B, 20-30 min: 40 to 80% B, 30 to 35 min: 80 to 100% B, 35 to 45 min: 100 to 0% B at a flow rate of 1.0 mL min-1 at 30°C. The elution profile was observed at 220 nm and the individual fractions were freeze-dried and tested for antimicrobial activity at the concentration of 10 mg mL-1.
For the identification of lipopeptides, LC–ESI–MS/MS analysis was performed using a Phenomenex ECC 18 column of 4.6 mm internal diameter × 250 mm length × 5µm particle size) in TOF/Q-TOF Mass Spectrometer (G6550B, Agilent technologies, Santa Clara, United States). The capillary voltage was set as 4000 V with dry gas flow at 11 l min-1 and temperature of 290°C. Each peak was measured in positive-ion mode and the masses of the individual peaks were screened in the m/z range of 200-2000. The lipopeptides were identified by matching with the exact calculated monoisotopic masses, adducts and their mass fragmentation patterns, according to previously published literature.
In-vitro susceptibility test
Susceptibility of spoilage microorganisms isolated from cardboard to LPE was evaluated by agar well diffusion method as per Ayed et al. (2015)11, with some modifications. NA and PDA were used for the growth of bacterial and fungal strains, respectively. Bacterial and fungal strains were diluted to 106 CFU mL-1 and 5 × 104 spores mL-1, respectively using a haemocytometer. Each bacterial culture was added to molten nutrient agar cooled to 40˚C and poured into petri dishes and 100 µL of fungal spores were spread on the PDA plates. Wells (6 mm diameter) were prepared and 50 μL of the test sample was added to them. The plates were then incubated for 24 h at 37˚C for bacteria and for 48 h at 30˚C for fungal strains. The radius of the zones of inhibition was recorded in millimetres. Arbitrary activity was determined against Thermoascus crustaceus CRS-15 and E. coli ATCC 35218. It was defined as the reciprocal of the highest dilution that formed a clear zone of inhibition and expressed as activity units (AU) per mL12.
Determination of minimum inhibitory concentration (MIC) of lipopeptides
The MIC of lipopeptides was calculated by using microdilution technique in 96-well microtiter plates (Thermo fisher scientific, Waltham, United States). The turbidity of the tested bacterial and fungal suspensions was adjusted to approximately 106 CFU mL-1 by comparing with 0.5 McFarland standard. Muller-Hinton Broth (MHB) (Himedia, Mumbai, India) was used for bacterial strains or Sabouraud Dextrose Broth (SDB) was used for fungal strains for dilution of inocula to 103 CFU mL-1. Initially, 100 μL of SDB or MHB was added in all of the plate wells. 100 μL of test solution was added to the first well and serially diluted to the next well to obtain samples of test concentrations from 8.625 mg mL-1 to 0.00042 mg mL-1. 100 μL of each inoculum suspension was added to the wells except for sterility control. 200 μL of MHB or SDB medium was added to sterility control and 100 μL of MHB or SDB and 100 μL of inoculum suspension was added to microbial growth control. As positive controls, standard antibiotic such as Azithromycin (Sigma-Aldrich, St. Louis, United States) and antifungal Ketoconazole (Sigma-Aldrich, St. Louis, United States) were used from 32 µg mL-1 to 0.0156 µg mL-1. Incubation was carried out at 37˚C for bacteria and 25˚C for fungi, and the results were observed after 2 days. The lowest concentration of the test samples that inhibited bacterial and fungal growth was measured as the MIC13. The experiment was conducted in triplicate against tested strains.
Fractional inhibitory concentration (FIC) index of lipopeptides with nisin and pediocin
The checkerboard technique was used to determine the combined antimicrobial activity of the LPE with ‘Nisin’ produced from Lactococcus lactis (LPE+Nisin) and ‘Pediocin’ from Pediococcus pentosaceus (LPE+Pediocin) using by calculating the FIC index13. 100 μL of MHB was distributed to each of the plate wells. 50 μL of both test samples at their MIC concentrations and two concentrations higher and lower than MIC were added horizontally and vertically. 10 μL of each of the inoculum suspensions were adjusted to 103 CFU mL-1 were added to the wells. Controls were same as MIC test. Incubation was carried out at 37˚C for bacteria and 25˚C for fungi, and the results were recorded visually. The fractional inhibitory concentration index (FIC) was determined as:
FIC1 = (MIC1 combined / MIC1 alone), FIC2 = (MIC2 combined / MIC2 alone)
FIC = FIC1 + FIC2
The results were interpreted as: FIC ≤ 0.5: synergistic activity (S), 0.5 < FIC ≤ 1: additive activity (AD), 1 < FIC ≤ 4: no interaction (I) and FIC > 4: antagonistic activity was observed.
Stability study
To determine the thermal stability of LPE, it was exposed to temperatures ranging from 4–120˚C for 30 s to 30 min and cooled to room temperature. The pH of LPE was varied from 2 to 12 by using 0.5 M of HCl and 0.5 M of NaOH solution and incubating it at room temperature for 1 hr. The residual antimicrobial activity of treated LPE was tested against the indicator strains. Untreated LPE samples were taken as controls. The residual activity at each data point was recorded in triplicate.
Application of lipopeptides and nisin for cardboard preservation
Cardboard-based packaging material was constructed of three separate layers: an outside fibreboard liner, an inside fibreboard liner and a middle-corrugated medium (fluting) secured together by starch as binding agent. Cardboard samples of dimension 5 cm ×5 cm were cut from the cardboard box and placed into bottom portion of the sterile petri dishes. Starch based glue was heated to 80˚C and LPE+Nisin was dissolved in it to the final concentrations of 1% and 2% (w/w) to obtain a homogenous mixture. Starch based glue was allowed to cool to 40˚C and microbial load (106 CFU mL-1) of isolates obtained from spoiled cardboard was added to it. The viscous paste formed was spread-coated between the fibreboard liners and corrugated medium for the assembly of corrugated cardboard sheets. Positive control was prepared by addition of commercially used salicylic acid (1% w/w) in the starch-based glue and in negative control, only microbial load was added. Plates were incubated at 25˚C, 37˚C and 55˚C at 50±5% r.h. and examined after every 5 days till microbial growth appeared. The efficacy of LPE+Nisin to prevent microbial spoilage was evaluated by visual observation, estimation of total microbial count and inhibitory activity assays of the treated cardboard surfaces at 25˚C.
Estimation of total microbial count, adsorption and release rates
The total microbial count on the tested cardboard samples was estimated by counting the bacterial and fungal colonies by placing the 10 mm ×10 mm sample from the treated cardboards on the NA and PDA plates after every 5 days of incubation. The ability of the LPE+Nisin to disperse throughout the coating was assessed by determining their adsorption and release rate from the cardboard surface as per method followed by Mauriello et al., (2005)14. The treated cardboard samples were removed after spread-coating step, after 2, 4, 6, 8, 10, 20 and 30 min and placed on NA plates seeded with E. coli ATCC 35218 and PDA plates seeded with T. crustaceus CRS-15. Based on the inhibition zones around the indicator strains, the adsorption rate was measured. For release rate, 10 mm × 10 mm pieces of the treated cardboards were placed in sterile distilled water and removed at 30, 60, 90 and 120 min and tested for antimicrobial activity.
Factory trial
The efficiency of LPE+Nisin for the preservation of cardboard-based packaging material was evaluated at “Parksons packaging Ltd, Pune. Starch-based glue was heated to 80-100˚C and mixed with LPE+Nisin at the concentration of 1%, 0.6% and 0.4% (w/w) for 30 min. LPE+Nisin at each concentration was placed in a tray and spread-coated between inner fibreboard and corrugated sheet by passing through roller machine to prepare 100 boxes. In total, 300 cardboard boxes of 0.65 m2 area were prepared and the final concentration of LPE+Nisin was calculated to be 800 mg m-2, 527.27 mg m-2 and 335.24 mg m-2 in each trial. All the boxes were kept at the storage facility at room temperature (20-25˚C, 50±5% r.h.). The efficacy of LPE+Nisin coating was evaluated by examining individual boxes after every 5 days over a period of 180 days by visual observation, estimation of total microbial count and inhibitory activity assays.
Identification of cardboard spoilage microflora
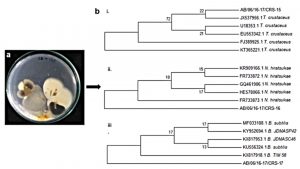
In this study, 16S rDNA genes from the isolated bacterial and fungal strains from the spoiled cardboard samples were amplified by PCR and compared against the GenBank database by BLAST analysis. Based on the phylogenetic analysis, isolate CRS-15, CRS-16 and CRS-17 were identified as Thermoascus crustaceus, Neosartorya hiratsukae and Bacillus subtilis, respectively (Fig. 1). Thermoascus crustaceus, is a thermophilic fungus which can cause peritonitis or pulmonary infections in immunocompromised patients and has high rates of morbidity and mortality15-17. Neosartorya hiratsukae, a close relative of Aspergillus fumigatus, is also a thermophilic fungus which is known to cause opportunistic infections such as cerebral aspergillosis, peritonitis and rhinosinusitis18-20. Bacillus spp. are capable of forming biofilms and thermoresistant spores and are most commonly found bacteria in the paper products3.
Fig. 1. Isolation of spoilage microorganism from cardboard using agar flooding method b. Phylogenetic tree of 16S rDNA gene sequences of i. T. crustaceus CRS-15 ii. N.hiratsukae CRS-16 iii. B. subtilis CRS-17
Inhibitory activity of lipopeptides against cardboard spoilage microorganisms
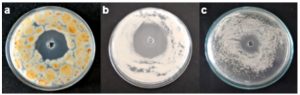
Inhibitory activity assays demonstrated that all the three strains isolated from cardboard were susceptible to LPE from Bacillus amyloliquefaciens MTCC 10456. Clear zones of inhibition of radii 7±0.56, 8±0.34 and 8±0.82 mm were observed against Thermoascus crustaceus CRS-15, Neosartorya hiratsukae CRS-16 and Bacillus subtilis CRS-17, respectively (Fig. 2). 0.1 M sodium phosphate buffer (pH 7.4) was tested as negative control, did not show any inhibitory activity.
Fig. 2. Antimicrobial activity of lipopeptides extract (LPE) for B. amyloliquefaciens MTCC 10456 by agar well diffusion asssay showing clear zone of inhibition against a T.crustaceus CRS-15 b. N.hiratsukae CRS-16 c. B.subtilis CRS-17
The worldwide need for packaged products such as food, medicines, chemicals and household appliances are increasing due to rapid urbanization. Paper-based cardboard boxes are one of the most widely used materials due to their flexibility, low weight, low cost, biodegradability, recyclability and environmental-friendly nature. Paper production or recycling takes place in an open unsterile environment and due to its natural composition, long-term storage conditions and transportation, there is a great risk of microbial spoilage, particularly from heat-resistant spores of bacteria and fungi, that causes decreased product quality, contaminated machinery and subsequent disease outbreaks2. Antibacterial agents such as organic acids, bacteriocins, enzymes, chitosan, plant extracts and antifungals such as sorbates, salicylic acid, glutaraldehyde and formaldehyde have been extensively tested in antimicrobial packaging systems. However, their usage is restricted due to narrow target specificity, low stability, environmental toxicity and development of resistance against pathogenic strains4,8,9. Thus, screening and identification of alternate antimicrobial agents, which can replace or be used in combination with existing compounds for preservation of paper-based packaging material against such thermotolerant spoilage microbes is necessary.
In the last few years, natural products such as bacteriocins and antimicrobial peptides produced by bacteria have gained attention for the bio-preservation of food products and packaging films. Members of marine Bacillus species represent the most promising strategy for addressing these issues as they are ubiquitously present in the environment and are considered as economically and ecologically valuable sources for the production of bioactive compounds as compared to any other sources21. Most of the studies regarding antimicrobial metabolites from Bacillus spp. have mentioned their application in food, agriculture, pharmaceutical or bioremediation, very few of them have done it in context of development of preservative coatings for paper-based packaging materials22. Thus, this work highlights the role of antagonistic metabolites from B. amyloliquefaciens MTCC 10456 as effective agents to inhibit thermotolerant bacteria and fungi, responsible for the spoilage of cardboard boxes and have inherent ability to cause human infections.
Identification of lipopeptides by mass spectrometry analysis
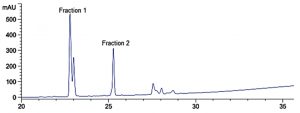
Lipopeptides produced from B. amyloliquefaciens MTCC 10456, isolated by salt-precipitation method were subjected to RP-HPLC for further separation, two fractions were collected at the retention times of 22.8 and 25.2 minutes and labelled as Fraction 1 and Fraction 2, respectively (Fig. 3). Fraction 1 exhibited inhibitory activity against T. crustaceus CRS_15 as evidenced by clear of zone of inhibition of radius 7 mm and fraction 2 exhibited inhibitory activity against E. coli ATCC 35218 as well as T. crustaceus CRS-15, resulting in zone of inhibition of radii 8 mm and 2 mm, respectively.
To determine the accurate molecular mass of the lipopeptides produced from B. amyloliquefaciens MTCC 10456, LC-ESI-MS/MS analysis was carried out (Table 1). The mass spectrum of fraction 1 revealed a major peak at molecular mass of 1031.5 [M+H]+ corresponding to molecular weight of C14 bacillomycin D (1030) and a peak at molecular mass of 1045.4 [M+H]+ corresponding to molecular weight of C15 bacillomycin D (1044). The peak at m/z 1031 fragmented in to masses of 1014. 51, 997.49 (loss of NH3 in each peak) and 979.45 (neutral loss of H2O). Ion peaks at 510 corresponded to loss of H2O from Thr -Ser -Glu -Pro -Asn chain, 673 for loss of H2O (Thr -Ser – Glu -Pro -Asn -Tyr -Asn) and 805 (Thr -Ser -Glu – Pro -Asn -Tyr -Asn -β-amino fatty acid) and fragment ions at m/z 617 (β-amino fatty acid-Asn -Tyr -Asn) and 948 (β-amino fatty acid-Asn -Tyr -Asn -Pro -Glu -Ser) were also observed, which confirmed the assignment of fraction 1 as C14 bacillomycin D consisting of -Asn -Tyr -Asn -Pro -Glu -Ser -Thr attached to β-amino fatty acid chain, which is a characteristic sequence for bacillomycin D family. Mass fragmentation of peak at m/z 1045 showed fragment ions at m/z of 1028.52, 1011.52 with a loss of NH3 in each peak (mass difference of 17) and 993.49 with a neutral loss of H2O molecule (mass difference of 18). Thus, both the peaks exhibited similar fragment ions which confirmed their homologous nature but consisting of β-amino fatty acid with C14 and C15 chains, respectively (Supplementary data, Fig. S1 and S2). These results are in agreement with previous reports which demonstrated that bacillomycin D could be produced by B. amyloliquefaciens strains and demonstrate strong antifungal activity23,24.
Table (1):
Lipopeptides produced by B. amyloliquefaciens MTCC 10456 and detected by LC-ESI-MS/MS analysis.
Fraction number |
Retention time (min) |
m/z |
Mass peak |
Molecular weight |
Possible assignment |
References |
|---|---|---|---|---|---|---|
1 |
22.8 |
1031.5, 1045.4 |
[M+H]+ |
1030, 1044 |
C14 Bacillomycin D, C15 Bacillomycin D |
23 |
2 |
`25.2 |
1030.63, 1044.65, 1058.6 |
[M+Na]+ |
1007, 1021, 1035 |
C13 Surfactin, C14 Surfactin, C15 Surfactin |
26 |
The mass spectra of fraction 2 showed intense mass peaks at masses 1030.63, 1044.65 and 1058.67 which were attributed to sodium adducts of C13 surfactin, C14 surfactin and C15 surfactin, when compared with published studies25. Protonated peaks [M+H] + were present at m/z values 1008.63, 1022.65 and 1036.67, which differed by a mass difference of 14, corresponding to -CH2 group. MS/MS fragmentation of the peak at m/z 1058.67 showed peaks at m/z of 1040, 1022 and 1007 with a neutral loss of H2O molecule. Ion peaks of m/z 391, 945, 832, 717 and 618 were observed which confirmed the assignment of fraction 2 as C15 surfactin consisting of Glu-leu/ile-leu-val-asp-leu-leu/ile peptide attached to β-amino fatty acid chain26 (Supplementary data, Fig. S3). Surfactin homologues were observed in fraction 2 responsible for conferring significant antibacterial and antifungal properties, which have been well documented in research reports27,28. LC-ESI-MS/MS has been proven to be an efficient technology with high sensitivity, accuracy and require low sample volumes which makes them ideal tools for rapid identification of antimicrobial peptides from diverse microorganisms. Using this technique, many members of Bacillus species have been screened for the production of antimicrobial peptides from their culture medium as well as from the cell pellet.
MIC determination and synergism study
MIC values of LPE against tested pathogens were determined by micro-broth dilution assay, as shown in Table 2 and 3. When LPE was combined with nisin (3:2 w/w), a significant reduction in MIC values was obtained against bacterial pathogens, as indicated by the FIC values. MIC values of LPE+Nisin were reduced by 69.5% against S. aureus ATCC 6538, 78.8% against E. coli ATCC 35218, 74.8% against S. enterica ATCC 12011, 90% against B. cereus ATCC 10876, 55% against L. monocytogenes ATCC 19115 and 50% against P. aeruginosa ATCC 9027. No interaction was observed between LPE with pediocin. Both, nisin and pediocin did not exhibit antifungal activity against any of the tested pathogens. Thus, synergism was not tested against fungal pathogens. Nisin is the most widely tested bacteriocin in active food packaging against bacterial pathogens and approved by United States Food and drug administration (USFDA). However, its narrow inhibition spectrum and inactivation at alkaline pH values limits its use for broad variety of packaging systems. Further research with higher concentrations or with combinations of antimicrobials has been suggested by recent reports9. In this study, combination of LPE with nisin asserted significant synergistic effect on the tested pathogens. Synergistic antibacterial effect is usually often observed when the two compounds act on different targets, thus increasing their efficacy to attack the host cells. Nisin specifically binds to lipid II in peptidoglycan layer and thus it is only efficient against Gram-positive bacteria, while Gram negative bacteria are protected by the outer membrane which is mainly composed of lipopolysaccharides that are negatively charged. However, when chelating agents e.g. EDTA are used to destabilize the outer membrane, nisin can efficiently inhibit Gram-negative bacteria31. Antibacterial mode of action of surfactin includes: binding of divalent cations in the cell membrane causing destabilization, formation of ion channels and insertion into bilipid layer to alter permeability32. Thus, a possible explanation of the synergistic effect between surfactin and nisin is that the surfactin affects the cell membrane integrity such that nisin can pass through outer membrane of Gram-negative bacteria. Presence of antifungal bacillomycin D did not affect the synergistic activity and thus usage of mixture of antimicrobials would not only increase the inhibition spectrum but also prevent overuse of one type of antimicrobial compounds, reduce the production cost as well as safety concerns associated with them.
Table (2):
Synergistic activity of lipopeptides extract (LPE) from B. amyloliquefaciens MTCC 10456 with nisin and pediocin against tested bacterial pathogens.
| Bacteria | ATCC No. | LPs | Nisin | Pediocin | LPE+Nisin | LPE+Pediocin | ||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL-1) | FIC2 | Activity | FIC | Activity | ||||
| S. aureus | 6538 | 4.31 | 4.25 | 10 | 0.51 | S | 3.32 | I |
| E. coli | 35218 | 4.31 | 25 | 50 | 0.35 | S | 2 | I |
| S. enterica | 12011 | 4.31 | 25 | 50 | 0.42 | S | 2 | I |
| B. cereus | 10876 | 1.69 | 9.3 | 25 | 0.17 | S | 2 | I |
| P. aeruginosa | 9027 | 8.62 | 25 | 50 | 0.69 | AD | 3.32 | I |
| L. monocytogenes | 19115 | 4.31 | 10.65 | 10 | 0.75 | AD | 3 | I |
2The fractional inhibitory concentration (FIC) index was calculated by the following formula:
FIC1 = (MIC1 combined / MIC1 alone), FIC2 = (MIC2 combined / MIC2 alone)
FIC = FIC1 + FIC2
The results were interpreted as: FIC ≤ 0.5: synergistic activity (S), 0.5 < FIC ≤ 1: additive activity (AD), 1 < FIC ≤ 4: no interaction (I) and FIC > 4: antagonistic activity was observed.
Table (3):
MIC values of lipopeptides extract (LPE) from B. amyloliquefaciens MTCC 10456 against fungal pathogens.
Fungi |
NCIM No. |
MIC of LPE (mg/mL-1) |
|---|---|---|
T. crustaceus |
CRS-15 |
0.08 |
N. hiratsukae |
CRS-16 |
0.08 |
A. fumigatus |
519 |
0.08 |
R. oryzae |
878 |
0.04 |
P. funiculosum |
S8 |
0.70 |
C. albicans |
3100 |
5.62 |
Stability profile
When LPE was subjected to pH values from 2 to 10, the highest antimicrobial activity was observed at pH 6 and 7. Activity was decreased to 80% and 90% at pH 2 and 10, respectively. The antimicrobial activity of LPE was retained in temperature range of 20˚C to 80˚C. However, activity was decreased to 60% and 40%, when exposed to 100˚C and 120˚C for 30 seconds, respectively. However, antimicrobial activity of LPE was lost after incubating at 121°C for 30 min (Supplementary data, Fig. S4). 100% of antimicrobial activity was retained when LPE was stored at 4˚C and -20˚C for 700 days. Thus, these results indicate long-term stability of LPE which makes them suitable for commercial use. These results are in correlation with those reported by Chen et al., (2017)29, and Zhang et al., (2018)33 which indicate towards the stable nature of antimicrobial peptides produced from Bacillus spp which is required for use in packaged products as they are exposed to different temperature and pH profiles during handling, storage and distribution.
Effect of combination of lipopeptides and nisin on the cardboard spoilage microflora
Visual observation and antimicrobial activity of treated cardboard surfaces
Cardboard samples treated with LPE+Nisin were visually observed after every 5 days to check for microbial contamination. In untreated cardboard samples, microbial growth started appearing after 14 days of incubation at 25˚C and 37˚C while in plates incubated at 55˚C, microbial growth was observed after 30 days of incubation (Fig. 4). Thus, treated cardboard samples incubated at 25˚C were used for subsequent experiments to simulate warehouse conditions. When the treated cardboard samples were tested for antimicrobial activity, clear zone of inhibition was observed around the cardboard surface. It indicated that the LPE+Nisin was uniformly distributed along the surface of the cardboard and retained its activity as observed by Mauriello et al., (2005)14. Interestingly, the treated cardboards coated with two different concentrations of LPE+Nisin (1% and 2% w/w) resisted the growth of microorganisms till 180 days of incubation period. These results are in correlation with study conducted by Lee et al., (2004)34 in which paperboard was coated with nisin for preservation of perishable foods.
Fig. 4. Application of LPE+Nisin for cardboard preservation a. Corrugated cardboard with ‘no inhibition’ of microbial growth. Cardboard coated with b. 1% (w/w) salicylic acid c. 15 (w/w) LPE+Nisin at 25°C d. 2 % (w/w) LPE+Nisin at 25 °C e. 1 % (w/w) LPE+Nisin incubated at 37 °C f. 2 % (w/w) LPE+Nisin incubated at 37 °C g. 1 % (w/w) LPE+Nisin incubated at 55 °C h. 2 % (w/w) LPE+Nisin incubated at 55°C observed after 180 days of incubation
Reduction in total microbial count
An initial microbial load of bacterial and fungal strains isolated from the spoiled cardboard samples (106 CFU gm-1) was added for monitoring accelerated spoilage of cardboard boxes. Reduction in 1 log cycle was observed after 5 days (5 × 105 CFU gm-1), 2 log cycles was observed after 10 days and a decrease in the order of approximately 4 log cycles of microbial population was observed (2 × 102 CFU gm-1) after 20 days as compared to the untreated cardboard samples in which 109 CFU gm-1 of total microbial population was observed (Fig. 5a). The reduction in the microbial growth can be attributed to the lipopeptides and nisin incorporated in the cardboard samples. These findings reveal that LPE+Nisin not only acts as a preservative coating but also contributes in reduction of the existing microbial load in the cardboard samples. These studies are in correlation with other reports of Nithya et al., (2013)22 and Mauriello et al., (2005)14 for preservation studies using other natural metabolites.
Adsorption and release rates
When binding of LPE+Nisin to the cardboard surface was assessed, the treated cardboard samples showed a steady increase in the antimicrobial activity till 6500 AU mL-1 against E. coli ATCC 35218 and 8000 AU mL-1 against T. crustaceus CRS-15 up to 10 min after which it remained constant (Fig. 5b). This observation indicated that LPE+Nisin gets adsorbed or absorbed to the cardboard surface in a short period of time and diffuses throughout the surface to form a coating as reported with other bacteriocins14. When the release of LPE+Nisin from the cardboard surface was determined, the water samples from 0 to 30 min showed steady increase in antimicrobial activity after which it remained constant at 2000 AU mL-1 against E. coli ATCC 35218 and 3000 AU mL-1 against T. crustaceus CRS-15, till 120 min. When the cardboard samples were tested from 30 to 120 min, they retained constant antimicrobial activity as evidenced by clear zone of inhibition around the indicator strains, implying that the LPE+Nisin was retained in the cardboard surface. These results showed that LPE+Nisin released only up to 30 min of incubation which indicated the suitability of treated cardboards for packaging purposes for an extended period of time (Fig. 5c). The interactions of the antimicrobial agents with the paperboard surface is necessary for the antimicrobial activity of the packaging surfaces. The rapid adsorption and the close alignment of lipopeptides on the cardboard surface may be contributed to its specific amphiphilic structure35. The hydrophilic composition of corrugated cardboard makes it likely to adsorb higher amounts of nisin and lipopeptides and greater retention of antimicrobial activity for long-term storage36.
Fig. 5. Evaluation of LPE+Nisin for cardboard preservation a. Reduction in microbial count of treated cardboard b. Binding of LPE+Nisin to cardboard surfact at different contact times c. Release of LPE+Nisin from the cardboard surface at different times
Factory trial
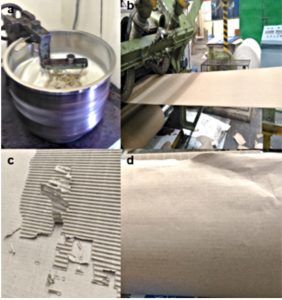
Cardboard samples of size 5 inches × 5 inches were cut from the manufactured boxes and were monitored in the laboratory for microbial spoilage. It was observed that the cardboard boxes treated with concentrations of 1% and 0.6% (w/w) resisted microbial spoilage and retained antimicrobial activity even after 180 days of incubation at 20-25˚C (Fig. 6). Cardboard boxes treated with 0.4% (w/w) of LPE+Nisin were susceptible to bacterial and fungal growth after 17 weeks which can be attributed to lower concentration of inhibitory peptides necessary to inhibit the microbial growth (Supplementary data, Fig S5). Thus, 0.6% (w/w) of LPE+Nisin can be used as an acceptable strategy for suppression of microbial growth and preservation of cardboard boxes during prolonged storage.
Fig. 6. Factory trial of LPE+Nisin mixture (0.06% w/w) on the cardboard boxes. A. Mixing of starch based glue with LPE+Nisin B. Spread coating of LPE+Nisin to corrugated cardboard on a roller tray C. Observation of spoilage free cardboard after 90 days D. Observation of spoilage free cardboad after 180 days
Seaweed-associated B. amyloliquefaciens MTCC 10456 demonstrated promising inhibitory activity against thermotolerant T. crustaceus, N. hiratsukae and B. subtilis, isolated from spoiled corrugated cardboard boxes made up of recycled paper. It could be attributed to the production of lipopeptides such as bacillomycin D and surfactin, which were isolated and identified by RP-HPLC and LC-ESI-MS/MS analysis, respectively. These lipopeptides in synergism with nisin, were successfully demonstrated as a preservative coating on corrugated cardboard boxes for protection against thermotolerant and spoilage microorganisms up to 180 days. This strategy can be implemented without any changes in the existing process flow since this formulation can be mixed with starch-based additive during the manufacturing of cardboard packaging boxes. This study provides an effective and scalable solution for preservation of paper-based packaging materials from microbial damage during storage. Further research can be carried out for physico-chemical characterization of cardboard surfaces after application of these metabolites.
Additional file: Additional Figures. S1 to S5. Additional Table S1.
ACKNOWLEDGMENTS
We would like to thank ‘Central instrumentation facility’, Savitribai Phule Pune University (SSPU) Maharashtra, India for mass spectrometric analysis of samples. We also thank the Analytical Sciences Team at Praj Matrix R & D center for RP-HPLC analysis of lipopeptides.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
YM: Conceptualization; Funding acquisition; Project administration; Resources; Writing – review & editing; Supervision. UV, RA, ZA: Data curation; Investigation; Methodology. UV: Formal analysis; Validation; Visualization; Roles/Writing – original draft.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analysed during this study are included in the manuscript and the supplementary files.
- Paper Packaging Market -Growth, Trends, and Forecast (2020-2025).html.
- Hladikova Z, Kejlova K, Sosnovcova J, et al. Microbial contamination of paper-based food contact materials with different contents of recycled fiber. Czech J Food Sci. 2015;33(4):308-312.
Crossref - Flemming H-C, Meier M, Schild T. Mini-review: microbial problems in paper production. Biofouling. 2013;29(6):683-696.
Crossref - Irkin R, Esmer OK. Novel food packaging systems with natural antimicrobial agents. J Food Sci Technol. 2015;52(10):6095-6111.
Crossref - Harwood CR, Mouillon J-M, Pohl S, Arnau J. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev. 2018;42(6):721-738.
Crossref - Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front Microbiol. 2019;10:302.
Crossref - Meena KR, Kanwar SS. Lipopeptides as the Antifungal and Antibacterial Agents: Applications in Food Safety and Therapeutics. BioMed Res Int. 2015;2015:1-9.
Crossref - Khwaldia K, Arab-Tehrany E, Desobry S. Biopolymer Coatings on Paper Packaging Materials. Compr Rev Food Sci F. 2010;9(1):82-91.
Crossref - Malhotra B, Keshwani A, Kharkwal H. Antimicrobial food packaging: potential and pitfalls. Front Microbiol. 2015;6.
Crossref - Guzinska K, Owczarek M, Dymel M. Investigation in the microbiological purity of paper and board packaging intended for contact with food. Fibres Text East Eur. 2012;6B(96):186-190.
- Ayed HB, Maalej H, Hmidet N, Nasri M. Isolation and biochemical characterisation of a bacteriocin-like substance produced by Bacillus amyloliquefaciens An6. J Glob Antimicrob Resist. 2015;3(4):255-261.
Crossref - Motta AS, Brandelli A. Characterization of an antibacterial peptide produced by Brevibacterium linens. J Appl Microbiol. 2002;92(1):63-70.
Crossref - de Castro RD, de Souza TMPA, Bezerra LMD, Ferreira GLS, de Brito Costa EMM, Cavalcanti AL. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: an in vitro study. BMC Complement Altern Med. 2015;15(1):417.
Crossref - Mauriello G, De Luca E, La Storia A, Villani F, Ercolini D. Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett Appl Microbiol. 2005;41(6):464-469.
Crossref - Alvarez E, Castillo A, Iturrieta I. Fungal peritonitis by Thermoascus crustaceus in a peritoneal dialysis patient from Chile. Rev Iberoam Micol. 2017;34(4):225-228.
https://doi.org/10.1016/j.riam.2017.01.004 - Mares M, Moroti-Constantinescu V-R, Voroneanu L, Doroftei F, Covic A, Mederle O-A. Invasive pulmonary infection due to Thermoascus crustaceus in a kidney transplant recipient. Infect Drug Resist. 2019;12:1929-1934.
Crossref - Oz Y, Kiraz N, Ozkurt S, Soydan M. Colonization of peritoneal catheter with a thermophilic fungus, Thermoascus crustaceus : a case report. Med Mycol. 2010;48(8):1105-1107.
Crossref - Guarro J, Kallas EG, Godoy P, et al. Cerebral Aspergillosis Caused by Neosartorya hiratsukae , Brazil. Emerg Infect Dis. 2002;8(9):989-991.
Crossref - Koutroutsos K, Arabatzis M, Bougatsos G, Xanthaki A, Toutouza M, Velegraki A. Neosartorya hiratsukae peritonitis through continuous ambulatory peritoneal dialysis. J Med Microbiol. 2010;59(7):862-865.
Crossref - Shivaprakash MR, Jain N, Gupta S, Baghela A, Gupta A, Chakrabarti A. Allergic fungal rhinosinusitis caused by Neosartorya hiratsukae from India. Med Mycol. 2009;47(3):317-320.
Crossref - Singh RP, Reddy CRK. Seaweed-microbial interactions: key functions of seaweed-associated bacteria. FEMS Microbiol Ecol. 2014;88(2):213-230.
Crossref - Nithya V, Murthy PSK, Halami PM. Development and application of active films for food packaging using antibacterial peptide of Bacillus licheniformis Me1. J Appl Microbiol. 2013;115(2):475-483.
Crossref - Ma Y, Kong Q, Qin C, et al. Identification of lipopeptides in Bacillus megaterium by two-step ultrafiltration and LC-ESI-MS/MS. AMB Expr. 2016;6(1):79.
Crossref - Gu Q, Yang Y, Yuan Q, et al. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Elliot MA, ed. Appl Environ Microbiol. 2017;83(19):e01075-17.
Crossref - Yun J-H, Cho D-H, Lee B, Kim H-S, Chang YK. Application of biosurfactant from Bacillus subtilis C9 for controlling cladoceran grazers in algal cultivation systems. Sci Rep. 2018;8(1):5365.
Crossref - Wang Y, Zhang C, Wu L, et al. Inhibitory Effect of Bacillus Subtilis WL-2 and Its IturinA Lipopeptides against Phytophthora Infestans. Microbiol. 2019.
Crossref - Hazarika DJ, Goswami G, Gautom T, et al. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019;19(1):71.
Crossref - Ndlovu T, Rautenbach M, Vosloo JA, Khan S, Khan W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Expr. 2017;7(1):108.
Crossref - Chen Y, Liu SA, Mou H, Ma Y, Li M, Hu X. Characterization of Lipopeptide Biosurfactants Produced by Bacillus licheniformis MB01 from Marine Sediments. Front Microbiol. 2017;8:871.
Crossref - Nastro RA, Arguelles-Arias A, Ongena M, et al. Antimicrobial Activity of Bacillus amyloliquefaciens ANT1 Toward Pathogenic Bacteria and Mold: Effects on Biofilm Formation. Probiotics & Antimicro Prot. 2013;5(4):252-258.
Crossref - Boziaris IS, Adams MR. Effect of chelators and nisin produced in situ on inhibition and inactivation of Gram negatives. Int J Food Microbiol. 1999;53(2-3):105-113.
Crossref - Carrillo C, Teruel JA, Aranda FJ, Ortiz A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta Biomembr. 2003;1611(1-2):91-97.
Crossref - Zhang QX, Zhang Y, He LL, Ji ZL, Tong YH. Identification of a small antimycotic peptide produced by Bacillus amyloliquefaciens 6256. Pestic Biochem Physiol. 2018;150:78-82.
Crossref - Lee CH, An DS, Lee SC, Park HJ, Leea DS. A coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and α-tocopherol. J Food Eng. 2004;62(4):323-329.
Crossref - Liu J-F, Mbadinga S, Yang S-Z, Gu J-D, Mu B-Z. Chemical Structure, Property and Potential Applications of Biosurfactants Produced by Bacillus subtilis in Petroleum Recovery and Spill Mitigation. Int J Mol Sci. 2015;16(3):4814-4837.
Crossref - Taboada-Rodriguez A, Garcia-Garcia I, Cava-Roda R, Lopez-Gomez A, Marin-Iniesta F. Hydrophobic properties of cardboard coated with polylactic acid and ethylene scavengers. J Coat Technol Res. 2013;10(5):749-755.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.