ISSN: 0973-7510
E-ISSN: 2581-690X
Multidrug-resistance in Staphylococcus aureus is a global health concern. Alternative strategies to battle antibiotic resistance with novel antimicrobials is of prime importance in the current times. Plant bioactive compounds in combination with antibiotics have proven effective for modulating the antibiotic resistance of various drug-resistant bacteria. Artemisia vulgaris L. is a common herbaceous plant used in traditional medicine. This study evaluated the synergistic activity of methanol extract of the leaves of Artemisia vulgaris L. with selected antibiotics directed against clinical isolates of multidrug-resistant Staphylococcus aureus. The plant extract concentration of 500 mg/ml exhibited the largest zone of inhibition of 23.33 ± 0.57 mm against the isolate SA 05. The minimum inhibitory concentration of the plant extract determined was 3.90 mg/ml and the minimum bactericidal concentration was 7.81 mg/ml against isolates SA 08 and SA 10, respectively. The MBC/MIC value of ≤4 exhibited a bactericidal effect of the extract against most of the tested clinical isolates. The methanol extract of A. vulgaris showed synergistic activity with oxacillin and clindamycin against all the clinical isolates of S. aureus. Synergistic activity was also exhibited with penicillin, gentamicin, and ciprofloxacin against most of the clinical isolates. Thirty phytocompounds were detected in the extract of A. vulgaris by Gas chromatography-Mass spectrometry analysis. Results have revealed potential antibiotic resistance modulatory property of A. vulgaris against multidrug-resistant S. aureus through synergistic action with antibiotics.
Artemisia vulgaris, Multidrug-resistant Staphylococcus aureus, GC-MS, Synergy, Antibiotics
Staphylococcus aureus is an important clinical pathogen in both the hospital and community settings. The infections caused by the bacterium have revealed an escalated disease and death rates.1 The “World Health Organisation Bacterial Priority Pathogen List 2024” has listed methicillin-resistant Staphylococcus aureus in the “high-priority pathogen category” for the research and development to control antibiotic resistance.2 Antibiotic resistance has become a major bottleneck for effective treatment of bacterial infections.3 The development of cost-effective and safer alternative antimicrobial drugs becomes an important requirement for treatment of infections.4 Plants have been utilised in traditional medicine since ancient civilizations.5 A large number of published research indicates that phytochemicals and antibiotics work synergistically, suggesting that this combination may be useful in treating drug-resistant illnesses.6,7 Artemisia vulgaris L. is a common herbaceous plant belonging to the Asteraceae family. It is an annual herb and a component used in traditional medicine. The plant is commonly termed as mugwort and is found in different parts of Europe, Asia, and North America.8 Infusions of plant stems and leaves have been used in traditional medicine to treat tumours, menopausal and menstrual symptoms, epilepsy, diabetes, dermatitis, bacterial infections, and insomnia.8 The study examined the antibacterial potential of A. vulgaris leaves methanol extract and its synergistic effect in conjunction with specific antibiotics directed against multidrug-resistant Staphylococcus aureus.
The research was conducted in the Department of Microbiology, School of Life Sciences, Sikkim University, India. The chemicals and consumables used in the study were procured from Merck, Germany, and HiMedia Laboratories Private Limited, India.
Collection and Identification of plant
The leaves of Artemisia vulgaris L. were collected from Geyzing, India (27°17’206’’ N, 088°14’039’’ E). The herbarium of the test plant was prepared and the plant was identified by the plant taxonomist at the Department of Botany, University of North Bengal, India. One copy of the herbarium with Accession No. 12633 was deposited at the herbarium of the Plant Taxonomy Division, University of North Bengal, India.
Plant extract preparation
The test plant leaves were collected, properly cleaned, and then left to dry in the shade for 20 days. Using a grinder, the dried leaves were chopped into small fragments and ground into a fine powder. The powder was again sieved to separate the bigger husks and stalks to get the fine powder (Figure 1). Methanol extract was prepared by Soxhlet extraction using 10 g of powdered material (1:10). Whatman No. 1 filter paper was used to filter the methanol extract, and a rotary vacuum evaporator was used to concentrate the mixture. The extract was dried and kept in vials at 4 °C.9
Test bacteria
A total of twenty MDR Staphylococcus aureus were used for the present study. These isolates have been identified in our earlier published study10 with respect to their multidrug-resistance antibiogram profile.
Determination of antibacterial activity
Using the agar-well diffusion method, the antibacterial activity of the extract of the leaves of A. vulgaris L. was determined.11 The surface of the MHA plates was inoculated with an inoculum of the test bacteria at OD 0.1 at 600 nm, corresponding to the 0.5 McFarland Standard. Plant extract (500 mg/mL) was pipetted into the punctured 8 mm wells, followed by the addition of vancomycin (30 mg/mL) and DMSO (10%) as the positive and negative controls, respectively. The incubation of the plates was done at 37 °C for 24 hours. By measuring the zone of inhibition, including the well diameter, the antibacterial activity of the tested plant extract was determined. Each experiment was performed in three sets.
Determination of MIC and MBC
The broth microdilution technique was used to determine the minimum inhibitory concentration (MIC) using 96-well plates at various concentrations of the plant extract.12 Two fold serial dilutions were made from column 1-10 to prepare the various concentrations (250 mg/ml to 0.48 mg/ml) of the plant extract in the 96 well microtiter plates. By immediately plating the contents of the 96 wells at concentrations greater than the MIC value in the MHA plates, the minimum bactericidal concentration (MBC) was ascertained. After incubating at 37 °C for 24 hours, the concentration at which no colony growth occurred was determined as the MBC value. The experiment was done in three sets.
Determination of synergistic activity
Synergistic activity of the test plant extract in conjunction with selected antibiotics was determined by the checkerboard technique13 with minor modifications. Prior to performance of the checkerboard assay, the MICs of four different antibiotics from selected classes were determined in triplicate. The antibiotics used were penicillin, oxacillin, gentamicin, ciprofloxacin, and clindamycin. After a 24-hour incubation period at 37 °C, 30 µl of 0.015% resazurin was added to each well and further incubated for an additional 2-4 hours to observe for any colour changes. The synergistic activity was determined by calculating the FIC index (FICI) of each combination.14
GC-MS analysis
Chemical characterization of the test plant extract was done using Gas Chromatography-Mass Spectrometry (GC-MS) instrument (QP 2010 Ultra SHIMADZU), at the Advanced Instrumentation Research Facility, Jawaharlal Nehru University, New Delhi, India.10
Determination of antibacterial activity
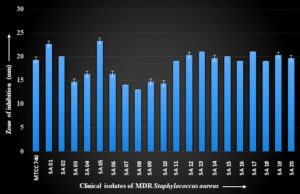
The clinical isolates of MDR S. aureus showed a zone of inhibition ranging in diameter from 13.00 ± 0.00 mm to 23.33 ± 0.58 mm with the methanol extract of A. vulgaris L. leaves. The isolate SA 05 showed the largest zone of inhibition. The results are shown in Figure 2.
Figure 2. Zone of inhibition with methanol extract of A. vulgaris L. leaves against clinical isolates of MDR S. aureus
Determination of MIC and MBC
The isolates SA 08 and SA 10 exhibited the lowest MIC and MBC values of 3.90 mg/ml and 7.81 mg/ml, respectively. The results are presented in Table 1. When the MBC/MIC ratio is ≤4, the drug is regarded as bactericidal.15 The test plant extract exhibited bactericidal activity against most of the clinical isolates of S. aureus.
Table (1):
MIC and MBC of methanol extract of Artemisia vulgaris L. leaves against clinical isolates of MDR S. aureus
No. |
Isolate |
MIC (mg/ml) |
MBC (mg/ml) |
MBC/MIC |
|---|---|---|---|---|
1 |
SA 01 |
15.62 |
125 |
8 |
2 |
SA 02 |
31.25 |
62.5 |
2 |
3 |
SA 03 |
15.62 |
31.25 |
2 |
4 |
SA 04 |
15.62 |
31.25 |
2 |
5 |
SA 05 |
7.81 |
15.62 |
2 |
6 |
SA 06 |
7.81 |
15.62 |
2 |
7 |
SA 07 |
7.81 |
31.25 |
4 |
8 |
SA 08 |
3.90 |
7.81 |
2 |
9 |
SA 09 |
7.81 |
15.62 |
2 |
10 |
SA 10 |
3.90 |
7.81 |
2 |
11 |
SA 11 |
7.81 |
31.25 |
4 |
12 |
SA 12 |
7.81 |
31.25 |
4 |
13 |
SA 13 |
7.81 |
31.25 |
4 |
14 |
SA 14 |
7.81 |
31.25 |
4 |
15 |
SA 15 |
7.81 |
31.25 |
4 |
16 |
SA 16 |
7.81 |
31.25 |
4 |
17 |
SA 17 |
7.81 |
31.25 |
4 |
18 |
SA 18 |
7.81 |
31.25 |
4 |
19 |
SA 19 |
7.81 |
31.25 |
4 |
20 |
SA 20 |
7.81 |
31.25 |
4 |
21 |
MTCC 740 S. aureus |
1.95 |
3.90 |
2 |
Determination of synergistic activity
When combined with clindamycin and oxacillin, the methanol extract exhibited synergistic effect against all clinical isolates of multidrug-resistant S. aureus with FICI <0.5. The results are shown in Table 2 and Table 3. The extract showed a synergistic effect with ciprofloxacin against all isolates except SA 14 and SA 15, which exhibited an additive effect (FICI = 1) as shown in Table 4. With the exception of SA 05, SA 14, and SA 15, which had an additive effect in conjunction with the antibiotic, the plant extract exhibited synergistic effects with penicillin against the majority of isolates (Table 5). Similarly, gentamicin showed a synergistic effect against most of the isolates except SA 05, SA 10, SA 12, SA 16, and SA 17 which exhibited additive effect (Table 6). These findings suggested that the combination of methanol extract of A. vulgaris leaves and antibiotics synergistically inhibited growth of most of the clinical isolates of MDR S. aureus.
Table (2):
Determination of synergistic activity of methanol extract of A. vulgaris L. leaves with clindamycin against clinical isolates of MDR S. aureus
| No. | Clinical MDR S. aureus | Minimum Inhibitory Concen. (MIC) | Fractional Inhibitory Concen. Index (FICI) | Result | ||
|---|---|---|---|---|---|---|
| Clindamycin (µg/mL) | A. vulgaris (mg/ml) | Clindamycin + A. vulgaris | ||||
| 1 | SA 01 | 0.48 | 15.62 | 0.03 | 0.12 | Synergistic |
| 2 | SA 02 | 0.48 | 31.25 | 0.03 | 0.12 | Synergistic |
| 3 | SA 03 | 0.48 | 15.62 | 0.03 | 0.12 | Synergistic |
| 4 | SA 04 | 0.48 | 15.62 | 0.03 | 0.12 | Synergistic |
| 5 | SA 05 | 0.48 | 7.81 | 0.03 | 0.12 | Synergistic |
| 6 | SA 06 | 0.48 | 7.81 | 0.06 | 0.24 | Synergistic |
| 7 | SA 07 | 0.48 | 7.81 | 0.03 | 0.12 | Synergistic |
| 8 | SA 08 | 0.48 | 3.9 | 0.03 | 0.12 | Synergistic |
| 9 | SA 09 | 0.48 | 7.81 | 0.03 | 0.12 | Synergistic |
| 10 | SA 10 | 0.48 | 3.9 | 0.06 | 0.24 | Synergistic |
| 11 | SA 11 | 0.12 | 7.81 | 0.01 | 0.2 | Synergistic |
| 12 | SA 12 | 0.12 | 7.81 | 0.03 | 0.5 | Synergistic |
| 13 | SA 13 | 0.12 | 7.81 | 0.01 | 0.2 | Synergistic |
| 14 | SA 14 | 0.12 | 7.81 | 0.03 | 0.5 | Synergistic |
| 15 | SA 15 | 0.12 | 7.81 | 0.01 | 0.2 | Synergistic |
| 16 | SA 16 | 0.12 | 7.81 | 0.03 | 0.5 | Synergistic |
| 17 | SA 17 | 0.12 | 7.81 | 0.03 | 0.5 | Synergistic |
| 18 | SA 18 | 0.12 | 7.81 | 0.01 | 0.2 | Synergistic |
| 19 | SA 19 | 250 | 7.81 | 31.25 | 0.24 | Synergistic |
| 20 | SA 20 | 0.12 | 7.81 | 0.03 | 0.5 | Synergistic |
| 21 | MTCC 740 | 0.12 | 1.95 | 0.03 | 0.5 | Synergistic |
Table (3):
Determination of synergistic activity of methanol extract of A. vulgaris L. leaves with oxacillin against clinical isolates of MDR S. aureus
| No. | Clinical MDR S. aureus | Minimum Inhibitory Concen. (MIC) | Fractional Inhibitory Concen. Index (FICI) | Result | ||
|---|---|---|---|---|---|---|
| Oxacillin (µg/mL) | A. vulgaris (mg/ml) | Oxacillin + A. vulgaris | ||||
| 1 | SA 01 | 31.25 | 15.62 | 7.8 | 0.5 | Synergistic |
| 2 | SA 02 | 31.25 | 31.25 | 3.9 | 0.24 | Synergistic |
| 3 | SA 03 | 31.25 | 15.62 | 1.95 | 0.12 | Synergistic |
| 4 | SA 04 | 31.25 | 15.62 | 1.95 | 0.12 | Synergistic |
| 5 | SA 05 | 31.25 | 7.81 | 1.95 | 0.12 | Synergistic |
| 6 | SA 06 | 31.25 | 7.81 | 1.95 | 0.12 | Synergistic |
| 7 | SA 07 | 31.25 | 7.81 | 1.95 | 0.12 | Synergistic |
| 8 | SA 08 | 31.25 | 3.9 | 1.95 | 0.12 | Synergistic |
| 9 | SA 09 | 15.62 | 7.81 | 0.97 | 0.12 | Synergistic |
| 10 | SA 10 | 31.25 | 3.9 | 3.9 | 0.24 | Synergistic |
| 11 | SA 11 | 1000 | 7.81 | 250 | 0.5 | Synergistic |
| 12 | SA 12 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 13 | SA 13 | 31.25 | 7.81 | 3.9 | 0.24 | Synergistic |
| 14 | SA 14 | 31.25 | 7.81 | 3.9 | 0.24 | Synergistic |
| 15 | SA 15 | 15.62 | 7.81 | 0.97 | 0.12 | Synergistic |
| 16 | SA 16 | 62.5 | 7.81 | 7.81 | 0.24 | Synergistic |
| 17 | SA 17 | 62.5 | 7.81 | 15.62 | 0.5 | Synergistic |
| 18 | SA 18 | 15.62 | 7.81 | 0.97 | 0.12 | Synergistic |
| 19 | SA 19 | 15.62 | 7.81 | 3.9 | 0.5 | Synergistic |
| 20 | SA 20 | 62.5 | 7.81 | 7.81 | 0.24 | Synergistic |
| 21 | MTCC 740 | 3.9 | 1.95 | 0.48 | 0.24 | Synergistic |
Table (4):
Determination of synergistic activity of methanol extract of A. vulgaris L. leaves with ciprofloxacin against clinical isolates of MDR S. aureus
| No. | Clinical MDR S. aureus | Minimum Inhibitory Concen. | Fractional Inhibitory Concen. Index (FICI) | Result | ||
|---|---|---|---|---|---|---|
| Ciprofloxacin (µg/mL) | A. vulgaris (mg/ml) | Ciprofloxacin + A. vulgaris | ||||
| 1 | SA 01 | 15.62 | 15.62 | 3.9 | 0.5 | Synergistic |
| 2 | SA 02 | 3.9 | 31.25 | 0.48 | 0.24 | Synergistic |
| 3 | SA 03 | 15.62 | 15.62 | 3.9 | 0.5 | Synergistic |
| 4 | SA 04 | 15.62 | 15.62 | 3.9 | 0.5 | Synergistic |
| 5 | SA 05 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 6 | SA 06 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 7 | SA 07 | 31.25 | 7.81 | 3.9 | 0.24 | Synergistic |
| 8 | SA 08 | 15.62 | 3.9 | 3.9 | 0.24 | Synergistic |
| 9 | SA 09 | 31.25 | 7.81 | 3.9 | 0.24 | Synergistic |
| 10 | SA 10 | 31.25 | 3.9 | 3.9 | 0.24 | Synergistic |
| 11 | SA 11 | 250 | 7.81 | 31.25 | 0.24 | Synergistic |
| 12 | SA 12 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 13 | SA 13 | 125 | 7.81 | 15.62 | 0.24 | Synergistic |
| 14 | SA 14 | 125 | 7.81 | 62.5 | 1 | Additive |
| 15 | SA 15 | 62.5 | 7.81 | 31.25 | 1 | Additive |
| 16 | SA 16 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 17 | SA 17 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 18 | SA 18 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 19 | SA 19 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 20 | SA 20 | 62.5 | 7.81 | 15.62 | 0.5 | Synergistic |
| 21 | MTCC 740 | 0.48 | 1.95 | 0.03 | 0.12 | Synergistic |
Table (5):
Determination of synergistic activity of methanol extract of A. vulgaris L. leaves with penicillin against clinical isolates of MDR S. aureus
| No. | Clinical MDR S. aureus | Minimum Inhibitory Concen. (MIC) | Fractional Inhibitory Concen. Index (FICI) | Result | ||
|---|---|---|---|---|---|---|
| Penicillin (µg/mL) | A. vulgaris (mg/ml) | Penicillin + A. vulgaris | ||||
| 1 | SA 01 | 250 | 15.62 | 31.25 | 0.24 | Synergistic |
| 2 | SA 02 | 15.62 | 31.25 | 1.95 | 0.24 | Synergistic |
| 3 | SA 03 | 125 | 15.62 | 15.62 | 0.24 | Synergistic |
| 4 | SA 04 | 62.5 | 15.62 | 7.81 | 0.24 | Synergistic |
| 5 | SA 05 | 31.25 | 7.81 | 15.62 | 1 | Additive |
| 6 | SA 06 | 250 | 7.81 | 62.5 | 0.5 | Synergistic |
| 7 | SA 07 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 8 | SA 08 | 125 | 3.9 | 31.25 | 0.5 | Synergistic |
| 9 | SA 09 | 250 | 7.81 | 62.5 | 0.5 | Synergistic |
| 10 | SA 10 | 250 | 3.9 | 62.5 | 0.5 | Synergistic |
| 11 | SA 11 | 250 | 7.81 | 31.25 | 0.24 | Synergistic |
| 12 | SA 12 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 13 | SA 13 | 125 | 7.81 | 15.62 | 0.24 | Synergistic |
| 14 | SA 14 | 125 | 7.81 | 62.5 | 1 | Additive |
| 15 | SA 15 | 62.5 | 7.81 | 31.25 | 1 | Additive |
| 16 | SA 16 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 17 | SA 17 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 18 | SA 18 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 19 | SA 19 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 20 | SA 20 | 62.5 | 7.81 | 15.62 | 0.5 | Synergistic |
| 21 | MTCC 740 | 1.95 | 1.95 | 0.24 | 0.24 | Synergistic |
Table (6):
Determination of synergistic activity of methanol extract of A. vulgaris L. leaves with gentamicin against clinical isolates of MDR S. aureus
| No. | Clinical MDR S. aureus Concen. | Minimum Inhibitory Concen. (MIC) | Fractional Inhibitory Concen. Index (FICI) | Result | ||
|---|---|---|---|---|---|---|
| Gentamicin (µg/mL) | A. vulgaris (mg/ml) | Gentamicin + A. vulgaris | ||||
| 1 | SA 01 | 15.62 | 15.62 | 1.95 | 0.24 | Synergistic |
| 2 | SA 02 | 0.97 | 31.25 | 0.06 | 0.12 | Synergistic |
| 3 | SA 03 | 7.81 | 15.62 | 0.97 | 0.24 | Synergistic |
| 4 | SA 04 | 7.81 | 15.62 | 0.97 | 0.24 | Synergistic |
| 5 | SA 05 | 31.25 | 7.81 | 15.62 | 1 | Additive |
| 6 | SA 06 | 3.9 | 7.81 | 0.97 | 0.5 | Synergistic |
| 7 | SA 07 | 15.62 | 7.81 | 3.9 | 0.5 | Synergistic |
| 8 | SA 08 | 15.62 | 3.9 | 3.9 | 0.5 | Synergistic |
| 9 | SA 09 | 62.5 | 7.81 | 15.62 | 0.5 | Synergistic |
| 10 | SA 10 | 7.81 | 3.9 | 3.9 | 1 | Additive |
| 11 | SA 11 | 62.5 | 7.81 | 15.62 | 0.5 | Synergistic |
| 12 | SA 12 | 31.25 | 7.81 | 15.62 | 1 | Additive |
| 13 | SA 13 | 15.62 | 7.81 | 3.9 | 0.5 | Synergistic |
| 14 | SA 14 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 15 | SA 15 | 0.97 | 7.81 | 0.12 | 0.24 | Synergistic |
| 16 | SA 16 | 62.5 | 7.81 | 31.25 | 1 | Additive |
| 17 | SA 17 | 62.5 | 7.81 | 31.25 | 1 | Additive |
| 18 | SA 18 | 31.25 | 7.81 | 7.81 | 0.5 | Synergistic |
| 19 | SA 19 | 125 | 7.81 | 31.25 | 0.5 | Synergistic |
| 20 | SA 20 | 62.5 | 7.81 | 15.62 | 0.5 | Synergistic |
| 21 | MTCC 740 | 0.01 | 1.95 | 0.002 | 0.45 | Synergistic |
GC-MS analysis
The GC-MS analysis detected 30 phytocompounds as shown in Table 7. Some of the compounds detected were mome inositol (72.07%), 24-Norursa-3,12-diene (5.35%), Bicyclo[4.1.0]heptan-3-ol, 4,7,7-trimethyl-, [1R-(1.alpha.,3.beta.,4.alpha.,6.alpha.)]- (2.47%), Phytol (2.28%), Neophytadiene (1.85%), 5-Acetylimino-7-acetylamino-8-5H-quinolone (1.46%), 24-Noroleana-3,12-diene (1.46%), (3E,10Z)-Oxacyclotrideca-3,10-diene-2,7-dione (1.18%), and Vitamin E (0.4%).
Table (7):
GC-MS analysis of methanol extract of A. vulgaris L. leaves
Peak |
Retention Time |
Peak area% |
Compound Name |
Molecular formula |
Molecular weight |
|---|---|---|---|---|---|
1 |
10.229 |
1.18 |
(3E,10Z)-Oxacyclotrideca-3,10-diene-2,7-dione |
C12H16O3 |
208 |
2 |
12.028 |
72.07 |
mome inositol |
C7H14O6 |
194 |
3 |
12.31 |
1.85 |
Neophytadiene |
C20H38 |
278 |
4 |
12.563 |
0.69 |
3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
C20H40O |
296 |
5 |
12.765 |
0.49 |
Heptadecanal |
C17H34O |
254 |
6 |
13.046 |
0.5 |
10-12-Pentacosadiynoic acid |
C25H42O2 |
374 |
7 |
15.031 |
2.28 |
Phytol |
C20H40O |
296 |
8 |
15.56 |
0.46 |
bufa-20,22-dienolide, 14,15-epoxy-3,5,16-trihydroxy- |
C24H32O6 |
416 |
9 |
16.484 |
1.2 |
9-Acetyl-S-octahydrophenanthrene |
C16H20O |
228 |
10 |
16.911 |
1.46 |
5-Acetylimino-7-acetylamino-8-5H-quinolone |
C13H11N3O3 |
257 |
11 |
17.097 |
0.13 |
cis-Arbusculone |
C9H14O2 |
154 |
12 |
17.442 |
0.27 |
2-Methyl-oct-2-enedial |
C9H14O2 |
154 |
13 |
17.83 |
0.11 |
1-cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl- |
C10H16O |
152 |
14 |
18.794 |
0.92 |
3-Buten-2-one, 4-(3-hydroxy-6,6-dimethyl-2-methylenecyclohexyl)- |
C13H20O2 |
208 |
15 |
19.068 |
0.58 |
9,12,15-Octadecatrienoic acid, 2-(acetyloxy)-1-(acetyloxy)methyl]ethyl ester |
C25H40O6 |
436 |
16 |
19.809 |
2.46 |
Bicyclo[4.1.0]heptan-3-ol, 4,7,7-trimethyl-, [1R-(1.alpha.,3.beta.,4.alpha.,6.alpha.)]- |
C10H18O |
154 |
17 |
20.621 |
0.79 |
2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19, 23-hexamethyl- |
C30H50 |
410 |
18 |
21.226 |
0.73 |
4a(2H)-Naphthalenemethanol, octahydro- |
C11H20O |
168 |
19 |
21.462 |
0.72 |
N-hentriacontanol-1 |
C31H64O |
452 |
20 |
23.09 |
0.16 |
2,2,4-trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol |
C30H52O |
428 |
21 |
23.772 |
0.43 |
10-Methylundec-2-en-4-olide |
C12H20O2 |
196 |
22 |
24.189 |
0.4 |
Vitamin E |
C29H50O2 |
430 |
23 |
26.359 |
0.35 |
Cholest-22-ene-21-ol, 3,5-dehydro-6-methoxy-, pivalate |
C33H54O3 |
498 |
24 |
28.289 |
0.67 |
Olean-12-en-3-ol, acetate, (3.beta.)- |
C32H52O2 |
468 |
25 |
29.386 |
1.46 |
24-Noroleana-3,12-diene |
C29H46 |
394 |
26 |
3.163 |
1.01 |
4,4,6a,6b,8a,11,11,14b-Octamethyl-1,4,4a,5,6,6a, 6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one |
C30H48O |
424 |
27 |
31.066 |
0.45 |
D:B-Friedo-B’:A’-Neogammacer-5-en-3-one |
C30H48O |
424 |
28 |
31.417 |
5.35 |
24-Norursa-3,12-diene |
C29H46 |
394 |
29 |
33.066 |
0.33 |
cholest-20(22)-en-3-one, 4,5-epoxy-11-hydroxy- |
C27H42O3 |
414 |
30 |
33.469 |
0.51 |
3,7,11,15-Tetramethylhexadec-2-en-1-yl acetate |
C22H42O2 |
338 |
Microorganisms resistant to antibiotics have emerged as a result of the widespread use of antibiotics. Drug-resistant pathogens have become a major hindrance in the treatment of infections. Many reviews have revealed phytochemicals from plants as possible substitutes for antibiotics against drug-resistant infections.16 It has been observed that several solvent extracts of Rhus chinensis Mill exhibited antibacterial and cell envelope-damaging qualities against Escherichia coli and S. aureus.17 The present study has examined the methanol extract of A. vulgaris L. leaves for its antibacterial activity and synergistic properties in conjunction with different test antibiotics against MDR Staphylococcus aureus.
It has been established that the phytochemicals have inhibitory effects on clinical isolates when administered as extracts.7 The methanol extract of the leaves of A. vulgaris exhibited a zone of inhibition ranging from 13.00 ± 0.00 mm to 23.33 ± 0.57 mm against the tested MDR Staphylococcus aureus. Dahiya and Purkayastha18 reported the antibacterial activity of various medicinal plants against MDR clinical isolates of both Gram-positive and Gram-negative bacteria. Recently, the MIC value of 2 mg/mL and MBC value of 5 mg/mL against S. aureus ATCC 25923 were reported using the ethanolic extracts of Artemisia annua L.19 The lowest MIC value was 3.90 mg/mL, and the MBC value was 7.81 mg/mL against the isolate SA 10. Methanolic extracts of A. vulgaris have demonstrated comparable effectiveness against pathogenic bacteria, including S. aureus (ATCC:25923), with MIC and MBC values of 12.5 mg/mL and 25 mg/mL, respectively.20 Methanol extract of A. vulgaris leaves was also shown to have a MIC value of 12.5 mg/ml against MRSA (ATCC 25923).21 The primary components of the essential oils (caryophyllene, germacrene D, and humulene) were found to have antibacterial and antifungal properties against S. aureus and Candida albicans, respectively.22,23 The antibacterial activity may be attributed to the synergistic interactions between the different phytoconstituents.24 However, different solvent fractions of this plant should also be examined for antibacterial properties.
Our findings demonstrated synergistic interaction of the test plant extract in combination with gentamicin and penicillin, respectively against almost all the isolates studied. Previous synergistic studies using combination of penicillin and Salvadora persica stem ethanol extract against S. aureus have shown an increase in the zone of inhibition from 18 mm to 21 mm.25 It has been reported that the flavonoids chrysosplenetin, penduletin, and chrysoeriol that were isolated from Artemisia rupestris L. have synergistic action with the fluoroquinolone-resistant strain SA1199B of S. aureus.26 It has been discovered that dieckol, which was isolated from Ecklonia stolonifera, works in concert with ampicillin and penicillin to inhibit MRSA.27 In our study, the reduction in MIC from 0.48 µg/ml to 0.03 µg/ml clearly showed a 16-fold reduction in MIC of clindamycin against nine test isolates. Combining oxacillin with the plant extract resulted in a similar 16-fold decrease in MIC against eight test isolates. MIC of oxacillin was decreased from 31.25 µg/ml to 1.95 µg/ml in the present study. According to reports, dieckol extracted from E. stolonifera and ampicillin together lowered the minimum inhibitory concentration against the MRSA from 512 µg/ml to 0.5 µg/ml.27 Plant extract combined with clindamycin and oxacillin showed synergistic activity against all isolates (FIC ≤0.5). Antagonistic activity was not seen for the plant extract and antibiotic combination against the tested isolates. Synergistic activity of plant extracts with antibiotics has been reported, which has been attributed to various phytochemicals present in the plant extracts.10 The results of our study have shown the antibiotic-potentiating property of the extract of A. vulgaris leaves against the clinical isolates of MDR S. aureus.
GC-MS analysis has revealed 30 compounds. Among the compounds, mome inositol was detected, with a maximum peak area of 72.07%. Some of the compounds detected have also been found in different medicinal plants to have potent antimicrobial activities. Mentha pulegium methanolic leaf extract with a high neophytadiene content was reported to be effective against Salmonella typhimurium ATCC CCM 538 and S. aureus ATCC 6538/P, with a MIC of 8 mg/mL.28 3,7,11,15-tetramethyl-2-hexadecen-1-ol is one of the main bioactive components in Solanum xanthocarpum methanolic extracts, which have demonstrated antibacterial and antioxidant qualities against Pseudomonas aeruginosa, Salmonella typhi, E. coli, and S. aureus.29 Phytol isolated from the aerial parts of Aster yomena had antibacterial properties by inducing oxidative stress in P. aeruginosa.30 A very small amount of vitamin E (Peak area of 0.4%) was present in the tested extract. Vitamin E, both water-soluble and lipid-soluble forms, can act as adjuvants to make the bacterium susceptible to the effects of antibiotics. It binds to the bacterial lipocalin protein (BcnA), which is generated by bacteria at sub-lethal antibiotic concentrations.31 Additional research could be done to isolate the pure bioactive components, elucidate their structure and screen for their potential pharmacological action.
We have demonstrated the antibacterial activity and antibiotic resistance modulatory activity of the extract of A. vulgaris L. leaves against clinical isolates of multidrug-resistant S. aureus through a synergistic interaction with the antibiotics. Therefore, the plant extract can be screened for the isolation of lead compounds for antibacterial medication development. Further exploration is required to know the mechanisms of synergistic action with resistant antibiotics against the clinical pathogen.
ACKNOWLEDGMENTS
The author(KHL) acknowledges the Ministry of Tribal Affairs, Government of India, for the National Fellowship for Scheduled Tribes (NFST). The authors are also thankful to the Health and Family Welfare Department, Government of Sikkim and Sir Thutob Namgyal Memorial -Multi Speciality Hospital (STNM-MSH), Sochakgang, Gangtok, India, Forest and Wildlife Department, Government of Sikkim, India, Department of Botany, University of North Bengal, India and Advanced Instrumentation Research Facility at Jawaharlal Nehru University, New Delhi, India, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
BS designed and conceputualized the study. KHL performed the experiments. BS and KHL analysed the data. KHL wrote the manuscript. BS supervised, reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Howden BP, Giulieri SG, Lung TWF, et al. Staphylococcus aureus host interactions and adaptation. Nat Rev Microbiol. 2023;21(6):380-395.
Crossref - World Health Organization, WHO bacterial priority pathogens list: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance, Geneva, Switzerland, WHO, 2024. https://www.who.int/publications/i/item/9789240093461
- Nwobodo DC, Ugwu MC, Anie CO, et al. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal. 2022;36(9):e24655.
Crossref - Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309-318.
Crossref - Hassan HMA. A short history of the use of plants as medicines from ancient times. Chimia (Aarau). 2015;69(10):622.
Crossref - Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials-a review. Plants. 2017;6(2):16.
Crossref - Bao M, Zhang L, Liu B, et al. Synergistic effects of anti-MRSA herbal extracts combined with antibiotics. Future Microbiol. 2020;15(13):1265-1276.
Crossref - Siwan D, Nandave D, Nandave M. Artemisia vulgaris Linn: an updated review on its multiple biological activities. Futur J Pharm Sci. 2022;8(1):47.
Crossref - Seidel V, Initial and bulk extraction. In: Sarker SD, Zahid L, Gray AI (Eds.), Natural products isolation, 2nd Edition. Humana Press, New Jersey. 2006:27-46.
Crossref - Limboo KH, Singh B. Antibiotic potentiating effect of Bauhinia purpurea L. against multidrug resistant Staphylococcus aureus. Front Microbiol. 2024;15:1385268.
Crossref - Perez C, Pauli M, Bazerque P. Antibiotic assay by agar-well diffusion method. Acta Biol Med Exp. 1990;15:113-115
- Elshikh M, Ahmed S, Funston S, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38(6):1015-1019.
Crossref - Bellio P, Segatore B, Mancini A, et al. Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine. 2015;22(2):223-230.
Crossref - Saiman L. Clinical utility of synergy testing for multidrug-resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis:’the motion for. Paediatr Respir Rev. 2007;8(3):249-255.
Crossref - Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther. 2020;20(1):1-11.
Crossref - AlSheikh HM Al, Sultan I, Kumar V, et al. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics. 2020;9(8):480.
Crossref - Poudyali B, Singh B. Antibacterial and cell envelope damaging properties of different solvent extracts of Rhus chinensis Mill against E. coli and Staphylococcus aureus. Indian J Tradit Knowl. 2020;19(2):428-434.
Crossref - Dahiya P, Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J Pharm Sci. 2012;74(5):443.
Crossref - Bordean ME, Ungur RA, Toc DA, et al. Antibacterial and phytochemical screening of Artemisia species. Antioxidants. 2023;12(3):596.
Crossref - Ahmadizadeh C, Monadi A, Rezaie A, Rad MG, Jafari B. Antibacterial activity of methanolic extract and essence of Sagebrush (Artemisia vulgaris) against pathogenic bacteria. Bull env pharmacol Life Sci J. 2014;3(2):121-125.
- Manandhar S, Luitel S, Dahal RK. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Trop Med. 2019;2019(1):1895340.
Crossref - Malik S, de Mesquita LSS, Silva CR, et al. Chemical profile and biological activities of essential oil from Artemisia vulgaris L. cultivated in Brazil. Pharmaceuticals. 2019;12(2):49.
Crossref - Pandeya S, Pudasaini A, Basyal D. Comparative Analysis on GCMS, Physicochemical and Anti-Microbial Properties of Aerial Parts of Plant Artemisia vulgaris Obtained from Two Different Altitudes. Int J Sci Res. 2019;8(11):1159-1162.
Crossref - Ayaz M, Ullah F, Sadiq A, et al. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem Biol Interact. 2019;308:294-303.
Crossref - Ahmed Z, Khan SS, Khan M, Tanveer A, Lone ZA. Synergistic effect of Salvadora persica extracts, tetracycline and penicillin against Staphylococcus aureus. African J Basic Appl Sci. 2010;2(1-2):25-29.
- Lan JE, Li XJ, Zhu XF, et al. Flavonoids from Artemisia rupestris and their synergistic antibacterial effects on drug-resistant Staphylococcus aureus. Nat Prod Res. 2021;35(11):1881-1886.
Crossref - Lee DS, Kang MS, Hwang HJ, et al. Synergistic effect between dieckol from Ecklonia stolonifera and b-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol Bioprocess Eng. 2008;13:758-764.
Crossref - Ceyhan-Guvensen N, Keskin D. Chemical content and antimicrobial properties of three different extracts of Mentha pulegium leaves from Mugla Region, Turkey. J Environ Biol. 2016;37(6):1341-1346
- Nithya M, Ragavendran C, Natarajan D. Antibacterial and free radical scavenging activity of a medicinal plant Solanum xanthocarpum. Int J food Prop. 2018;21(1):313-327.
Crossref - Lee W, Woo ER, Lee DG. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic Res. 2016;50(12):1309-1318.
Crossref - Naguib MM, Valvano MA. Vitamin E increases antimicrobial sensitivity by inhibiting bacterial lipocalin antibiotic binding. Msphere. 2018;3(6):10-1128.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.