ISSN: 0973-7510
E-ISSN: 2581-690X
This research investigates the synergistic impact of Trichoderma asperellum and biochar in sustainable plant disease management. Through a series of in vitro assays, dual culture techniques, and poison food methods, the investigation reveals that combining T. asperellum with biochar significantly inhibits the growth of Pythium aphanidermatum, achieving up to 85.92% inhibition at optimized concentrations. Additionally, biochar supplementation enhances cellulase enzyme activity and protein production, with the highest levels observed at 3% biochar. The integration of biochar within submerged fermentation systems establishes a microhabitat conducive to microbial enzyme synthesis, boosting ecological efficacy and supporting environmentally friendly disease control. The findings underscore the potential of this approach to reduce reliance on synthetic fungicides, improve agricultural productivity, and foster eco-friendly pest management. Future research should aim to elucidate the underlying molecular mechanisms, optimize biochar formulations, and conduct field-scale validations to ensure practical applicability across diverse agro-ecosystems.
Biochar, Cellulase Activity, Pythium aphanidermatum, Sustainable Agriculture, Trichoderma asperellum
Microbial enzymes play a dual role as biocatalysts in essential metabolic processes and as facilitators of microbial survival within specific ecological niches.1 Rhizosphere associated microorganisms are well known for their ability to enhance plant growth and inhibit the activity of plant pathogens.2 These microorganisms can improve plant resistance to plant pathogens through multiple strategies such as antagonism for space and nutrient, parasitism, activation of Microbe-induced plant immunity, along with the secretion of enzymes that degrade pathogen cell walls (e.g., chitinase, β-1,3 glucanase, amylase, protease, and cellulase). Among these, synthesis of hydrolytic enzymes is considered a highly effective biocontrol strategy to inhibit phytopathogens present in the rhizosphere environment.3 In response to phytopathogen invasion, rhizosphere microbes such as Trichoderma, Bacillus, and Pseudomonas produce several enzymes including chitinases, β-glucanases, cellulases, and proteases.4 Filamentous fungi serve as highly effective biocontrol agents owing to their significant secretion of extracellular enzymes.5 Within this group, Trichoderma spp. stand out for their exceptional enzymatic activity and have been widely used in the manufacture of cellulase owing to their strong cellulolytic potential.6 Notably, Trichoderma asperellum has demonstrated superior antagonistic activity against Pythium species, significantly reducing pathogen growth and disease incidence compared to other Trichoderma species.7-9 Trichoderma has been used extensively as an antagonist against phytopathogens. It uses several biocontrol mechanisms such as mycoparasitism, coiling, competition for nutrient and space, production of cell wall degrading enzymes and antibiotics, and triggering plant defence mechanisms.10 Several advanced studies (e.g., artificial mutation and genetic engineering) have been carried out to enhance cellulase production from microbes.11-13 Furthermore, there are several other methods for culture technologies. Currently, there are only two basic culture techniques used for the secretion of cellulase: Solid-State Fermentation (SSF) and Liquid-Based Submerged Fermentation (SmF). The Solid Substrate Fermentation is conducted in conditions with minimal microorganisms and liquids cultivating over the surface of the growth medium, during the Submerged culture fermentation process is carried out using a substrate immersed in liquid medium.14,15 Submerged fermentation (SmF) offers better process control, scalability, and ease of automation, making it ideal for industrial enzyme production, though it requires high energy and may cause product dilution.16 Solid-State Fermentation (SSF), closer to fungi’s natural habitat, yields higher enzyme titers with lower energy use and can utilize agro-waste, but faces scale-up challenges due to difficult parameter control and complexity.17 The choice between SmF and SSF depends on the organism, product type, and industrial need. The controlled processing conditions and simplicity of product recovery offered by submerged fermentation (SmF) provide industrial advantages over solid-state fermentation (SSF), which often delivers highly concentrated enzymes.14 Several important parameters that contribute to SmF include pH, nutrition sources (both carbon and nitrogen), incubation period, temperature, dissolved oxygen, and aeration.18 Hence, numerous studies have focused on optimizing the culture environment to enhance cellulase synthesis in Submerged Fermentation.18,19
For the optimization of cellulase production in submerged culture fermentation, additional innovative aspects were investigated, integrating the advantages of solid-state fermentation within the submerged fermentation framework. Using a similar approach, this work aimed to assess the impact of microbial habitat alteration through biochar addition into the submerged fermentation (SmF) system on cellulase production.18 Biochar is a very porous, carbon-dense material produced via pyrolysis under oxygen-restricted conditions from diverse biomass sources, including agricultural and forestry residues.20 Due to its rich functional group composition, extensive surface area, and high porosity, biochar is extensively utilized in environmental applications such as water treatment, soil improvement, carbon sequestration, and the removal of pollutants.21 Biochar may also enhance the biological activity of soil. Due to its extremely porous surface and easily degradable carbon and nitrogen, biochar has been found to enhance the growth of soil microbes and fungi, along with boosting enzymatic activity.19 According to the recent studies, biochar plays a vital role in the reduction of both biotic and abiotic stresses. It can improve systemic resistance, change rhizospheric chemistry, and act as a host for beneficial microorganisms. Biochar also releases chemicals with allelopathic properties that suppress phytopathogens, weeds, and insect pests.22 Although many studies suggest biochar could boost soil or sludge cellulase activity, research on its use in cellulase production is limited.23 Trichoderma species are recognized for their ability to degrade chitin, lignin, cellulose, and hemicellulose, attributed to their production of hydrolytic enzymes. Studies of gene expression are widely used for analysis of enzyme activities.24 The cellulolytic enzymes of Trichoderma are primarily composed of β-glucosidases and exo, endo-β-1,4-glucanses, which are used to degrade biomass and hydrolyse β-1,4-glycosidic bonds in cellulose. Consequently, Trichoderma species have the opportunity to use cellulose as a carbon source when colonizing various ecological niches where this polysaccharide is present.25 This hydrolytic enzyme inhibits the growth of plant pathogens. Hydrolytic enzymes can impede pathogen growth, thereby facilitating a reduction in the duration of the composting or decomposition process.26 To prevent environmental hazards from pesticides and crop damage due to phytopathogens, it is necessary to develop new plant disease management approaches.
Although previous research has revealed the individual potential of Trichoderma spp. utilized as biological control agents and biochar as a soil amendment, limited research has explored the integrated use of biochar and Trichoderma asperellum for increased cellulase enzyme production and biocontrol activity. Additionally, while biochar is known to influence microbial activity in soils, its role in modifying microbial habitats under submerged fermentation (SmF) conditions to boost enzyme secretion has not been adequately investigated. This study addresses this gap by evaluating the synergistic effect of biochar and T. asperellum on enzyme activity and pathogen suppression under controlled in vitro conditions.
Pathogen extraction and identification
A virulent isolate of Pythium aphanidermatum, the pathogen that causes disease in tomato seedlings (i.e., Root rot and damping-off), was recovered from infected specimens in the farming fields of Lovely Professional University. To obtain a pure culture, the isolate was purified using hyphal tip isolation method.27 This involved transferring a plug from the outer edge of a colony grown on potato dextrose agar (PDA), a medium for fungal growth to a 2.5% water agar medium. After the colony reached approximately 1 cm in diameter, the agar medium was inverted and incubated until the mycelium penetrated the agar surface. After that, a tiny piece of agar with one hyphal strand was removed and transferred to a PDA slant for maintenance at 28 ± 2 °C, with regular subculturing. The culture was kept at 4 °C for subsequent use.28
Microorganism and culture maintenance
Trichoderma asperellum (PP256386) were obtained from 21 different rhizospheric soil samples taken from uncultivated field of Punjab.29 To isolate Trichoderma strain, 10 g of rhizospheric soil were mixed into 100 ml conical flask consisting of 90 mL autoclaved water and were kept on Vortex mixer for 15 minutes at 200 revolutions for homogenization. Once homogenization is done the samples were diluted serially up to 10-6.30 A 100 µl suspension of every sample was put on Petri dishes containing RBA (Rose Bengal Agar) selective medium and was used for obtaining a single spore of Trichoderma. After the inoculation of soil suspension, the plates were maintained at 25 °C, for continuous incubation of three days and the pure colony was transferred to potato dextrose agar (PDA) plates, for obtaining pure culture.31 Based on their macro and micromorphological features like pigmentation, conidiophore, conidial shape, etc., the isolates were first classified as members associated with the genus Trichoderma. The plates were then stored at 4 °C in the refrigerator until further use.
Preparation of culture filtrate
For the preparation of filtered culture, the medium of Trichoderma isolate was maintained in PDB at 28 °C for a period of 7-10 days in a shaking incubator maintained at 120-150 rpm. After the incubation period, the liquid culture was passed through Whatman No. 1 paper to separate and collect the mycelial biomass. The acquired filtrate was further sterilized using a membrane filter (0.22 µm) to ensure the removal of any microbial contaminants and was subsequently stored at 4 °C for future use.32,33
Preparation of different concentrations
The crude extract of fungus Trichoderma was first dried to obtain powder form. Different concentrations of 1.0%, 2.5%, 5.0% and 10% were prepared by weighting extract 10 mg, 25 mg, 50 mg, and 100 mg of dried crude extract and dissolved each in 1 ml of sterilized water, resulting in solutions of 10, 25, 50, and 100 mg/ml, respectively. Each mixture was vortexed for 5 minutes to ensure complete solubilization before application in the agar well diffusion assay.
Antimicrobial well diffusion technique
Various extract concentrations were produced in order to assess Trichoderma asperellum antifungal effectiveness. Ten milligrams of crude extract was diluted in one millilitre of sterile distilled water at the 10 mg/mL concentration. Crude extract amounts of 25 mg, 50 mg, and 100 mg were similarly dissolved in 1 mL of sterile distilled water to obtain final concentrations of 25 mg/mL, 50 mg/mL, and 100 mg/mL, respectively. Before use in the agar well diffusion assay, every solution underwent five minutes of vortexing to guarantee total solubility. PDA was also made through mixing 39 g of PDA powder with one litre of sterile water, then sterilized using high-pressure steam at 121 °C for 15 minutes. This medium was then chilled to 45-50 °C, placed into sterile 90 mm Petri plates, and let to set. From an actively developing culture, a 7 mm mycelial disc of Pythium was taken and positioned at the middle of every PDA plate. The pathogen was equally distributed over the plate using a sterile cotton swab to create a homogeneous lawn; the plates were left to dry for 15 to 20 minutes before treatment application. Under sterile conditions, uniformly sized wells (5 mm in diameter) were created in the agar medium using a disinfected cork borer for the agar well diffusion method. While a negative control-sterile distilled water-was included in one well, four different concentrations of T. asperellum extract were distributed into separate wells. After 48-72 hours of development at 25 ± 2 °C, the zones of inhibition were quantified with a digital Vernier calliper. The experiment was performed three times, and statistical analysis involved recorded mean inhibition values.
Biochar used in Trichoderma asperellum culture
Biochar was prepared from hardwood using an electric tubular furnace. The hard wood biomass was procured from the LPU (Lovely Professional University, Phagwara) Agricultural field (31.2560° N, 75.7051° E). It was oven-dried overnight (12 h at 80 °C), grinded and kept in an airtight container to create an oxygen-deficient environment, and shifted to an electric tubular furnace (Nabertherm, Germany) which was heated at 500 °C, for 4 h. The synthesized BC was sieved using different mesh sizes (100, 200, and 300 mm). Biochar generally shows a surface area that falls between roughly 1.4 and 6.87 m²/g,34 while its pore sizes can range from 1.32 to 2.51 µm.35 The physicochemical properties recorded 7.79 pH and 2.22 dS/m of electrical conductivity (EC). The composition includes 79.0% carbon (C), 3.0% hydrogen (H), 0.2% nitrogen (N), 0.6% sulphur (S), and 15.0% oxygen (O). The ash content measures at 2.2%. The ratio of hydrogen to carbon is measured at 0.46, and the ratio of oxygen to carbon stands at 0.14. Furthermore, the (O + N)/C ratio stands at 0.19, while the (O + N + S)/C ratio is recorded at 0.20.34
Integration of biochar in Smf
To assess the impact of biochar application on cellulase enzyme production, different concentrations of biochar (1%, 2%, 3%, 4% and 5%) were added in PDB media. For comparison, without biochar, Trichoderma culture served as control. Each experimental group contained 50 mL cultures in 100 mL conical flasks, repeated thrice. The culture medium used for assessing cellulase activity comprising of yeast extract 15 grams per litre, monopotassium phosphate 5 grams per litre, dipotassium phosphate 5 grams per litre, magnesium sulfate heptahydrate three grams per litre, and streptomycin three percent weight by volume, adjusted to 5.0 of an initial pH. All flasks were sterilized for 30 minutes at 121 °C to ensure aseptic conditions. After sterilization, an inoculum comprising 5% (w/w) of the preculture was introduced into each flask. The cultures were incubated at 28 °C with continuous agitation at 150 revolutions per minute for a duration of 21 days. Agitation speed, dissolved oxygen (DO), and aeration are critical parameters influencing microbial growth and enzyme productivity in submerged fermentation. Agitation at 120-180 rpm ensures uniform mixing and adequate oxygen dispersion, while minimizing shear damage to filamentous fungi like Trichoderma asperellum.36 In this study, 150 rpm was selected as optimal based on preliminary experiment. Dissolved oxygen should be maintained above 30% saturation to support aerobic metabolism and consistent cellulase production.37 Aeration rates between 0.5-1.0 vvm (volume of air per volume of medium per minute) are typically suitable for maintaining DO levels and promoting high enzyme yields.38 However, higher aeration must be managed carefully to avoid excessive foaming and loss of volatile metabolism. Optimizing these parameters enhances the efficiency of submerged fermentation under biochar amended conditions. To measure the changes in enzyme activity 1 mL of broth was sampled every 3 days. The filtration of samples was conducted by means of Whatman No. 1 paper, and the resulting supernatant was collected for analytical purposes.
Assay method
Enzymatic activity assay and quantification of reducing sugars using the DNS method
The experiment involved the preparation of reagents for enzymatic activity assays, including a Carboxymethyl Cellulose (CMC) solution, which was prepared by dissolving 1 gram of CMC in 100 millilitres of 50 mM of sodium citrate buffer (pH 4.8), and subsequently sterilized either by filtration or autoclaving. The sodium citrate buffer was made through dissolution of 14.7 grams of sodium citrate dihydrate in deionized water, adjusting the pH to 4.8, and making up with sterile water to formulate the final volume. The enzyme supernatant was obtained by growing Trichoderma in a CMC-based liquid medium, filtering the culture, and storing the supernatant on ice. The reaction was set up by adding one millilitre of one percent CMC solution (substrate) and 1 mL of enzyme supernatant (source of endoglucanase) in a reaction tube, followed by gentle mixing. For enzymatic activity, the sample was maintained at 50 °C for a duration of 30 minutes under controlled conditions with occasional mixing to maintain uniformity. To stop the reaction, 3 mL of DNS reagent was immediately added. DNS assay was utilized for the quantification of reducing sugars, with the DNS reagent which is made up of 3,5-Dinitrosalicylic acid (1 g), NaOH (20 g), Rochelle salt (30 g), phenol (0.2 mL), and sodium sulfite (0.5 g), dissolved to one litre of sterile water.
Enzyme activity
Somogyi-Nelson technique (Nelson, 1944) was used to assess endoglucanase activity. A 2% carboxymethyl cellulose solution in 0.1 M of (pH 5.0) sodium citrate (Na3C6H5O7) substrate was used. The reaction mixture comprised of the CMC solution 45 µl and enzyme extract of 5 µl, which was incubated for 30 minutes at 60 °C. Following incubation, copper reagent 50 µl was introduced to the mixture, which was then heated to boiling for 10 minutes to halt enzyme activity. Subsequently, Nelson’s arsenomolybdate reagent 50 µl, along with distilled water of 850 µl, was mixed into the solution. The supernatant absorbance value was measured at 650 nm to quantify the released reducing sugars.
Cellobiohydrolase and β-Glucosidase activities were measured with 10 mM solutions of pNP-α-d-glucopyranose and pNP-β-D-cellobioside, respectively. Both substrates were dissolved in 0.1 M Na3C6H5O7 buffer (pH 5.0). The experimental solution was made up of buffer (160 µl), substrate (20 µl), and enzyme extract (20 µl), making a total of 200 µl. The reaction mixture was maintained at 65 °C for 15 minutes. Upon completion of incubation, to stop the reaction, 50 µl of sodium carbonate (Na2CO3) was added, and using a spectrophotometer, absorbance was measured at 405 nm.
SA U/mg = (Enzymatic activity (U/mL))/(Protein concentration (mg/mL))
Protein quantification was performed according to the standard procedure outlined in NREL TP-510-42628 (2008) for measuring Filter Paper Unit (FPU) activity. The reaction mixture was prepared using 0.1 M sodium citrate buffer (1 ml), enzyme solution (0.5 ml) and filter paper (0.05 g) and then transferred into a sterilized conical flask and stored at a specific temperature 50 °C for one hour. Following the incubation period, DNS reagent (3.0 mL) was mixed to the reaction solution and was kept in a water bath for 5 min to terminate the chemical reaction. The resulting mixture was separated using a centrifuge machine set at 13,000 rpm, 10 min. The spectrophotometer was used to measure the absorbance (at 540 nm) of supernatant and the concentration of the released product was calculated using a standard calibration curve. One enzyme activity unit (U) denoted as that quantity of the enzyme which secretes 1 µmol of glucose for EG and FPU and p-Nitrophenol for BGL and CBH per minute of reaction under experimental setup.
Protein assay
The amount of protein in Trichoderma was quantified using the Bradford assay. The assay solution included distilled water (400 µl), protein assay reagent (100 µl), and enzyme extract (10 µl), which was kept at room temperature for incubation (5 min). Absorbance readings were taken at 540 nm using a spectrophotometer. Protein concentration was calculated by plotting the graph using absorbance against standard curves made using BSA. Duplicate measurements were taken to ensure reproducibility and accuracy.
Statistical analysis
All experimental data were obtained in triplicate and are presented as mean ± standard error (SE). Statistical analyses were performed using IBM SPSS Statistics software (version 22) and R statistical environment (version 4.4.1). One-way analysis of variance (ANOVA) was employed to assess the significance of treatment effects to assess the enzyme activity (Endoglucanase, β-glucosidase, Cellobiohydrolase). When significant differences were observed (p < 0.05), Tukey’s Honest Significant Difference (HSD) post-hoc test was applied to compare the means across treatment groups. The threshold for statistical significance was set at p < 0.05 for all analyses. Graphical representations of the results were generated using OriginPro 2022 software to visually illustrate treatment effects.
Dual culture test and agar diffusion test
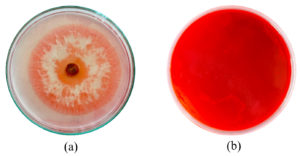
In dual culture technique, by measurement of radius growth, it was found that Trichoderma asperellum was capable of inhibiting the pathogen (P. aphanidermatum) when grown in potato dextrose medium by ≥75.00% as shown in Figure 1. To reconfirm the efficacy of Trichoderma asperellum against the test pathogen, agar well diffusion test was conducted. The agar well diffusion assay image shows a pathogen colony with a distinct zone of inhibition surrounding the wells, indicating the antagonistic effect of Trichoderma asperellum. The clear zones around the wells suggest that Trichoderma produces extracellular compounds that inhibit Pythium growth. The results clearly showed (Table 1 and Figure 1) that minimal growth inhibition (28.4 ± 1.2%) was recorded with concentration 10 mg/mL, whereas the highest growth inhibition (85.92 ± 0.0 was observed with concentration 100 mg/mL.
Table (1):
Growth inhibition zone of T. asperellum metabolites against P. aphanidermatum
Crude Extracts (mg/mL) |
Inhibition (%) ± SD |
|---|---|
10 |
28.40 ± 1.2 |
25 |
41.38 ± 2.5 |
50 |
72.53 ± 1.0 |
100 |
85.92 ± 0.0 |
Figure 1. Image (a) represents Pure Culture of Trichoderma asperellum. (b) represents the antagonistic effect of P. aphanidermatum with T. asperellum. (c) Effect of metabolites on the radius growth of P. aphanidermatum (a) 1% (w/v) solution (b) 2.5% (w/v) solution (c) 5% (w/v) solution (d) 10% (w/v) solution
Evaluation of cellulase activity of Trichoderma asperellum
Trichoderma asperellum showed the medium cellulase activity whereas the control did not show any enzymatic activity Figure 2.
Assessment of biochar influence on the activity of cellulase
The variation in enzymatic activities due to different concentration of biochar (1%-5%) at the flask scale, along with the corresponding maximum values (Table 2).
Table (2):
Flask-scale observations on day 9 revealed the enzymatic activity and protein concentration of T. asperellum under varying levels of biochar
Treatment |
Endoglucanase (U/mL) |
β-glucosidase (U/mL) |
Cellobiohydrolase (U/mL) |
Protein (U/mL) |
|---|---|---|---|---|
TA + BC 1% |
23.54 + 0.33 |
4.21 + 0.02 |
0.89 + 0.01 |
2.03 + 0.02 |
TA + BC 2% |
29.65 + 0.46 |
4.39 + 0.08 |
1.21 + 0.01 |
2.23 + 0.01 |
TA + BC 3% |
36.42 + 0.77 |
4.52 + 0.01 |
1.39 + 0.05 |
2.56 + 0.01 |
TA + BC 4% |
35.43 + 0.43 |
4.53 + 0.01 |
1.36 + 0.01 |
2.53 + 0.02 |
TA + BC 5% |
31.43 + 0.48 |
4.48 + 0.01 |
1.31 + 0.01 |
2.48 + 0.01 |
Control (TA ) |
20.30 + 0.43 |
2.07 + 0.09 |
0.50 + 0.08 |
1.42 + 0.01 |
*TA = Trichoderma asperellum; BC = Biochar; p value: 0.001 (significant at p < 0.05); ANOVA test shows statistically significant differences between different treatments
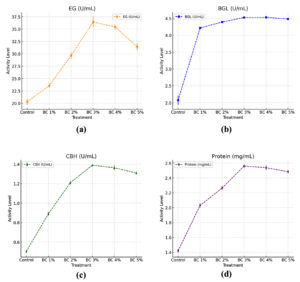
The graphs illustrate the impact of different biochar (BC) concentrations (1%-5%) on the activity of three cellulolytic enzymes-EG, BGL, and CBH-as well as total protein content in the sample. The results indicate that biochar enhances cellulase activity in a concentration-dependent manner, with an optimal level observed at 3% BC Figure 3.
Figure 3. T. asperellum enzyme activities and protein concentration recorded under different biochar (BC) concentrations in conical flask cultures (a) Enzyme activity of EG, (b) Activity of BGL, (c) Activity of CBH, (d) Protein concentration
Effect of biochar concentration on endoglucanase activity
EG activity increased gradually with increasing biochar concentrations, reaching a maximum at 3% biochar (36.42 ± 0.77 U/mL), which exceeded the control by 1.79-times (20.30 ± 0.43 U/ml). The activity at 1%, 2%, and 3% biochar was 1.16, 1.46, and 1.79 times higher, respectively, compared to the control. However, at higher concentrations (4% and 5%), the activity slightly declined to 35.43 ± 0.43 U/mL and 31.43 ± 0.48 U/mL, respectively, suggesting an ideal range for biochar enhancement of EG production.
Effect of biochar concentration on β-glucosidase activity
BGL activity also increased with biochar amendment, with the maximum activity recorded at 4% biochar (4.53 ± 0.01 U/mL), which was 2.19-fold higher than the control (2.07 ± 0.09 U/mL). The activity at 1%, 2%, 3%, and 5% BC remained relatively stable, ranging from 4.21 ± 0.02 to 4.52 ± 0.01 U/mL, indicating that even at low biochar levels, BGL activity was significantly enhanced compared to the untreated.
Assessment of biochar concentration on cellobiohydrolase activity
CBH activity showed a peak at 3% biochar (1.39 ± 0.05 U/mL), which was 2.78-fold higher than the control (0.50 ± 0.08 U/ml). At 1% and 2% biochar, CBH activity increased to 0.89 ± 0.01 and 1.21 ± 0.01 U/mL, respectively. However, a slight reduction in CBH activity was recorded at 4% and 5% BC (1.36 ± 0.01 and 1.31 ± 0.01 U/mL), suggesting an inhibitory effect at higher biochar concentrations.
Assessment of biochar on protein concentration during enzyme production
The protein concentration increased progressively with biochar supplementation, reaching a maximum at 3% BC (2.56 ± 0.01 mg/ml), which exceeded the control by 1.8 times (1.42 ± 0.01 mg/mL). At 1% and 2% biochar, the protein concentration was 2.03 ± 0.02 and 2.23 ± 0.01 mg/mL, respectively. A slight decline was detected at 4% and 5% biochar (2.53 ± 0.02 and 2.48 ± 0.01 mg/mL), which followed a similar trend as EG and CBH activities.
This study demonstrates that Trichoderma asperellum effectively suppresses the mycelial spread of Pythium aphanidermatum, exhibiting the maximum percentage of inhibition recorded in the dual culture assay. These findings indicate that Trichoderma is capable of producing various antimicrobial compounds. A major mechanism of action of Trichoderma is as a microbial antagonist in mycoparasitism, wherein it produces a suite of lytic enzymes, including cellulase, chitinase, xylanase, and glucanase, to degrade the cell walls of target pathogens. Numerous studies have shown that antagonistic microbes such as Trichoderma spp. secrete enzymes that degrade cellulose, chitin, and glucan-core structural materials of fungal and oomycete cell walls.39-41 Viterbo et al.42 demonstrate that Trichoderma produced chitinase, glucanase, and protease during antagonism. Likewise, numerous studies have demonstrated that Trichoderma’s antagonistic effect on soil-borne pathogens is strongly linked to its production of extracellular lytic enzymes.43-47 According to a report, T. asperellum-derived chitinase and cellulase enzymes play a major role in its antagonistic interplay and it was involved for the mycelial growth inhibition of Colletotrichum gloeosporioides and Phytophthora capsici.48 This study found that the synthesized enzymes effectively suppressed the mycelial growth of Pythium aphanidermatum in vitro.
In the context of inhibitory interactions, organic metabolites comprising both non-volatile and volatile compounds are synthesised by species of Trichoderma.49,50 These compounds are known to have a crucial impact on microbial competition and defence. It has been well established that non-volatile metabolite such as flavonoid and phenolic compounds, effectively supress the fungal growth and the germination of phytopathogens.51,52 The present study demonstrated that non-volatile organic compounds synthesised by T. asperellum substantially lowered the mycelial growth of Pythium aphanidermatum under a controlled environment condition, suggesting their involvement in antagonistic suppression. Similar findings were reported by Umar et al.53 who found that crude organic compounds from T. ghanense and T. citrinoviride effectively suppressed both spore germination and P. aphanidermatum mycelial growth.
The microbe-inhibiting effects of Trichoderma secondary metabolites are likely due to the combined influence of both VOCs (non-volatile and volatile). Numerous species of Trichoderma are known to secrete VOCs that suppress soil-borne pathogens.50,54 VOCs not only contribute to pathogen inhibition but also enhance Trichoderma’s resistance to environmental stresses, potentially protecting its own cell walls from hydrolytic enzymes released by competing organisms. Moreover, in dual culture assays, the synthesis of VOCs was observed to increase when Trichoderma was confronted with a pathogen.55 Beyond initial enhancement, assessing the stability and longevity of cellulase activity in biochar-amended cultures over extended periods (e.g., 30-45 days) is essential. Meta-analyses have shown that cellulolytic activity tends to increase more in studies spanning 20-50 days by approximately 59% compared to shorter durations (<15 days) which saw only 42% increases.56 However, longer-term biochar amendments (≥1 year) often shift microbial enzyme profiles enhancing ligninase activity while reducing cellulase activity indicating possible microbial adaptation or substrate depletion over time.57
This study demonstrated that Trichoderma asperellum (PP256386) significantly inhibited Pythium aphanidermatum through the action of extracellular enzymes and both volatile and non-volatile metabolites. Biochar supplementation enhanced protein concentration and cellulase activity, with the highest enzymatic function observed at 3% biochar. The concentration of nutrients is influenced by the pyrolysis conditions and the type of feedstock material used for the preparation of biochar.58 Lower temperature pyrolysis tends to preserve organic compounds that are more labile which aid in microbial compatibility. In contrast, high temperature pyrolysis produces stable biochar that are less bio-active due to low nutrient surface reactivity.59 The integration of biochar into the submerged fermentation system created a microhabitat that boosted enzyme synthesis by T. asperellum. Recent studies examining the broader impact of biochar on increasing microbial function, including systemic resistance in plants, and mitigating biotic and abiotic stressors continue to support the more localized enhancement.22 These results emphasize the synergistic potential of biochar and Trichoderma for sustainable disease control. In addition to the synergism observed in this study, we found that the use of biochar in association with Trichoderma exerts great environmental and economic benefits for sustainable agriculture.60 Biochar also plays a key role in carbon sequestration and in the improvement of long-term soil health, which can be observed via better nutrient retention, microbial colonization, and soil structure.61 We also observed that it is a factor in the reduction of our reliance on synthetic fungicides, which in turn decreases the environmental impact due to pesticide overuse.61 It is low cost to produce both biochar and Trichoderma, which makes them very accessible to smallholders and resource-limited farming systems.60 Molecular studies such as RNA-Seq or qRT-PCR can be employed to analyse the expression of cellulolytic genes like cbh1 (cellobiohydrolase I), egl1 (endoglucanase I), and bgl1 (β-glucosidase) in Trichoderma asperellum under biochar-amended conditions.62 Studies have shown that biochar can modulate microbial gene expression and enhance the abundance of glycoside hydrolase transcripts, contributing to improved enzyme secretion and carbon metabolism.63 Gene expression profiling can thus clarify the regulatory influence of biochar on T. asperellum enzyme pathways.64 While the present study demonstrated strong antagonism of Trichoderma asperellum and biochar against Pythium aphanidermatum, evaluating this combination against a broader spectrum of soil-borne pathogens such as Fusarium oxysporum, Rhizoctonia solani, and Sclerotium rolfsii is essential. Such assessments would help determine the consistency and robustness of the synergistic effect under diverse pathosystems and cropping conditions, thereby confirming its potential for broad-spectrum biological control.65 In addition to controlling fungal pathogens, Trichoderma species have shown considerable promise in the biocontrol of plant-parasitic nematodes, especially root-knot nematodes (Meloidogyne spp.) in tomato. Several strains of Trichoderma harzianum and T. asperellum have demonstrated the ability to reduce nematode populations, gall formation, and associated plant damage by producing chitinases, secondary metabolites, and by inducing systemic resistance in host plants.66 This broad biocontrol potential reinforces the ecological relevance of Trichoderma and suggests that its synergistic use with biochar may also offer nematode suppression, an avenue worth exploring in future research.67 However, issues including biochar’s variation in effectiveness among various soil types, requirement for additional formulation and application optimization still exist.68 To ensure the successful implementation of biocontrol, a number of issues need to be resolved, such as biopesticide’s shelf life, the lack of knowledge and awareness of biocontrol techniques, regulatory registration for commercialization, and appropriate agricultural applications.69 Further exploration is essential to understand the biological mechanisms involved and validate this strategy under field conditions.
This study demonstrated that biochar supplementation significantly enhances cellulase enzyme production by Trichoderma asperellum in a concentration-dependent manner, with optimal enzyme activity and protein production observed at 3% biochar. EG, BGL, and CBH activities were markedly improved compared to the control, although higher biochar concentrations (≥4%) led to a slight decline, suggesting an optimal range for enzyme stimulation. Additionally, Trichoderma asperellum exhibited strong biocontrol potential against Pythium aphanidermatum, achieving ≥75% inhibition in dual culture and forming distinct inhibition zones in the agar well diffusion assay, with the highest effect observed at 100 mg/mL concentration.
These findings highlight the dual benefits of biochar as both an enhancer of microbial enzyme activity and a potential soil amendment for biological disease control. However, several critical scientific challenges remain unresolved. The precise molecular and physiological mechanisms by which biochar influences microbial metabolism and biocontrol efficacy are still poorly understood. Furthermore, the variability in properties of biochar depending on feedstock and pyrolysis conditions presents a challenge in standardizing its application. Research should focus on elucidating the signalling pathways involved in biochar-mediated stimulation, evaluating synergistic interactions with other soil microbes, and conducting long-term field studies to assess environmental sustainability, cost-effectiveness, and scalability of this approach in diverse agro-ecosystems.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Arora NK, Mishra J, Mishra V, eds. Microbial Enzymes: Roles and Applications in Industries. Singapore, Springer Nature. 2020;11.
Crossref - Li J, Wang C, Liang W, Liu S. Rhizosphere microbiome: The emerging barrier in plant-pathogen interactions. Front Microbiol. 2021;12:772420.
Crossref - Saravanakumar K, Yu C, Dou K, Wang M, Li Y, Chen J. Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp. cucumerinum. Biol Control. 2016;94:37-46.
- Riseh RS, Vatankhah M, Hassanisaadi M, Barka EA. Unveiling the role of hydrolytic enzymes from soil biocontrol bacteria in sustainable phytopathogen management. Front Biosci (Landmark Ed). 2024;29(3):105.
Crossref - Soccol CR, Marin B, Raimbault M, Lebeault JM. Breeding and growth of Rhizopus in raw cassava by solid state fermentation. Appl Microbiol Biotechnol. 1994;41:330-336.
Crossref - Dimawarnita F, Andhayu GS, Faramitha Y, Mufidah E. Production of crude enzyme from Trichoderma sp. BIO Web of Conf. 2024;99(1):02004.
Crossref - Mbarga JB, Ten Hoopen GM, Kuate J, et al. Trichoderma asperellum: A potential biocontrol agent for Pythium myriotylum, causal agent of cocoyam (Xanthosoma sagittifolium) root rot disease in Cameroon. Crop Prot. 2012;36:18-22.
Crossref - Chen S, Daly P, Anjago WM, et al. Genus-wide analysis of Trichoderma antagonism toward Pythium and Globisporangium plant pathogens and the contribution of cellulases to the antagonism. Appl Environ Microbiol. 2024;90(9):e00681-24.
Crossref - Vasumathi S, Aiyanathan KEA, Nakkeeran S. Biodiversity and molecular characterization of Trichoderma spp. and exploring its synergistic action for the management of cucumber damping off incited by Pythium aphanidermatum. J Pure Appl Microbiol. 2017;11(1):487-495.
Crossref - Oyesola OL, Tonjock RK, Bello AO, Taiwo OS, Obembe OO. Trichoderma: A review of its mechanisms of action in plant disease control. 2024; Preprints.
Crossref - Chen G, Liu B, Zhang Y, Zhang S, Chen Y. Engineering Trichoderma reesei for hyperproduction of cellulases on glucose to efficiently saccharify pretreated corncobs. J Agric Food Chem. 2020;68(45):12671–12682.
Crossref - Pant S, Ritika, Nag P, et al. Employment of the CRISPR/Cas9 system to improve cellulase production in Trichoderma reesei. Biotechnol Adv. 2022;60:108022.
Crossref - Lv D, Zhang W, Meng X, Liu W. Single mutation in transcriptional activator Xyr1 enhances cellulase and xylanase production in Trichoderma reesei on glucose. J Agric Food Chem. 2023;71(31):11993–12003.
Crossref - Singh B, Anu A, Singh D, et al. Cellulase production by Myceliophthora thermophila in solid-state fermentation and its utility in saccharification of rice straw. New Energy Exploit Appl. 2022;1(2):10-17.
Crossref - Korsa M, Tadesse S, Mekonnen S. Application of the solid-state fermentation process and its variations in PHA production: A review. Bioprocess Biosyst Eng. 2023;46(1):1-15.
Crossref - Nazir M, Iram A, Cekmecelioglu D, Demirci A. Approaches for producing fungal cellulases through submerged fermentation. Frontiers in Bioscience-Elite, 2024;16(1):5.
- Ezejiofor TIN, Enebaku UE, Ogueke C. Waste to wealth-value recovery from agro-food processing wastes using biotechnology: a review. British Biotechnology Journal, 2014;4(4):418.
- Myeong S, Yun J. Culture of Trichoderma sp. with biochar to produce high-activity cellulase in a laboratory. BioResources. 2024;19(2):2029. https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_19_2_2029_Myeong_Culture_Trichoderma
- Infanzón-Rodríguez MI, Del Moral S, Castro-Martínez C, et al. Multi-response optimization using the desirability function of exoglucanases, endoglucanases and β-glucosidases production by Aspergillus niger ITV-02 from delignified sugarcane bagasse. Sugar Tech. 2023;25(1):86-98.
Crossref - Sajjadi B, Chen WY, Egiebor NO. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev Chem Eng. 2019;35(6):735-776.
Crossref - Zeghioud H, Fryda L, Djelal H, Assadi A, Kane A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J Water Process Eng. 2022;47:102801.
Crossref - Arshad U. Biochar application: A sustainable approach for mitigating biotic and abiotic stresses in plants. Integrative Plant Biotechnology, 2024;2(1):1-22.
- Feng J, Yu D, Sinsabaugh RL, et al. Trade-offs in carbon-degrading enzyme activities limit long-term soil carbon sequestration with biochar addition. Biol Rev Camb Philos Soc. 2023;98(4):1184-1199.
Crossref - Giovannoni M, Gramegna G, Benedetti M, Mattei B. Industrial use of cell wall degrading enzymes: the fine line between production strategy and economic feasibility. Front Bioeng Biotechnol. 2020;8:356.
- Strakowska J, Błaszczyk L, Chełkowski J. The significance of cellulolytic enzymes produced by Trichoderma in opportunistic lifestyle of this fungus. J Basic Microbiol. 2014;54(S1):S2-S13.
Crossref - Jadhav HP, Shaikh SS, Sayyed RZ. Role of hydrolytic enzymes of rhizoflora in biocontrol of fungal phytopathogens: an overview. In: Rakshit A, Meena VS, Parihar M, Singh HB, eds. Rhizotrophs: Plant Growth Promotion to Bioremediation. Singapore: Springer; 2017:183-203.
Crossref - Joseph R, Darrisaw C, Lloyd A, Hoel D, Keyhani NO. Isolation of a novel Pythium species, P. thermoculicivorax, and Trichoderma sp. from natural enzootic mosquito larval infections. J Fungi. 2024;10(3):199.
Crossref - Muthukumar A, Eswaran A, Sanjeevkumas K. Exploitation of Trichoderma species on the growth of Pythium aphanidermatum in chilli. Braz J Microbiol. 2011;42:1598-1607.
Crossref - Kumari R, Kumar V, Arukha AP, et al. Screening of the biocontrol efficacy of potent Trichoderma strains against Fusarium oxysporum f. sp. ciceri and Sclerotium rolfsii causing wilt and000000000 collar rot in chickpea. Microorganisms. 2024;12(7):1280.
Crossref - Akhtar R, Showkat S, Saini N. Isolation and Identification of soil born fungi from agricultural fields of Dehradun, India.
- Shrestha A, Limay-Rios V, Brettingham DJ, Raizada MN. Maize pollen carry bacteria that suppress a fungal pathogen that enters through the male gamete fertilization route. Front Plant Sci. 2024;14:1286199.
Crossref - Baazeem A, Almanea A, Manikandan P, et al. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J Fungi. 2021;7(5):331.
Crossref - Mishra A, Dixit S, Ratan V, et al. Identification and in silico screening of biologically active secondary metabolites isolated from Trichoderma harzianum. Ann Phytomed. 2018;7(1):78-86.
Crossref - Jiang S, Nguyen TA, Rudolph V, et al. Characterization of hard- and softwood biochars pyrolyzed at high temperature. Environ Geochem Health. 2017;39:403-415.
Crossref - Yargicoglu EN, Sadasivam BY, Reddy KR, Spokas K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015;36:256-268.
Crossref - Umar A, Abid I, Elshikh MS, Dufossé L, Abdel-Azeem AM, Ali I. Agitation role (Dissolved Oxygen) in production of laccase from newly identified Ganoderma multistipitatum sp. nov. and its effect on mycelium morphology. BMC microbiology, 2023;23(1): 280.
- Singh S, Du Preez JC, Pillay B, Prior BA. The production of hemicellulases by Thermomyces lanuginosus strain SSBP: influence of agitation and dissolved oxygen tension. Applied microbiology and biotechnology, 2000;54:698-704.
- Lojananan N, Cheirsilp B, Intasit R, Billateh A, Srinuanpan S, Suyotha W, Boonsawang P. Successive process for efficient biovalorization of Brewers’ spent grain to lignocellulolytic enzymes and lactic acid production through simultaneous saccharification and fermentation. Bioresource Technology, 2024;397: 130490.
- Meena B, Marimuthu T, Vidyasekaran P, Velazhahan R. Biological control of root rot of groundnut with antagonistic Pseudomonas fluorescens strains. J Plant Dis Protect. 2001;108:368-381.
- Gruber S, Kubicek CP, Seidl-Seiboth V. Differential regulation of orthologous chitinase genes in mycoparasitic Trichoderma species. Appl Environ Microbiol. 2011;77(20):7217-7226.
- Saravanakuma K, Dou K, Lu Z, et al. Enhanced biocontrol activity of cellulase from Trichoderma harzianum against Fusarium graminearum through activation of defense-related genes in maize. Physiol Mol Plant Pathol. 2018;103:130-136.
- Viterbo A, Ramot O, Chernin L, Chet I. Significance of lytic enzymes from Trichoderma spp. in the biocontrol of fungal plant pathogens. Antonie Van Leeuwenhoek. 2002;81:549-556.
- Marco LJ, Valadares-Inglis MC, Felix RC. Production of hydrolytic enzymes by Trichoderma isolates with antagonistic activity against Crinipellis perniciosa the causal agent of witches’ broom of cocoa. Braz J Microbiol. 2003;34:33-38.
- Zhang F, Yang X, Ran W, Shen Q. Fusarium oxysporum induces the production of proteins and volatile organic compounds by Trichoderma harzianum T-E5. FEMS Microbiol Lett. 2014;359:116-123.
- Kredics LZ, Antal A, Szekeres L, et al. Extracellular proteases of Trichoderma species. Acta Microbiol Immunol Hung. 2005;52:169-184.
- Szekeres A, Kredics L, Antal Z, Kevei F, Manczinger L. Isolation and characterization of protease overproducing mutants of Trichoderma harzianum. FEMS Microbiol Lett. 2004;233:215-222.
- Gajera HP, Bambharolia RP, Patel SV, Khatrani TJ, Goalkiya BA. Antagonism of Trichoderma spp. against Macrophomina phaseolina: evaluation of coiling and cell wall degrading enzymatic activities. J Plant Pathol Microbiol. 2012;3:149.
- De la Cruz-Quiroz R, Roussos S, Rodríguez-Herrera R, Hernandez-Castillo D, Aguilar CN. Growth inhibition of Colletotrichum gloeosporioides and Phytophthora capsici by native Mexican Trichoderma strains. Karbala Int J Mod Sci. 2018;4(2):237-243.
- Lorito M, Woo SL, Harman GE, Monte E. Translational research on Trichoderma: from ‘omics to the field. Annu Rev Phytopathol. 2010;48(1):395-417.
- Vinale F, Sivasithamparam K, Ghisalberti LE, et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol J. 2014;8(Suppl-1, M5):127-139.
- Mazzei P, Vinale F, Woo LS, et al. Metabolomics by proton high-resolution magic-angle-spinning nuclear magnetic resonance of tomato plants treated with two secondary metabolites isolated from Trichoderma. J Agric Food Chem. 2016;64:3538-3545.
- Pakora GA, Mpika J, Kone J, et al. Inhibition of Phytophthora species, agents of cocoa black pod disease, by secondary metabolites of Trichoderma species. Environ Sci Pollut Res. 2017;25:29901-29909.
Crossref - Al-Shuaibi BK, Kazerooni EA, Al-Maqbali DA, et al. Biocontrol potential of Trichoderma ghanense and Trichoderma citrinoviride toward Pythium aphanidermatum. J Fungi. 2024;10(4):284.
- Vinale F, Sivasithamparam K, Ghisalberti EL, et al. Trichoderma–plant–pathogen interactions. Soil Biol Biochem. 2008;40:1-10.
- El-Hasan A, Schöne J, Höglinger B, Walker F, Voegel TR. Assessment of the antifungal activity of selected biocontrol agents and their secondary metabolites against Fusarium graminearum. Eur J Plant Pathol. 2018;150:91-103.
- Kerner P, Struhs E, Mirkouei A, Aho K, Lohse KA, Dungan RS, You Y. Microbial responses to biochar soil amendment and influential factors: a three-level meta-analysis. Environmental science & technology, 2023;57(48):19838-19848.
- Feng J, Yu D, Sinsabaugh RL, et al. Trade offs in carbon degrading enzyme activities limit long term soil carbon sequestration with biochar addition. Biol Rev. 2023;98(4):1184-1199.
Crossref - Sharma S, Mukherjee S, Bolan S, et al. Biochar as a potential nutrient carrier for agricultural applications. Curr Poll Rep. 2025;11(1):1-36.
Crossref - Zhang H, Voroney RP, Price GW. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol Biochem. 2015;83:19-28. doi
Crossref - Kumari R, Kumar V, Koul B, Abul Farah M, Mishra AK. Synergistic effects of Trichoderma and biochar on the biocontrol of two soil-borne phytopathogens in chickpeas. Front Microbiol. 2025;16:1583114.
Crossref - Saleem I, Riaz M, Mahmood R, et al. Biochar and microbes for sustainable soil quality management. In: Kumar A, Singh J, Ferreira LFR, eds. Microbiome under Changing Climate. 2022:289-311.
Crossref - Mohamad Sobri MF, Abd-Aziz S, Abu Bakar FD, Ramli N. In-silico characterization of glycosyl hydrolase family 1 β-glucosidase from Trichoderma asperellum UPM1. Int J Mol Sci. 2020;21(11):4035.
Crossref - Zhang Y, Sun C, Wang S, et al. Stover and biochar can improve soil microbial necromass carbon, and enzymatic transformation at the genetic level. GCB Bioenergy. 2022;14(10):1082-1096.
Crossref - Chen J, Jiao N, Ran Y, et al. Assessing effect of Trichoderma asperellum T16 on management of Bursaphelenchus xylophilus. Industrial Crops and Products. 2024;215:118628.
Crossref - Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43-56.
Crossref - Sharon E, Bar-Eyal M, Chet I, Herrera-Estrella A, Kleifeld O, Spiegel Y. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopathology. 2001;91(7):687-693.
Crossref - Annapurna M, Bhagawati B, Kurulkar U. Biochemical mechanism of native fungal bioagents in the management of root-knot nematode Meloidogyne incognita on tomato. Int J Curr Microbiol Appl Sci. 2018;7(11):380-395.
Crossref - Luo P, Zhang H, Chen Y, Li M, Wang J, Liu Q. Biochar-based fertilizers: Advancements, applications, and future directions in sustainable agriculture – A review. Agronomy. 2025;15(5):1104.
Crossref - Bakr R, Abdelmoteleb A, Mendez-Trujillo V, Gonzalez-Mendoza D, Hewedy O. The potential of beneficial microbes for sustainable alternative approaches to control phytopathogenic diseases. Microbiol Res. 2025;16(5):105.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.