ISSN: 0973-7510
E-ISSN: 2581-690X
Candida is a yeast like fungus. It causes candidiasis which is a spectrum of disease from cutaneous, mucosal, systemic & multisystem dissemination. It is a coloniser of mucocutaneous surfaces of body but it is an opportunistic fungus causing severe type of infection. Objective of the study was to detect colonization of Candida species in diabetic patient & to detect virulence factors i.e., phospholipase, proteinase, esterase activity & homolysis activity in isolated strains of Candida from colonised diabetic patients. Throat swab and urine sample were collected from diabetic patients visiting the General Medicine OPD/Ward diagnosed with diabetes mellitus before six months and was submitted in microbiology department. Candida species were identified tested for production of virulence factors. Total 30 diabetes mellitus patients diagnosed 6 months before. In 41-65 years age groups, Candida colonization was found predominantly, 63% patients. Amongst these 78% had fasting sugar levels greater than the normal limits. The study revealed a strong correlation between higher fasting blood sugar levels and Candida colonization. The predominant isolate was Candida albicans in throat followed by Candida glabrata. Out of 27 Candida species isolates, 16 strains showed production of virulence factors. Amongst these 44 % (7/16) strains were positive for proteinase production, 38 % (6/16) were positive for esterase production, 13% (2/16) strains were positive for hemolysin and single strain (6%, 10/16) showed the production of phospholipase. The study concludes that Candida albicans was the predominant colonising species found followed by Candida glabrata in diabetic patients. Non-Candida albicans species can be seen as an emerging colonizing species in the diabetic patients and thereby are increasingly gaining clinical importance. Colonized or commensal Candida species showed in vitro production of virulence factor.
Diabetes, Candida, Immunocompromised, Colonization, Proteinase, Esterase, Hemolysin
Candida is yeast like fungus. It causes candidiasis which is a spectrum of disease from cutaneous, mucosal, systemic & multisystem dissemination. Candida albicans and other species of Candida are member of the normal human commensal,1 but in diabetic patients it forms severe colonization. This type of colonization makes them more prone to develop deeper mucosal colonization with further dissemination via blood.2 It may be due to immune suppression, cancer chemotherapy, intravascular catheters, cardiothoracic or gastrointestinal surgery, and endocrine disorder like diabetes mellitus or HIV infections.3
Rodrigues et in his study analysed the development of candidiasis in diabetic patients by outlining various characteristics of Candida sp. Including hydrolytic enzymes & biofilm & correlated with the development of candidiasis.4
Life threatening infections like candidemia usually acquired in hospital when colonised patients are introduced with IV or urinary catheters.
Candida is having some virulence factors which is having role in proliferation. So, the study was planned to detect the colonization of Candida species in diabetic patients and detection of virulence factors like phospholipase, proteinase, esterase & hemolysin in the isolated strains of Candida species.
Objectives
Primary Objectives
- To detect colonization of Candida species in diabetic patient
- To detect virulence factor phospholipase, proteinase, esterase activity & hemolysin activity in isolated strains of Candida from colonised diabetic patients.
Secondary Objectives
- To identify Candida species isolated from colonized diabetic patients
The Cross-sectional Study was conducted at tertiary care hospital after approval Institutional Ethics Committee. The study duration was 15th July to 1st September 2019. It involved human patients diagnosed with diabetes mellitus of age >18 years before 6 months. Patients were asked for the written informed consent to participate in the study. Two samples (throat swab/oropharyngeal swab, urine sample) were collected at the time of enrolment. Samples were submitted in the department of Microbiology. It was processed for isolation, identification & detection of virulence factor by following the standard protocol. The sample was processed for microscopy with Gram stain& KOH mount.
Culture of samples on two sets of SDA & SDA with Chloramphenicol (incorporated to inhibit bacterial growth) were inoculated. One set will be incubated at 37°C & another at room temperature i.e.,25°C to 29°C in BOD incubators. Identification of colony was done with Gram stain, Germ tube test, chlamydospore formation, sugar assimilation, sugar fermentation method& by inoculating it on HiCrome agar.
If growth of yeasts from at least two different sites were considered as coloniser. virulence factors were detected in the isolates by detecting enzymatic activity.5-8
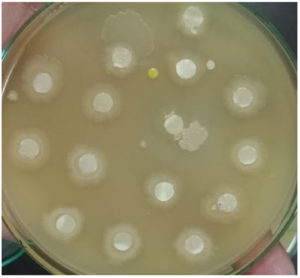
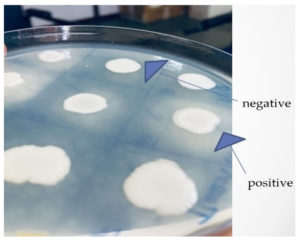
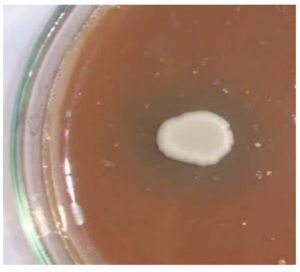
The phospholipase activity was carried out in egg yolk agar medium. The proteinase was detected in Bovine serum albumin medium. Esterase activity was detected by using Tween 80 medium. Hemolysin will be detected by SDA with Sheep blood agar. Yeast suspension was inoculated onto plates & incubated at 37 °C for48 h. Hemolysis was considered as positive hemolytic activity. ATCC C. albicans 10231 strain will be used as Control strain in the study.
This study was carried out in the Department of Microbiology. The clinical samples were collected from diabetic patients who visited the Medicine department in the study duration. Total 30 diabetes mellitus patients diagnosed 6 months before, were included in the study. The predominant age group observed was 41-65 years (80%) and amongst these 63% (15/24) were females. Fasting sugar levels ≥126 was seen in 77% (24/30) & postprandial sugar levels ≥200 was seen in 53% (16/30) of patients. Amongst these 47% (14/30) were having both fasting & postprandial sugar levels above the normal limits.
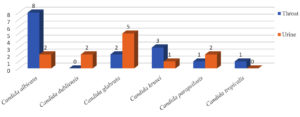
Following the standard protocol, samples were processed for isolation Candida species were isolated and identified. Total 19 patients had colonization of Candida species. Total 27 Candida strains were isolated from clinical samples of 19 patients (Figure 4). These were processed for identification of species & production of virulence factors.
The predominant isolate was Candida albicans in throat followed by Candida glabrata in urine as shown in (Figure 4). Amongst the 19 patients with colonization,79% (15/19) patients had fasting sugar level more than the normal limits. When fasting blood sugar is more than normal limit there is strong correlation for candida colonization was observed (Pearson’s test) shown in Table 1.
Table 1. Production of virulence factors by isolated species of Candida
Candida species → Virulence factors ↓ |
Candida albicans (n=10) |
Candida glabrata (n=7) |
Candida krusei (n=4) |
Candida parapsilosis (n=3) |
Candida tropicalis (n=1) |
|---|---|---|---|---|---|
Phospholipase |
0 |
1 |
0 |
0 |
0 |
Proteinase |
2 |
1 |
3 |
1 |
0 |
Esterase |
2 |
3 |
1 |
0 |
0 |
Hemolysin |
0 |
1 |
0 |
1 |
0 |
Total |
4 (18.75%) |
6 (37.5%) |
4 (25%) |
2 (12.5%) |
0 (0%) |
Note: In parentheses depicts percentage out of vertical total
Candida isolates were processed for detection of virulence factors-Phospholipase, Proteinase, Esterase and Hemolysin by following the mentioned methodology. (Figure 1 to 3).
Out of 27 Candida species isolated from throat swabs and urine samples, 16 strains showed production of virulence factors. Amongst these 44 % (7/16) strains were positive for Proteinase production, 38 % (6/16) were positive for Esterase production, 13% (2/16) strains were positive for Hemolysin and single strain showed the production of Phospholipase. (Table 1)
Esterase production was predominantly seen in Candida glabrata 50% (3/6) followed by Candida albicans 33% (2/6), Candida krusei 17% (1/6).
Hemolysin was produced by one strain of Candida glabrata and one strain of Candida parapsilosis.
Phospholipase production was observed only by a single strain of Candida glabrata.
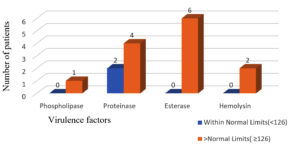
Virulence factors production in candida coloniser was seen comparatively more in patients with fasting sugar level more than normal limit. (Figure 5).
Figure 5. Number of patients showing production of virulence factors with respective fasting sugar level
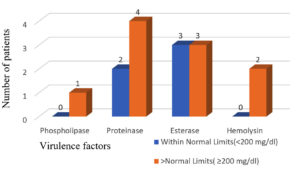
Similar findings were observed for Proteinase production in case of post prandial sugar levels, but Esterase production was observed to be equal in patients having post prandial sugar levels within normal limits and above normal limits. (Figure 6).
Figure 6. Number of patients showing production of virulence factors with respective postprandial sugar level
There is strong correlation of production of virulence factor for candida isolates from patients having postprandial blood sugar more than >200 mg/dl. (Figure 6).
All the four virulence factors were not produced by any isolate but multiple virulence factors production was seen in the various Candida isolates (Table 2).
Table (2):
Distribution of Production of virulence factors by isolated species of Candida.
Patients with candida species showing all the virulence factors production |
0 (0%) |
Patients with candida species showing 3 virulence factors production but no proteinase |
1 (3.3%) |
Patients with candida species showing 2 virulence factors production (Proteinase & Esterase) |
2 (6.6%) |
Patients with candida species showing any one of the virulence factors production |
8 (26.6) |
Total patients having colonized strains producing virulence factors |
11 (36.6%) |
Total |
30 (100%) |
In parentheses depicts percentage out of vertical total
Colonization means that a person has the organism somewhere on their body but does not have an infection or symptoms of infection.2 Diabetes mellitus (DM) is a metabolic disorder that predisposes individuals to fungal infections, due to an immunosuppressive effect on the patient, also making them susceptible to infections those related to Candida species. The study revealed a strong correlation between higher fasting blood sugar levels and Candida colonization. (Pearson test). Similar findings were revealed by studies carried by Kumar et al.,9 Khazal et al.10 and Lotfi-Kamran et al.11 This is due to the higher levels of blood glucose which lead to increased adherence of Candida species to buccal and mucosal surfaces due to the presence of various adhesive molecules in Candida. Pallavan B et al. found that increase in the oral candidial colonization in diabetic patients as compared to non-diabetic individuals.12 Rizzi et al observed that there is a high risk of Urinary tract infections and of Genital infections in diabetic patients. Only genital infections were found to be associated with poor glycemic control.13
Kumar et al found C. albicans as the predominant species followed by C. tropicalis and C. krusei.9 This difference obtained in the isolation percentage of different Candida species may be due to the difference in distribution of Candida in various geographical areas, the time of sampling, or the use of different methods for yeast recovery.9,14-16 The predominant isolate was Candida albicans followed by Candida glabrata. Study conducted at All India Institute of Medical Sciences, New Delhi, India, in diabetic patients with vulvovaginal candidiasis showed C. glabrata as the primary species responsible for vulvovaginal candidiasis in the patients.15,16
In a study by Gomes et al Candida albicans was the most frequent isolate and had higher virulence in diabetic patient.17 In a study by Pandey et al.18 in which Extracellular hydrolytic enzyme activities of the different Candida spp. isolated from the blood of the Intensive Care Unit- patients was determined, hemolysin activity was shown by 89.86% of the isolated Candida spp., whereas other extracellular enzymes such as proteinase, phospholipase, and esterase were produced only by 84.72%, 55.69%, and 37.97% of the strains, respectively was produced.18
Proteinase production was predominantly seen in Candida krusei followed by Candida albicans, Candida glabrata & Candida parapsilosis. Pandey et al.18 found that strong proteinase activity was shown by the C. albicans (93.75%), followed by the C. tropicalis (87.5%).19
Esterase production was predominantly seen in Candida glabrata 50% (3/6) followed by Candida albicans 33% (2/6), Candida krusei 17% (1/6). Pandey et al found that 56% C. albicans showed esterase activity and 54% C. tropicalis showed the esterase activity.18 Similarly, Kumar et al. reported C. albicans and C. tropicalis species with the highest rate of esterase activity.19 No activity was observed in the isolated strains of the C. glabrata and C. krusei, which are considered as the intrinsic fluconazole resistant. Fatahinia et al., reported esterase activities of higher level in C. glabrata isolated from diabetic patients having oral candidiasis.20
Hemolysin was produced by one strain of Candida glabrata and one strain of Candida parapsilosis Pandey et al.18 observed that hemolysin activity was shown by 95.8% of C. tropicalis and 90.9% strains of the C. krusei strains. Only 87.5% strain of C. albicans produced hemolysin activity. Hemolysin and proteinase enzymes were produced by >80% of the isolated Candida spp. Similar findings of higher hemolysin production have been reported by Riceto et al.21 Present study demonstrated higher production of proteinase which was similar to the findings by Pandey et al.18 and Riceto et al.21 However, hemolysin was produced only by one strain of Candida glabrata and one strain of Candida parapsilosis.
However, phospholipase was produced by only one strain of Candida glabrata. Earlier studies also show that Candida albicans and Candida glabrata represent the two most commonly isolated Candida species worldwide.22,23
The difference in production of virulence factors obtained may be due to intra- and interspecies differences in the virulence of Candida species strains.24,25 Genetic variation in the ribosomal DNA (rDNA) internal transcribed spacer (ITS) region has been studied among fungi.26 This may also be responsible for the difference obtained in the production of virulence factors within different species and for the difference in present study from other studies.
The isolates of C. albicans are the most virulent and produce the most extracellular virulence factors; The expression of proteases, hemolysins, and esterases is correlated with the virulence of Candida isolates.27
The expression of virulence factors among Candida species, may vary depending on the infecting species, geographical origin, type of infection, the site and stage of infection, and host reaction.28 Studies like this can explore the colonizing newer emerging Candida species for example C. auris which is resistant to many antifungals can be identified. The study is useful to know the presence or absence of virulence factors in colonized strains of Candida. This becomes extremely important when patient condition is deteriorated, & Candida is isolated from the colonized site the dissemination of isolated colonized strain may occur which may be fatal. The immunostimulatory factors that the virulence factors offer stimulate dendric cell activation, T cell infiltration, and activation. The risk of resistance developing in Candida infections can be decreased by focusing on virulence factors.29 Pawar et al detected PLB gene in 97.3% C. albicans isolates.30
The study concluded that Candida albicans is the predominant species causing colonization followed by Candida glabrata. The candida isolates showed production of proteinase predominantly followed by esterase. If the patient is colonized with intrinsically drug resistant Candida species like C. auris or C. glabrata, it will help to take preventive measures accordingly, when patients get admitted. Testing of virulence factors may help to know its potential role in invasiveness of Candida.
ACKNOWLEDGMENTS
The authors would like to thank Technical Staff of Department of Microbiology, AIIMS, Raipur, India for the technical help to carry out this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by the Indian Council of Medical Research, New Delhi, India under ICMR short term studentship project for the year 2019-2020.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hazen KC, Howell SA. Candida, Cryptococcus, and other yeasts of medical importance. In Manual of Clinical Microbiology, 8th edition. Eds, PR Murray, EJ Baron, JH Jorgensen, MA Pfaller, & RH Yolken. Washington, DC: ASM Press, 2003: pp. 1693–1711.
- Reiss E.Fundamentals Of Medical Mycology, 1 sted, Wiley-Blakwell. 2012.
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 201315;4(2):119-28.
Crossref - Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. Infections in Patients with Diabetes Mellitus. J Clin Med. 2019;8(1):76.
Crossref - Manns JM, Mosser DM, Buckley HR. Production of a hemolytic factor by Candida albicans. Infect Immun. 1994;62(11):5154-5156.
Crossref - Yenisehirli G, Bulut Y, Tuncoglu E. Phospholipase, proteinase and hemolytic activities of Candida albicans isolates obtained from clinical specimens. Mikrobiyol Bul. 2010;44(1):71-7. Turkish. PMID: 20455401.
- Khedidja B, Abderrahman L. Selection of orlistat as a potential inhibitor for lipase from Candida species. Bioinformation. 2011;7(3):125-129.
Crossref - Trofa D, Agovino M, Stehr F, et al. Acetylsalicylic acid (aspirin) reduces damage to reconstituted human tissues infected with Candida species by inhibiting extracellular fungal lipases. Microbes Infect. 2009;11:1131-1139.
Crossref - Deepa A, Kumar, Muralidhar S, et al. Species Diversity, Antifungal Susceptibility, and Virulence Attributes of Candida Colonising the Oral Cavities of Adult Diabetic Patients. J Mycol. 2014;2014:395041.
Crossref - Khazal FA, Mahan A, Al-Hasnawi HH. Oral carriage rate of Candida species indiabetic patients. Kindy Col Med J. 2006;.3 (1):8-11.
- Lotfi-Kamran MH, Jafari AA, Falah-Tafti A, Tavakoli A, Zadeh MHF. Candida colonisation on the denture of diabetic and non-diabetic patients. Dental Research Journal 2009;6(1):23-27.
- Pallavan B, Ramesh V, Dhanasekaran BP, Oza N, Indu S, Govindarajan V. Comparison and correlation of candidal colonization in diabetic patients and normal individuals. J Diabetes Metab Disord. 2014;13:66.
Crossref - Rizzi M, Trevisan R. Genitourinary infections in diabetic patients in the new era of diabetes therapy with sodium-glucose cotransporter-2 inhibitors. Nutr Metab Cardiovasc Dis. 2016;26(11):963-970.
Crossref - Al-Attas SA, Amro SO. Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Ann Saudi Med. 2010;30(2):101-108.
Crossref - Goswami R, Dadhwal V, Tejaswi S, et al. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic status. J Infect. 2000;41(2):162-166.
Crossref - Goswami D, Goswami R, Banerjee U, et al. Pattern of Candida species isolated from patients with diabetes mellitus and vulvovaginal candidiasis and their response to single dose oral fluconazole therapy. J Infect. 2006;52(2):111-117.
Crossref - Gomes CC, Guimaraes LS, Pinto LCC, Camargo GADCG, Valente MIB, Sarquis MIM. Investigations of the prevalence and virulence of Candida albicans in periodontal and endodontic lesions in diabetic and normoglycemic patients. J Appl Oral Sci. 2017;25(3):274-281.
Crossref - Pandey N, Gupta MK, Tilak R. Extracellular hydrolytic enzyme activities of the different Candida spp. isolated from the blood of the Intensive Care Unit-admitted patients. J Lab Physicians. 2018;10(4):392-396.
Crossref - Kumar CPG, Menon T, Sundararajan T, et al. Esterase activity of Candida species isolated from immunocompromised hosts. Rev Iberoam Micol. 2006;23(2):101-103.
Crossref - Fatahinia M, Poormohamadi F, Zarei Mahmoudabadi A. Comparative study of esterase and hemolytic activities in clinically important Candida species, isolated from oral cavity of diabetic and non-diabetic individuals. Jundishapur J Microbiol. 2015;8:e20893.
Crossref - Riceto EB, Menezes Rde P, Penatti MP, Pedroso Rdos S. Enzymatic and hemolytic activity in different Candida species. Rev Iberoam Micol. 2015;32(2):79-82.
Crossref - Azie N, Neofytos D, Pfaller M, Meier-Kriesche H-U, Quan S-P, Horn D. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73(4):293-300.
Crossref - Khatib R, Johnson LB, Fakih MG, Riederer K, Briski L. Current trends in candidemia and species distribution among adults: Candida glabrata surpasses C. albicans in diabetic patients and abdominal sources. Mycoses. 2016;59(12):781-786.
Crossref - Marcos-Zambrano LJ, Bordallo-Cardona MA, Borghi E, et al. Candida isolates causing candidemia show different degrees of virulence in Galleria mellonella. Med Mycol. 2020;58(1):83-92.
Crossref - Merseguel KB, Nishikaku AS, Rodrigues AM, et al. Genetic diversity of medically important and emerging Candida species causing invasive infection. BMC Infect Dis. 2015;15:57.
Crossref - Mroczynska M, Brillowska-Dabrowska A. Virulence of Clinical Candida Isolates. Pathogens. 2021;10(4):466.
Crossref - Czechowicz P, Nowicka J, Gosciniak G. Virulence Factors of Candida spp. and Host Immune Response Important in the Pathogenesis of Vulvovaginal Candidiasis. Int J Mol Sci. 2022;23(11):5895.
Crossref - Deorukhkar SC, Saini S, Mathew S. Virulence Factors Contributing to Pathogenicity of Candida tropicalis and Its Antifungal Susceptibility Profile. Int J Microbiol. 2014;2014:456878.
Crossref - Staniszewska M. Virulence Factors in Candida species. Curr Protein Pept Sci. 2020;21(3):313-323.
Crossref - Pawar MY, Hatolkar SM, Misra RN. Phenotypic and molecular detection of virulence factor genes SAP4 and PLB in Candida albicans isolates from the Western part of India. Medical Journal Armed Forces India. 2022;78:3:271-276.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.