ISSN: 0973-7510
E-ISSN: 2581-690X
The current interest of scientific study aims at survival mechanisms of the cyanobacteria on the extreme habitats (i.e. building facades and monuments) growing under adverse conditions. The present investigation points towards finding out indigenous which are tolerant of conflicting environmental conditions, such as pH, temperature and calcium carbonate. Three isolates of cyanobacteria Scytonema coactile, Scytonema geitleri and Lyngbya aerugineo–coerulea from a cave, building façade, and temple, Orissa respectively were examined. Tolerance to stress at different pH and temperature were evaluated by quantifying cyanobacteria growth at different time intervals. Tolerance to CaCO3 was studied by subjecting the isolates to the desired concentration 0.0001 – 1% w/v. Each organism was grown for 15 days at 25°C ± 1°C under continuous light intensity (7.5 W/m2) and then harvested, succeeded by SDS gel-electrophoresis protein analysis. Results revealed that three isolated cyanobacteria species from different sub-aerial habitats responded in a specific manner to different stress conditions and to various concentration of CaCO3 concerning protein synthesis. A 30 and 38 kDa protein was overproduced by all isolates under pH and temperature stress, whereas for CaCO3 stress, the protein of 16 and 22 kDa was overproduced by Lyngbya aerugineo–coerulea respectively which concluded that the survival of the isolates under stress conditions depends on specific protein synthesis. Generally, isolates tolerant to different stress may be due to specific protein synthesis for their survival to extreme habitats.
Cyanobacteria, environmental stress, extreme habitats, tolerance, adaptation (CaCO3– Calcium Carbonate)
Very few studies focus on phototrophic biofilms which are the important colonizers in a wide range of extreme environments including soils, rocks, modern and ancient building facades (Gaylarde et al., 2001; Garcia-Pichel et al., 2001; Hoffmann, 2002). Such Biofilms occurring as greenish, blackish or blackish-brown mat are mostly dominated by oxygenic phototrophic Cyanobacteria, particularly sheath forming ones, and produce extracellular polymeric substances (EPS) (Wimpenny et al., 2000; Jiao et al., 2010) suiting to the environmental conditions prevailing on the surfaces (Saarela et al., 2004). Several cyanobacteria forming such phototrophic mats have been found on the building facades and monuments in different locations of eastern India exposed to intense solar radiation and extreme dryness. In such habitats, the organisms receive water from leakage area, roof guttering, and inadequate drainage of flat areas or adjacent water courses. During the rainy season, the average value of rainfall in Orissa state, on the east coast of India is 100mm to 330mm between mid months June to August followed by a long dry season of winter. The air temperature ranges from 15°C to 28°C between September to January and summer air temperature ranges from 28°C to 45°C between February to mid-June. In such a situation, they have to withstand not only long periods of matrix water stress but also extremes of prolonged desiccation, high temperature, and solar radiation. Reports are supporting that several microorganisms, biofilms, and microbial mats growing on and within different substratum, composed of K, Na, Ca, Fe, Ag, Pb, and P contribute to the selective bioleaching of the component material. It has also been found out that precipitation of calcium salt by cyanobacterial cells growing on limestone and migration of calcium from and to neighboring sites is an important mechanism in stone degradation by these organisms (Schultze-Lam and Beveridge, 1994; Ascaso et al., 1998; Ortega-Morales et al., 2000). They deposit CaCO3 in the presence of light and solubilize it at night due to a change in carbonate concentrations. Such mobilization of calcium ions and the trapping of released particles of calcite in the gelatinous sheath of cyanobacterial cells lead to degradation of calcareous stone (Pentecost, 1988; Warschied, 1991; Rossi et al., 2002; Crispim et al., 2006).Hence studying the metabolic activities of such organisms to various environmental regimes, e.g. pH and temperature prevailing on the facades, would throw light on their survival mechanism under extreme environmental conditions.
In the present study, the effect of stress, pH, and the temperature was evaluated on three cyanobacteria species isolated from three different habitats. To find out the cyanobacterial species those were associated on the building facades and stone monuments, the present investigation was also undertaken to examine growth behavior of three cyanobacteria species Scytonema coactile, Scytonema geitleri and Lyngbya aerugineo–coerulea isolated from sub-aerial habitats to various concentrations of Calcium carbonate commonly found in various concentrations on the lime-washed building facades. In addition to these, the further objective was to find out the protein profile of each organism in the control condition and the change, if any, that occur when they are exposed to different environmental variables especially looking at the protein profile which was repressed, induced or overproduced under the stress conditions.
Cyanobacterial isolates
Microbial crusts were collected from a blackish-brown crust on the limestone surface in the twilight zone of Gupteswar cave, Odisha; from the blackish crust on the lime-washed building facade of Utkal University campus, Bhubaneswar and the brownish crust on the stone surface of Bhaskareswar temple, Bhubaneswar, Odisha
(Fig. 8a-c).Scrapped crust samples were collected and stored in pre-sterilized screwcapped Tarson bottles and transported to the laboratory for analysis. The samples were wetted with sterile water for 12 h and examined under the light microscope. A pinch of the pre-wetted crust was transferred to the BG-11 medium (Rippka et al., 1979) with or without combined nitrogen and to agarplates (1.2 % w/v) and incubated at 25 ± 1°C under illumination with continuous light from fluorescent tubes at an intensity of 7.5 W/m2. The organisms appearing in the plates were isolated, made axenic with repeated sub-culturing following Venkataraman (1969), and were identified using the monographs (Desikachary, 1959; Komarek and Hauer, 2015). The organisms at their exponential growth phase were used in all the experiments. Three cyanobacteria species Scytonema coactile, isolated from the blackish-brown crust on the limestone surface in the twilight zone of Gupteswar cave, Odisha; Scytonema geitleri, from the blackish crust on the lime-washed building facade of Utkal University campus, Bhubaneswar and Lyngbya aerugineo–coerulea, from the brownish crust on the stone surface of Bhaskareswar temple, Bhubaneswar, Odisha were isolated and used in the experiments to understand their metabolic activity under various environmental variables. Growth, chlorophyll a and carotenoid (Mackinney, 1941; Jessen, 1978), cell-protein (Lowry et al., 1951) and extracellular carbohydrate content (Herbert et al., 1971) and changes in the protein profile of the organisms in response to the environmental variables were estimated following.
Effect of pH and temperature stress in cyanobacteria growth
Tolerance of cyanobacterial isolates were evaluated by quantification of cyanobacteria growth (optical density at 750nm) in BG – 11 medium with different pH of 4, 5, 6, 7, 8, 9 and 10 respectively after 15 days of Cyanobacteria growth. Tolerance of isolates to different temperatures (35°C, 45°C, 55°C and 65°C) was also evaluated simultaneously. The control conditions were considered to be pH 7 and 25°C.
Effect of Calcium carbonate in cyanobacteria growth
Tolerance to various concentrations of Calcium carbonate was evaluated. The stock solution of Calcium carbonate was prepared with distilled water, sterilized separately from the mineral salts and added aseptically to the culture medium to obtain the desired concentration (0.0001, 0.0005, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5 and 1%, w/v). The organisms were grown up to 15 days at 25 ± 1°C under a continuous light intensity of 2000lux and then harvested.
Analysis of Protein profiling
Changes in the protein patterns induced by pH, temperature, and calcium carbonate were detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS – PAGE) analysis of total cell proteins. Cell treatment and electrophoresis were done as described by Lowry et al., 1951.
Statistical analysis
To compare tolerance of different isolates to distinct conditions, the optical density obtained for each isolate after 15 days at 25°C, pH 7 was considered as 100% growth. Data represent the average of three replicates per treatment. The mean value of 3 replicates of each experiment ± S.D has been presented.
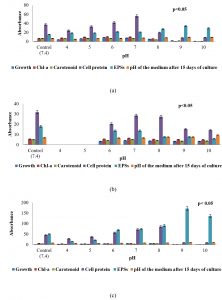
The analysis of biochemical constituents of the three selected sub-aerial Cyanobacteria expressed in µg/ml were given in (Fig. 1a-c; 2a-c; 3a-c). Increase or decrease of growth, chlorophyll-a,carotenoids, soluble cell-protein content of the cells as well as quantity of extracellular carbohydrates released by these organisms to different conditions of pH and temperature stress and in different concentration of calcium carbonate over their respective control was examined after incubating for 15 days at 25°C under 2000lux light intensity. All these three isolated, purified from the different substratum of a cave, building facades and temple from the eastern region of India is shown in (Fig. 8a-c). The results in respect to different conditions of pH and changes in pH medium after 15 days of incubation of culture which is represented in (Fig. 1a-c). S. coactile grew over a wide range of pH, mostly at acidic range from 4 to 7 whereas on other hand, two species isolates S. geitleri and Lyngbya aerugineo–coerulea from exteriors of building and temple showed their maximum growth at pH 7 or 8, but pH above 9 was found to be detrimental or lethal. None of the pHs, however, encouraged the synthesis of carotenoid in its cells and the pH which was adverse to the growth triggered the release of extracellular carbohydrate into the medium. However, the chlorophyll-a and soluble cell protein content were increased significantly at pH 7. Interestingly the range of pH from 9 and 10 encouraged the release of extracellular carbohydrate significantly revealed maximum as compared to control (S. coactile and Lyngbya aerugineo-coerulea).
Fig. 1. Growth response, chlorophyll – a, carotenoid, soluble cell-protein, and extracellular polysaccharides content of three sub-aerial cyanobacteria, (a) Scytonema coactile(limestone,Gupteswar cave), (b) Scytonema geitleri (lime-washed wall, Building) and (c)Lyngbya aerugineo-coerulea(Stone carving,BhaskareswarTemple) submitted to different conditions of pH and changes in pH medium after 15 days of incubation of culture.
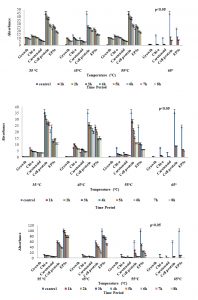
Fig. 2. Growth response, chlorophyll – a, carotenoid, soluble cell-protein, and extracellular polysaccharides content of three sub-aerial cyanobacteria, (a) Scytonema coactile (limestone,Gupteswar cave), (b) Scytonema geitleri (lime-washed wall, Building) and (c) Lyngbya aerugineo-coerulea (Stone carving, Bhaskareswar Temple) upon exposure to various temperature. Homogenized suspension of the organism was subjected to heat treatment (Temp. 35°C-65°C for up to 8h) and then cultured at 25 ± 1°C for 15 days.
Analysis of tolerance of isolates to different temperature stress, 35°C, 45°C, 55°C for 8 hours and at 65°C for 3 hours revealed that three isolates endure the 35°C – 55°C better than 65°C temperature after subsequently cultivating for 15 days at 25°C under 2000lux light intensity presented in (Fig. 2a-c). As expected for cyanobacteria (Haubner et al., 2006), most isolates grew better at 25°C. However, with the increase of duration of exposure to a higher temperature, showed significantly decrease on growth, chlorophyll-a,soluble cell protein,and extracellular carbohydrate content in all three isolates as compared to control which is depicted in (Fig. 2a-c). However, at a higher temperature beyond 35°C maybe not favorable for extracellular carbohydrate liberation by any of the test organisms.It was also found that all three isolates grew slowly with a pre-exposure to 65°C for up to 3 hours. However, prolonged incubation beyond 3 hours was detrimental to the growth of all the three experimental isolates.
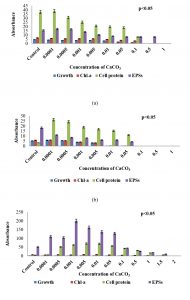
Fig. 3. Growth response, chlorophyll – a, carotenoid, soluble cell-protein, and extracellular polysaccharides content of three sub-aerial cyanobacteria, (a) Scytonema coactile (limestone, Gupteswar cave), (b) Scytonema geitleri (lime-washed wall, Building) and (c) Lyngbya aerugineo-coerulea (Stone carving, Bhaskareswar Temple) upon treatment to different concentrations of Calcium carbonate. Cultures were incubated at 25 ± 1°C under 7.5 W/m² light intensity up to 15 days.
To evaluate the stress tolerance of three isolates were exposed to different concentrations of Calcium carbonate (CaCO3) compared to control (Fig. 3a-c). Analysis results showed that upon treatment with calcium carbonate, growth, chlorophyll-a as well as cell-protein content of all the three isolates decreased and the decrease was proportionate with increasing concentration of the chemical in the medium. S. coactile tolerated up to 0.1% of CaCO3 and beyond leading to cell bleaching and lethal of the organism whereas, other isolates, S. geitleri from the lime-washed wall of the building could survive up to 0.05% of CaCO3 which was almost 10 times less in concentration which S. coactile, isolated from a cave could tolerant. However, on the other hand, the cyanobacterium L. aerugineo–coerulea from exteriors stone carving of the temple was able to tolerate and grew even in presence of 1.5% CaCO3 in the medium. It was also observed that exposure to the low concentration of CaCO3 up to 0.005% in S. coactile and up to 0.05% in the culture of L. aerugineo–coerulea showed an increased in extracellular carbohydrate content but was not observed in isolates (S. geitleri). These findings indicated that cyanobacterium occurring in the caves and on the stone carving of the temple possesses the capability to utilize the salt at low concentrations which triggered liberation EPS leading to its binding on the exposed surfaces on which they occur. Each isolates are having its uniqueness showed specific under adverse environmental stress.
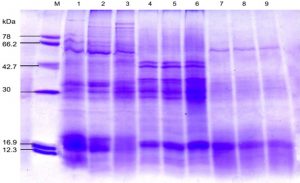
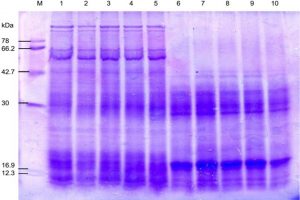
Fig. 4. SDS-PAGE of the protein profiling of three sub-aerial cyanobacteria isolates Scytonema coactile, Lane 2-3; S. geitleri, Lane 5-6and Lyngbya aerugineo-coerulea, Lane 8-9 grown at pH 6 and 9 up to 15 days. An equal amount of proteins were loaded into each well. Control (pH – 7.4), Lane 1,4 and 7; M. Molecular mass of standard proteins in kDa.
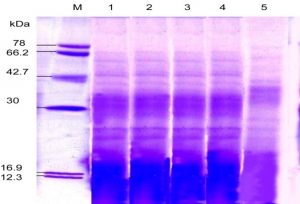
Fig. 5. SDS-PAGE of the protein profiling of three sub-aerial cyanobacteria isolates: Scytonema coactile Lane 2-5 and S.geitleri Lane 7-10 after exposure to different duration of temperature for 8 hours (35°C, 45°C, 55°C) and 3 hours (65°C), then incubated for 15 days under continuous illumination (2000 lux) at 25±2°C. An equal amount of proteins were loaded into each well. Control (25°C), Lane 1, 6; M. Molecular mass of standard proteins in kDa.
Table (1):
Changes in the protein profile of three sub-aerial cyanobacteria strains, Scytonema coactile, S. geitleri and Lyngbya aerugineo-coerulea has grown at specific pH 6 and 9 under continuous illumination (2000 lux) at 28±2°C up to 15 days.
| Organisms | a pH of the culture | Repression of protein (kDa) |
Induction of protein (kDa) |
Overproduction of protein (kDa) |
|---|---|---|---|---|
| Scytonema coactile | 6 | 18 | 30 | 30 |
| 9 | 18, 28, 38 | 48, 54, 102 | 30, 102 | |
| Scytonemageitleri | 6 | 18 | 16 | |
| 9 | 18 | 16, 28, 30, 36, 38,48, 62 | ||
| Lyngbyaaerugineo- coerulea | 6 | 38 | ||
| 9 | 18, 28, 38, 44, 58 |
Table (2):
Changes in the protein profile of three sub-aerial cyanobacteria strains, Scytonema coactile, S. geitleri and Lyngbya aerugineo-coerulea, after exposure to different duration of temperature (35°C, 45°C, 55°C) for 8 hours and 3 hours at (65°C), and then incubated under continuous illumination (2000 lux) at 28±2°C up to 15 days.
| Organisms | Growth condition (Temperature) |
Repression of protein (kDa) |
Induction of protein (kDa) |
Overproduction of protein (kDa) |
|---|---|---|---|---|
| Scytonema coactile | 35˚C | 18 | 48 | |
| 45˚C | 18, 28 | 48 | ||
| 55˚C | 22, 28, 36, 40, 78,99 | 48 | ||
| 65˚C | 12,18, 36, 40, 62,68, 78, 96, 99 | 48 | 30 | |
| S.geitleri | 35˚C | 38 | 18 | |
| 45˚C | 38 | 18, 36 | ||
| 55˚C | 38 | 16, 18, 36 | ||
| 65˚C | 16, 38 | 36 | 18, 30, 36 | |
| Lyngbyaaerugineo-coerulea | 35˚C | |||
| 45˚C | ||||
| 55˚C | ||||
| 65˚C | 18, 22, 34, 44, 58,62 | 42, 66, 78 | 38 |
Table (3):
Changes in the protein profile of three sub-aerial cyanobacteria strains, Scytonema coactile, S. geitleri and Lyngbya aerugineo-coerulea grown in presence of different concentrations of calcium carbonate (%) under continuous illumination (2000 lux) at 28±2°C up to 15 days.
| Organisms | Concentrations of calcium carbonate (%) | Repression of protein (kDa) | Induction of protein (kDa) | Overproduction of protein (kDa) |
|---|---|---|---|---|
| Scytonema coactile | 0.001 | 48, 54, 90, 96, 99 | 22 | |
| 0.01 | 8, 28, 34, 36, 38, 42, 48, 62, 68, 78, 90, 96, 112 | 22 | ||
| 0.1 | 8, 18, 28, 30, 34, 36, 38, 42, 48,54,62, 66, 68, 78, 90, 96, 99,102 | 22 | ||
| Scytonema geitleri |
0.001 | 12, 18, 62 | ||
| 0.01 | 12, 18, 34, 62 | |||
| 0.1 | 12 , 18, 30, 34, 36, 38, 40, 42,62 | |||
| Lyngbyaaerugineo-coerulea | 0.001 | 28, 34, 38, 40, 42, 58, 62 | 24 | |
| 0.1 | 28, 34, 36, 38, 42, 58, 62 | 24 | ||
| 1 | 28, 34, 36, 38, 42, 58, 62 | 24 | 16, 22 |
Further analyses were carried out based on the results concerning the effect of pH, temperature and calcium carbonate on metabolic activities; three isolates were chosen for SDS-PAGE analysis of the protein profiles. SDS-PAGE analysis of isolates generated reproducible protein profiles is represented in Table 1- 3 and Fig. 4-7. Qualitative and quantitative differences in the protein patterns of isolates were detected upon their growth at acidic/alkaline of pH 6 and 9 respectively, when compared with the control conditions (pH 7.4) were represented in (Table – 1; Fig. 4), several proteins were repressed e.g. 18 kDa proteins in all the isolates and besides low molecular weight protein-like 58, 44, 38 and 28kDa proteins in these two isolates, S. coactile and L. aerugineo–coerulea were repressed. None of the proteins were induced in S. geitleri and L. aerugineo–coerulea when cultured on acidic and alkaline pH, whereas S. coactile induce the 30kDa protein at pH 6 and 102, 54 and 48 kDa proteins at pH 9 which possibly are the key factors allowing the organism to tolerate a wide range of pH un-like the other two isolates. Both the Scytonema species also overproduced certain proteins when grown at acidic/alkaline pH. In S. coactile the low and high molecular weight proteins (102 and 30 kDa) were overproduced whereas, in S. geitleri similarly 16 kDa protein was overproduced in acidic pH including several low molecular weight proteins e.g. 48, 38, 36, 30 and 28 kDa and one chaperonin 62 kDa were overproduced when the isolates were cultured at pH 9 which possibly allowed the organism to thrive at the alkaline pH. However, the detected changes are distinct, depending on the isolate and growth conditions.
Fig. 6. SDS-PAGE of the protein profile ofsub-aerial cyanobacteria isolate: Lyngbya aerugineo-coerulea Lane 2-5 after exposure to different duration of temperature for 8 hours (35°C, 45°C, 55°C) and 3 hours (65°C), and then incubated for 15 days under continuous illumination (2000 lux) at 25±2°C. An equal amount of proteins were loaded into each well. Control (25°C), Lane 1; M. Molecular mass of standard proteins in kDa.
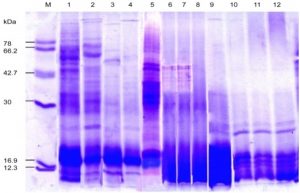
Fig. 7. SDS-PAGE of the protein profiling of threesub-aerial cyanobacteria isolates: Scytonema coactile, Lane 2-4 S. geitleri, Lane 6-8, and Lyngbya aerugineo-coerulea, Lane 10-12 grown in presence of different concentration of calcium carbonate (%) in the medium up to 15 days. An equal amount of proteins were loaded into each well. Control (Lane 1, 5, 9); Molecular mass of standard proteins in kDa.
In different isolates, proteins with the same molecular weight were overproduced under stress temperature shown in (Table – 2; Fig. 5-6). For example, isolates S. coactile, only one 30 kDa protein was overproduced and S. geitleri revealed the simultaneous overproduction of three proteins (36, 30and 18 kDa) upon exposure to higher temperatures at 65°C. However, other isolates, L. aerugineo–coerulea 38 kDa protein was overproduced which possibly confers protection and supports their important cellular functions. Some proteins were detected after submitting the three isolates to temperature stress at 35 – 65°C possibly were synthesized de novo, suggesting their importance in the survival and growth of sub-aerial cyanobacteria in stress conditions. For instance, with S. coactile,48 kDa proteins were detected or induced after exposure to a temperature at 35 – 65°C whereas, isolate S. geitleri showed a 34 kDa proteins and other three proteins (78, 66 and 42 kDa) were detected. High tolerance to temperature stress (65°C) was not related to the number of induced proteins.
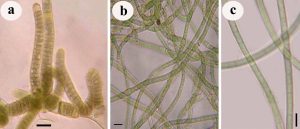
Fig. 8. Photograph showing three different sub-aerial cyanobacteria species isolates, a. Scytonema coactile (blackish-brown crust on the limestone surface in the twilight zone of Gupteswar cave), b. Scytonema geitleri (blackish crust on the lime-washed building facade) and c. Lyngbya aerugineo–coerulea (brownish crust on the stone surface of Bhaskareswar temple)from the eastern region of India.
Upon exposure to different concentrations of calcium carbonate, however, several proteins were repressed and the number increased with increasing concentration in three isolates (Table -3; Fig. 7). However, showed one 22 kDa and 24 kDa protein were selectively induced in two isolates, S. coactile as well as L. aerugineo–coerulea respectively, and in the other species, two proteins 16 kDa and 22 kDa were overproduced in presence of higher concentration of CaCO3. On the contrary, none of the proteins were overproduced in either of the Scytonema species when grown in presence of CaCO3 in the culture. These results showed that the selected strains growing under similar cultural conditions expressed a specific protein either repressed or induced or overproduced in response to a specific stressor conferring protection to the organisms to overcome the stress in terms of their growth pattern and metabolic activities.
Cyanobacteria strains isolated from exteriors surface of buildings and monuments were examined for growth and biochemical contents about pH, temperature and different concentrations of CaCO3 stress tolerance. Our results showed remarkable variation concerning these attributes amongst the three isolates studied. All the three isolates isolated from different sites did not show uniform growth responses when cultivated under defined pH regimes varying from 4 to 10. The isolates, S. geitleri from cave tolerated and grew exclusively at acidic range (pH 4 -7) and whereas another species under the same genus, S. geitleri from the lime-washed wall of building grew in near neutral range, however, survived well up to pH 9, and further only former species was capable of excreting extracellular carbohydrate which is one of the primary substances might be helping the organisms to adhere the substratum and grow under the adverse environment of the caves. On the contrary, other species, L. aerugineo–coerulea grew mostly at the neutral range, produced copious mucilaginous substances at ranging pH 8-10 which possibly suggesting that these substances help the organism to thrive on the exposed surfaces of the stone carving of the temple from where it was isolated. Further, the tendency to change the media pH towards acidic range upon growth by the two Scytonema species indicates their biodeteriogenic potential. There are reports that the pH of the culture medium always tends to change to alkaline range. Such a change in attributed to the influence of extracellular products of cyanobacteria which are composed of amino acids, polypeptides, carbohydrates, hormones and vitamins (Whitton, 1965; Singh, 1974; Adhikary and Pattnaik, 1981; Grant, 1982; Stranger-Johannessen, 1988; Paul and Rout, 2017). However, upon the growth of both the Scytonema species in culture, the pH of the medium did not change to the alkaline range, rather decreased to the acidic range after 15 days of culture and the organism continued to grow in an acidic environment. Previous studies have shown that the biocorrosion process on stones can also be established by alkaline reaction (Philippisand Vincenzini, 1998). The major damaging agents for deterioration are the microorganisms like green algae, fungi, chemo-organatrophic bacteria, cyanobacteria and litho-autotrophic bacteria found on the building walls made of concrete as well as of stones cause damage by excretion of organic acids like oxalic and glucouronic acid which degrade the carbonaceous material of the stone (Gaylarde and Morton, 1999; Krumbein and Jens, 1981; Krumbein, 1988; Marthe and Choudhury, 1975; Diereks et al., 1991). Several nitrifying microorganisms have also been found on historic sandstone buildings secrete nitric acids as metabolic end products also cause corrosion of the rocks (Bock et al., 1988; Sand, 1987).
Generally, isolates irrespective of the habitats from where they were isolated, all were sensitive to higher temperature >35°C through there exists a variation in the degree of tolerance from species to species. Even exposure to a temperature up to 55°C up to 8 hours did not destroy these organisms, as they revived upon subsequent culture showed their tolerance capacity to elevated temperature. All the three species of cyanobacteria showed healthy filaments enclosed by a distinct sheath, however, could survive and grow at a reduced rate in comparison to control might be their capacity to survive the adverse conditions of the tropics during summer months. The cyanobacterial forms belonging to genus Scytonema and Lyngbya, which forms dominant crust /mats on the exposed substratum of stone and facades of buildings, possessed well defined colored sheath layers around their trichome. Such layer has been shown to retain moisture (Bravery, 1988; Hoffmann, 1989; Wessels and Büdel, 1995) and allow the organisms to lose or gain water slowly in a controlled way from their cells which probably is an important factor in preventing damage to the cells during dehydration and/or desiccation period (Tripathi and Talpasayi, 1980; Grili-Caiola et al., 1996), which might also be a critical factor allowing these organisms to tolerate and survive after exposure to a higher temperature. Differential mechanisms of tolerance towards the stressed environment have been reported in the literature, which might be responsible for the variability observed in the parameters examined amongst the isolates.
Many of the facades of buildings and monuments showed a characteristic form of biodeterioration caused by cyanobacteria and algae on the exposed rocks, stones, and limestone surfaces. They affect the stability of the limestone matrix, and further, through chemical corrosion of the stone forming minerals such as oxidation and hydration reactions as well as dissolution of carbonates and solubilization of some elements from silicate bearing minerals, enhance the deterioration process. The constructed materials of buildings with calcareous substratum are usually found covered by biofilms. In such environments, the physical parameters, among which the more important are high levels of humidity and light intensity, promote the growth of some species of sub-aerial calciphytic cyanobacteria that can precipitate calcium carbonate forming extracellular encrustations. Previous studies have reported that precipitation of extra-cellular calcium carbonate is a common phenomenon due to algal growth in caves and therefore it represents a serious threat to these structures due to calcium deposition (Ortega-Calvo et al., 1995, Pitrini and Ricci, 1993). Another reason is most of the building walls and even ancient stone monuments are periodically washed by lime to clear the greenish/blackish mats temporarily from the exposed surfaces. Some evidence suggested that speleothem carbonate formation comprised mineralization of microbes in their cells and sheath possible on the exposed surfaces of stone sculptured monuments (Baskar et al., 2007).
It has been documented that most organisms including cyanobacteria and algae investigated to date respond to stress by synthesizing a new set of proteins (Bhagawat and Apte, 1989; Adhikary, 2003; Ahikary et al., 2013). The results obtained in this work showed that three different species of cyanobacteria S. coactile, S. geitleri and L. aerugineo–coerulea isolated from different sub-aerial habitats responded similar pattern to pH and temperature stress, and when exposed to various concentrations of CaCO3 concerning protein synthesis. Upon subjected to either of these stressors, specific proteins were repressed, induced, or overproduced in the cells conferring protection to the organism to cope with the stressor. When a protein is repressed, it is assumed that the organism is susceptible to injury. Similarly, when any protein is overproduced or induced it might be enabling the organism to tolerate stress, adapt, and grow normally performing usual physiological functions. Despite the report on the occurrence of stress proteins very little is known about their precise physiological function. Specific stress polypeptides were detected in Phormidium autumnale and Chroococcidiopsis sp. when exposed to drought stress. Also when exposed to salt stress polypeptide of similar size were induced suggesting that both salt stress and drought stress cause similar response (Hershkovitz et al., 1991). In Synechococus sp. PCC 6301, several proteins with an apparent molecular weight of 91, 79, 74, 65, 61, 49, 45, 24, 22, 18, 16, 14, and 12 kDa were affected by temperature shift (Barbelyet al., 1985). Similar repression of several proteins was observed in the experimental organisms isolated from the twilight zone of a cave, building facades and surface of a stone monument in response to various environmental variables. Also, induction and accumulation of proteins have been demonstrated to be closely correlated with the development of stress tolerance (Potts, 1994). However, might be changed in protein expression contributes to the survival process. Changes in the expression of a large number of proteins occur due to a particular stress, yet only some of these proteins are probably involved in stress tolerance. It may also be possible that the synthesis of a protein indicates sensitivity to a stressor rather than being a part of the tolerance mechanism. In general, the results of the present work indicate that protein synthesis and modification in all these experimental organisms from three different sub-aerial habitats is an adaptive behavior so far as they can tolerate a specific pH level, temperature, or calcium carbonate concentration for their survival.
The present study focused on the survival strategy of cyanobacteria which mainly depends on the nature of the substratum and environment. These factors allow the micro-organisms to form sub-aerial biofilms on extreme habitats. This leads to the characterization of species on different habitats based on ecological requirements. Moreover, it was observed that the most representative genera occurring in unfavorable environments develop different strategies to survive in such conditions due to the production of sheath composed of extracellular polymeric substances as a protection against desiccation, ensuring the maintenance of moisture by balancing changes in humidity and temperature which permits cyanobacteria to resists drought periods. Due to the presence of these colloidal polymeric substances, the cyanobacteria biofilms cause various changes in the substratum by biogeophysical and biogeochemical process and leads to biodeterioration.
ACKNOWLEDGMENTS
The authors thank the Department of Science and Technology, Government of Odisha for funding support through the Research grant to LKS, Department of Botany, College of Basic Science and Humanities, Odisha University of Agriculture and Technology and Dept. of Biotechnology, Gangadhar Meher University for providing laboratory facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both the authors have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
Funding was received from Department of Science and Technology, Govt. of Odisha of vide letter No-202827.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Adhikary SP, Sethi SK, Sahu JK. Recovery of Chlorophyll-a pigment and nitrogenase activity of different population sizes of Cyanobacteria after exposure to commercial fertilizers. In: Microbiology Applications, Rath, C.C. Har Krishan Bhalla & Sons (Ed.), New Delhi, India. 2013:365-379.
- Adhikary SP, Pattanaik H. Effect of organic carbon sources on the liberation of extracellular amino acid by nitrogen fixing blue green alga, Westiellopsis prolifica Janet in light and dark. Indian J Bot. 1981;4:60-69.
- Adhikary SP. Heat shock proteins in the terrestrial epilithiccyanobacterium Tolypothrix byssoidea. Biol Plant. 2003;47:125-128.
Crossref - Ascasco C, Wiezchos J, Castello R. Study of the biogenic weathering of calcareous litharenite stones caused by lichen and endolithic microorganisms. Int Biodeterior Biodegrad. 1998;42:29-38.
Crossref - Barbely G, Suranyi G, Korcz A, Palifi Z. Effect of heat shock on protein synthesis in the cyanobacterium Synechococcus sp. strain PCC 6301. J Bacteriol. 1985;161:1125-1130.
Crossref - Baskar S, Baskar R, Kaushik A. Evidences for microbial involvement in the genesis of speleothem carbonates, Borra caves, Visakhapatnam, India. Curr Sci. 2007;92:350-355.
- Bhagawat AA, Apte SK. Comparative analysis of proteins induced by heat shock, salinity and osmosis stress in the nitrogen fixing cyanobacteriumAnabaena sp. strain L-31. J Bact. 1989;171(9):5187-5189.
Crossref - Bock E, Sand W, Meincke M, et al. Biologically induced corrosion of natural stones-strong contamination of monuments with nitrifying organisms. In: Biodeterioration. Houghton DR, Smith RN, Eggins HOW. [Eds.]: Elsevier Applied Science, New York and London, 1988:436 – 440.
Crossref - Bravery AF. Biodeterioration of paint – a state of the art review. In: Biodeterioration 7. Houghton DR, Smith RN, Eggins HOW. [Eds.] Elsevier. 1988.
Crossref - Crispim CA, Gaylarde PM, Gaylarde CC, Neilan BA. Deteriogenic cyanobacteria on historic buildings in Brazil detected by culture and molecular techniques.Int Biodeterior Biodegrad. 2006;57(4):239-243.
Crossref - Desikachary TV. Cyanophyta: Indian Council of Agricultural Research, New Delhi. 1959:686.
- Diereks M, Sand W, Bock E. Microbial corrosion of concrete. Experentia. 1991;47:514-516.
Crossref - Rossi F, Micheletti E, Bruno L, Adhikary SP, Albertano P, De Philippis R. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling. 2012;28:215-224.
Crossref - Garcia-Pichel F, Lopez-Cortes A, Nubel U. Phylogenetic and morphological diversity in cyanobacteria in soil deserts crusts from the Colorado Plateau. Appl Environ Microbiol. 2001;67:1902-1910.
Crossref - Gaylarde CC, Morton LHG. Deteriogenic biofilms on buildings and their control: a review. Biofouling. 1999;14:59-74.
Crossref - Gaylarde PM, Gaylarde CC, Guiamet PS, Gomez de Saravia SG, Videla HA. Biodeterioration of Mayan building at Uxmal and Tulum, Mexico. Biofouling. 2001;17:41-45.
Crossref - Grant C. Fouling of terrestrial substrates by algae and implications for control- a review. Int Biodeterior Bull. 1982;18:57-65.
- Grilli-Caiola M, Billi D, Friedman EI. Effect of desiccation on envelopes of the cyanobacterium Chroococcidiopsis sp. (Chroococcales). Eur J Phycol. 1996;31:97-105.
Crossref - Haubner N, Schumann R, Karsten U. Aeroterrestrial microalgae growing in biofilms on facades-Response to temperature and water stress. Microbiol Ecology. 2006;51:285-293.
Crossref - Hershkovitz N, Oren A, Post A, Cohen Y. Induction of water stress proteins in cyanobacteria exposed to matric or somotic water stress. FEMS Microbiol Lett. 1991;83:169-172.
- Herbert D, Phipps PJ, Strange RE. Chemical Analysis of Microbial Cells. In: Methods In Microbiology Vol. (V) B (Ed.) Morris Jr, Ribbons Dw. New York: Academic Press. 1971;209-344.
Crossref - Hoffmann L. Algae of terrestrial habitats.Botanical Rev. 1989;55:77-105.
Crossref - Hoffmann L. Caves and other low-light environments: aerophitic photoautotrophic microorganisms. In: Encyclopedia of environmental microbiology. Bitton, G. [Eds.]: JohnWileyand Sons, New York. 2002;835-843.
Crossref - Jenssen A. Chlorophyll and carotenoid. In: Handbook of phycological methods. Physiological and biochemical methods. Hellebust JA, Craigie JS [Eds.]: Cambridge University press, Cambridge, UK. 1978;59-70.
- Jiao Y, Cody GD, Harding AK, et al. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl Environ Microbiol. 2010;76(9):2916-2922.
Crossref - Komarek J, Hauer T. CyanoDB.cz-On-line database of cyanobacterialgenera.World-wide electronic publication, University of South Bohemia and Institute of Botany. 2015.
- Krumbein WE, Jens K. Biogenic rock varnishes of the Negev desert (Israel), an ecological study of iron and manganese transformation by cyanobacteria and fungi. Oecologia. 1981;50:25-38.
Crossref - Krumbein WE. Biotrasformation in monuments – a sociobiological study. Durability of building Materials. 1988;5:359-382.
- Lowry OH, Rosenbrough NT, Farr AL, Randall RJ.Protein measurement with Folin-Phenol reagent. J Biol Chem. 1951;193:265-275.
- Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315-322.
- Marathe KV, Chaudhari PR. An example of algae as pioneers in the lithosphere and their role in rock corrosion. J Ecology. 1975;63:65-69.
Crossref - Ortega-Calvo JJ, Arino X, Hernandez-Marine M, Saiz-Jimenez C. Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci Total Environ. 1995;167:329-341.
Crossref - Ortega-Morales BO, Gaylarde CC, Englert GE, Gaylarde PM. Analysis of salt – containing biofilms on limestone buildings of the Mayan culture at Edzna, Mexico. Geomicrobiol J. 2005;22:261-268.
Crossref - Pentecost A. Growth and calcification of the cyanobacteriumHomoeothrix crustacean. J Gen Microbiol. 1988;134:2665-2671.
Crossref - Pereira S, Zille A, Micheletti E, Moradas-Ferreira P, De Philippis R, Tamagnini P. Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. 2009;33:917-941.
Crossref - Philippis RD, Vincenzini M. Extracellular polysaccharides from cyanobacteria and their possible applications.FEMS Microbiology Reviews. 1998;22:151-175.
Crossref - Pietrini AM, Ricci S. Occurrence of a calcareous blue green alga, Scytonemajulianum (Kutz.)Meneghini, on the frescoes of a church carved from the rock in Matera, Italy. Cryptogamic Botany. 1993;3:290-295.
- Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755-805.
Crossref - Rippka R, Deruelles J, Waterbury JB, Herdman M, Stainer RY. Generic assignments, strains histories and properties of pure cultures of cyanobacteria. JGen Microbiol. 1979;111:1-61.
Crossref - Saarela M, Alakomi HL, Siuhko ML, Maunuksela L, Raaska L, Mattila-Sandholm T. Heterotrophic microorganisms in air and biofilm samples from roman catacomb, with special emphasis on actinobacteria and fungi. Int Biodeterior Biodegrad. 2004;54:27-37.
Crossref - Sand W. Importance of hydrogen sulfide, thiosulfate and methyl mercaptan for growth of thiobacilli during simulation of concrete corrosion. Appl Environ Microbiol. 1987;53:1645-1648.
Crossref - Schultze-Lam S, Beveridge TJ. Physicochemical characteristics of the mineral forming S-layer from the cyanobacterium Synechococcus strain GL 24. Appl Environ Microbiol. 1994;60:447-453.
- Sethi SK, Samad LK, Adhikary SP. Cyanobacteria and micro-algae in biological crusts on soil and sub-aerial habitats of eastern and north-eastern region of India. Phykos. 2012;42(1):1-9.
- Singh PK. Effect of pH on growth and nitrogen fixation in Aphanothece (Cyanophyta). Oikos. 1974;25:114-116.
Crossref - Stranger-Johannessen M. The anti-microbial effects of pigments in corrosion protective paints. In: Biodeterioration 7. Houghton DR, Smith RN, Eggins HOW [Eds.]: Elsevier, London. 1988:372 – 377.
Crossref - Swain N, Adhikary SP. Dependence of growth of Microcystisaeruginosa (Kutz. Emend. Elenkin) on pH of culture medium. J Freshwater Biol. 1991;3:131-137.
- Tripathi SN, Talpasayi ERS. Sulphydryls and survival of sub-aerial blue green algae. Curr Sci. 1980;49:31-32.
- Venkataraman GS. The cultivation of algae.Indian Council of Agricultural Research, New Delhi. 1969:319.
- Warscheid T, Oelting M, Krumbein WE. Physico-chemical aspects of biodeterioration process on rocks with special regard to organic pollutants. Int Biodeterior Bull. 1991;28:37-48.
Crossref - Wessels DCJ, Budel B. Epilithic and cryptoendolithic cyanobacteria of clarens sandstone cliffs in the Golden gate highlands natural park, South Africa. Bot Acta. 1995;108:220-226.
Crossref - Whitton BA. Extracellular products of blue green algae. J Gen Microbiol. 1965;40:1-11.
Crossref - Wimpenny J, Manz W, Szewzyk U. Heterogeneity in biofilms. FEMS Microbiol Rev. 2000;24:661-671.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.