ISSN: 0973-7510
E-ISSN: 2581-690X
Burns are a significant problem with high associated morbidity and mortality worldwide. Bacteremia is a serious complication that is important in increasing the overall fatality rate in burn injury patients. In this study, we report a case of Streptococcus mitis and Klebsiella pneumoniae mixed infection in severe burns in a 40-year-old female. The patient was transferred from an outside hospital to the emergency unit after burn injuries in a gas explosion accident at home. The patient underwent surgical debridement and antibiotics treatment but died due to sepsis and multiorgan failure. Our case study will help to underscore the important role of Streptococcus mitis and Klebsiella pneumoniae as human opportunistic pathogens in severe burn injury patients.
Burns, Infection, Streptococcus mitis, Klebsiella pneumoniae
Burn injuries are a global health problem. World Health Organization estimated 180,000 deaths every year are caused by burns; The majority occur in low-middle-income countries.1 Burns occur mainly at home and the workplace. Infections pose a significant challenge in healthcare settings, leading to prolonged hospital stays, increased morbidity, and even mortality among burn patients. Whether caused by thermal, chemical, electrical, or radiation sources, burns compromise the skin’s natural barrier function, rendering it susceptible to microbial invasion. The ensuing infection can impede wound healing, exacerbate tissue damage, and result in systemic complications, underscoring the critical need for a comprehensive understanding of the underlying mechanisms and effective management strategies.
Bacteremia represents a formidable complication in the management of severe burn patients, precipitating a cascade of systemic inflammatory responses and often culminating in grave clinical outcomes. Burn injuries inflict profound physiological derangements, compromising the skin’s barrier function and creating a nidus for microbial colonization and invasion.2 Consequently, the bloodstream becomes a conduit for disseminating pathogens, fueling the development of bacteremia and associated septic complications.
Clinically, bacteremia in severe burn patients heralds a perilous course characterized by hemodynamic instability, end-organ dysfunction, and an increased risk of mortality.3 Prompt recognition and management of bacteremia are imperative to mitigate its harmful consequences and improve patient outcomes. However, diagnosing bacteremia in the context of burn injuries poses unique challenges, given the confounding effects of burn-related inflammation on traditional diagnostic parameters such as fever, leukocytosis, and inflammatory markers.4 Hereby, we presented the Streptococcus mitis and Klebsiella pneumoniae mixed infection in severe burns patient.
Case Presentation
A female, 40 years old, was transferred from an outside hospital to the emergency department Dr. Soetomo General Hospital due to a gas explosion at home 6 hours before admission. The patient had been resuscitated with RL fluid 3500 cc in the prior hospital. On admission, she was conscious, and she had been diagnosed with severe burns (56%), superficial to dermal full thickness in the face, neck, anteroposterior torso, bilateral arms, and bilateral legs. Her blood pressure was 122/64 mmHg, with a heart rate of 128 beats/minute and a respiratory rate of 22 times/minute using nasal oxygen (4 liter/minute). The patient was intubated due to inhalation trauma. In addition, she underwent excisional debridement and wound preparation procedures. Nutritional supplements were provided through the feeding tube.
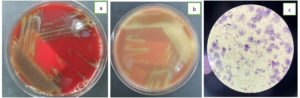
The blood, urine, and wound superficial pus samples had been sent to the clinical microbiology laboratory for culture examination. The cultures from all samples were sterile. On day 6, the patient was febrile. The blood examination showed leukocytes at 16,400, Procalcitonin was 4.57, and CRP was 14.92. The aspirate sputum from the endotracheal tube, the blood from both sides and the tissue from wound debridement were sent to the clinical microbiology laboratory for culture examination. The blood specimens were cultured in BACTEC (BD, USA) and showed positive result in less than 24 hours. Then, the specimens from BACTEC (BD, USA) were subcultured to the blood agar, chocolate agar, and MacConkey agar. The blood and chocolate agar showed alpha-hemolytic colonies (Figure 1a and 1b), and there was no bacteria growth on the MacConkey agar. The bacterial Gram staining showed Gram-Positive cocci (Figure 1c). VITEK II (BioMerieux, Hazelwood, MO, USA) was used for microorganism isolation and antibiotic susceptibility test. The aspirate sputum culture showed Klebsiella pneumoniae, the blood cultures drawn from the right and left sides showed Streptococcus mitis and the wound tissue culture was sterile. The patient was treated with Cefuroxim 750 mg every 8 hours intravenous. On day 12, the patient died due to sepsis and multiorgan failures.
Severe burns inflict extensive tissue damage, prompting the release of proinflammatory cytokines, damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs), collectively contributing to the dysregulated host immune response observed in burn patients.2 This immunocompromised state, compounded by factors such as impaired neutrophil function, complement depletion, and disruption of mucocutaneous defenses, predisposes burn patients to opportunistic infections, including bacteremia.5
The pathogenesis of bacteremia in severe burn patients is multifaceted, encompassing a spectrum of microbial etiologies ranging from common skin commensals to nosocomial pathogens in healthcare environments. Burn wound colonization is a primary reservoir for these pathogens, facilitating their hematogenous dissemination and seeding distant sites, thereby perpetuating a cycle of systemic infection and organ dysfunction.6
Streptococcus mitis, a part of the Viridans Group Streptococci (VGS), is commonly found as part of the oral microbiota in humans. On sheep blood agar, Streptococcus mitis is alpha-hemolytic. While it is typically considered a commensal bacterium and rare-causing disease, it can act as an opportunistic pathogen under certain conditions, particularly in individuals with compromised immune systems or in specific host environments such as burn wounds.7 In this patient, Streptococcus mitis could enter the blood system due to burn injuries and mucosal lesions from the oral cavity.
Streptococcus mitis may contribute to pathogenicity in burn infections through various virulence factors, including adhesion and biofilm formation, extracellular enzymes, toxin production, and immunomodulatory factors.7 Streptococcus mitis possesses surface adhesins that enable it to adhere to host tissues and form biofilms. Biofilm formation on burn wounds can promote bacterial colonization, enhance resistance to host immune defenses, and facilitate persistent infection. The virulence factors like IgA1 protease and zinc metalloprotease C, neuraminidases A and B, autolysin, pneumolysin, several choline-binding proteins, and PavA were present in some strains of Streptococcus mitis.8 These enzymes may degrade host proteins, disrupt tissue integrity, and promote the spread of infection within the burn wound and to surrounding tissues.
Some strains of Streptococcus mitis can produce toxins such as hemolysins, which can lyse host cells and contribute to tissue necrosis. These toxins may exacerbate tissue damage, impair wound healing, and promote the systemic spread of infection, leading to septic complications in burn patients. Streptococcus mitis cell surface consists of a lipid bilayer, cell wall peptidoglycan (PG), cell wall-associated teichoic acids (WTA), membrane-associated lipoteichoic acids (LTA), and a variety of proteins that can modulate host immune responses.9 Based on the bioinformatic study, most Streptococcus mitis strains should contain the same type IV LTA as Streptococcus pneumoniae.10 In addition, some strains of Streptococcus mitis can express serotype-1 capsule, similar to serotype-1 from Streptococcus pneumoniae.11 These can influence inflammatory signaling pathways and contribute to the dysregulated immune response observed in burn patients. These immunomodulatory factors may exacerbate tissue inflammation, impair wound healing, and promote systemic complications associated with burn infections.12
Klebsiella pneumoniae is a Gram-negative bacterium commonly associated with hospital-acquired infections, including those occurring in severe burn patients. Klebsiella pneumoniae is one of the most predominant bacteria in burn infections after Pseudomonas aeruginosa and Acinetobacter baumannii.13 In the context of burn injuries, Klebsiella pneumoniae can exhibit several virulence factors that contribute to its pathogenicity and exacerbate the clinical course in affected individuals. Klebsiella pneumoniae is known for its ability to produce a thick polysaccharide capsule, which serves as a major virulence determinant. In severe burn patients, the presence of a robust capsule can enhance the ability of Klebsiella pneumoniae to colonize burn wounds and disseminate systemically, leading to invasive infections and septic complications.14
Klebsiella pneumoniae can adhere to host tissues and medical devices, facilitating colonization of burn wounds and other anatomical sites.15 Moreover, Klebsiella pneumoniae can form biofilms on abiotic surfaces, such as catheters, endotracheal tubes, and wound dressings, as well as on biotic surfaces within the burn wound environment. Biofilms are highly structured microbial communities that display increased resistance to antimicrobial factors and host defenses.16 Biofilm formation poses a significant challenge in managing burn patients.
Klebsiella pneumoniae can secrete various virulence factors, including capsules, exopolysaccharides associated with mucoviscosity, lipopolysaccharides (LPSs), adhesins, and iron uptake systems.17 Klebsiella pneumoniae can secrete siderophores and scavenge iron from host proteins, allowing Klebsiella pneumoniae to thrive in iron-limited environments such as the burn wound. The ability to obtain iron is critical to the growth and replication of bacteria.18 There are four iron carriers in Klebsiella pneumoniae; enterobactin, yersiniabactin, salmochelin, and aerobactin. Enteromycin has the highest affinity for iron and is considered the primary iron absorption system.19 Hemolysins lyse host cells and contribute to tissue damage. At the same time, proteases degrade host proteins and extracellular matrix components, promoting bacterial dissemination and tissue destruction in severe burn patients.
Due to its enhanced resistance and recently focused hypervirulence, Klebsiella pneumoniae has been of more significant concern worldwide in the past decades.20 Klebsiella pneumoniae is notorious for its ability to acquire antimicrobial resistance through various mechanisms, including the production of ג-lactamases, efflux pumps, and alterations in outer membrane proteins. This resistance can limit the efficacy of commonly used antibiotics in treating Klebsiella pneumoniae infections, complicating the management of burn patients and necessitating alternative antimicrobial agents or combination therapies.14 Klebsiella pneumoniae can modulate host immune responses by producing immunomodulatory molecules such as lipopolysaccharide (LPS) and capsular polysaccharides. These molecules can trigger excessive inflammation, leading to tissue damage and organ dysfunction in severe burn patients.2
Cefuroxime is a second-generation cephalosporin antibiotic with a broad spectrum and can be active against gram-negative and gram-positive bacteria, including Streptococcus Spp and Klebsiella pneumoniae.21 Cefuroxime is generally recommended for burn patients because it has relatively good effectiveness, is cheaper, and does not promote drug resistance. Cefuroxime is an antibiotic usually used in Dr. Soetomo General Academic Hospital for burn patients.22
Streptococcus mitis and Klebsiella pneumoniae have different virulence factors and mechanisms of pathogenesis, but their interactions within the host environment can influence disease outcomes. Understanding the virulence factors employed by Streptococcus mitis and Klebsiella pneumoniae in burn infections is crucial for devising effective therapeutic strategies to mitigate bacterial pathogenicity, prevent complications, and improve clinical outcomes in affected individuals. Further research into the molecular mechanisms underlying Streptococcus mitis and Klebsiella pneumoniae virulence in burn patients is warranted to inform the development of targeted interventions for managing infections caused by these opportunistic pathogens.
Streptococcus mitis and Klebsiella pneumoniae are opportunistic pathogens capable of causing infections. They may coexist or even synergize to exacerbate the severity of infections, particularly in immunocompromised individuals, including in severe burn injury patients.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Written informed consent was obtained from the participant before enrolling in the study.
- World Health Organization. Burns. Fact Sheets. 2023. https://www.who.int/news-room/fact-sheets/detail/burns. Accessed March 3, 2024.
- Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG. Burns: Pathophysiology of Systemic Complications and Current Management. J Burn Care Res. 2017;38(1):e469-e481.
Crossref - Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388(10052):1417-1426.
Crossref - Yan J, Hill WF, Rehou S, Pinto R, Shahrokhi S, Jeschke MG. Sepsis criteria versus clinical diagnosis of sepsis in burn patients: A validation of current sepsis scores. Surg (United States). 2018;164(6):1241-1245.
Crossref - Burgess M, Valdera F, Varon D, Kankuri E, Nuutila K. The Immune and Regenerative Response to Burn Injury. Cells. 2022;11(19):3073.
Crossref - Lee HG, Jang J, Choi JE, et al. Blood stream infections in patients in the burn intensive care unit. Infect Chemother. 2013;45(2):194-201.
Crossref - Mitchell J. Streptococcus mitis: walking the line between commensalism and pathogenesis. Mol Oral Microbiol. 2011;26(2):89-98.
Crossref - Kilian M, Riley DR, Jensen A, Bruggemann H, Tettelin H. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. MBio. 2014;5(4):1-9.
Crossref - Hanson BR, Neely MN. Coordinate regulation of Gram-positive cell surface components. Curr Opin Microbiol. 2012;15(2):204-210.
Crossref - Gisch N, Peters K, Thomsen S, Vollmer W, Schwudke D, Denapaite D. Commensal Streptococcus mitis produces two different lipoteichoic acids of type I and type IV. Glycobiology. 2021;31(12):1655-1669.
Crossref - Lessa FC, Milucky J, Rouphael NG, et al. Streptococcus mitis Expressing Pneumococcal Serotype 1 Capsule. Sci Rep. 2018;8(1):1-9.
Crossref - Taneja N, Chari PS, Singh M, Singh G, Biswal M, Sharma M. Evolution of bacterial flora in burn wounds: key role of environmental disinfection in control of infection. Int J Burns Trauma. 2013;3(2):102-107. PMID: 23638328
- Soedjana H, Nadia J, Sundoro A, et al. The profile of severe burn injury patients with sepsis in hasan sadikin bandung general hospital. Ann Burns Fire Disasters. 2020;33(4):312-316.
- Freystätter C, Radtke C, Ihra G, Thalhammer F, Fochtmann-Frana A. Sepsis caused by multidrug-resistant klebsiella pneumoniae infection in a 23-year-old burn patient: case report and literature review. Ann Burns Fire Disasters. 2018;31(2):113-117.
- Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:1-15.
Crossref - Guerra MES, Destro G, Vieira B, et al. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front Cell Infect Microbiol. 2022;12:1-13.
Crossref - Riwu KHP, Effendi MH, Rantam FA, Khairullah AR, Widodo A. A review: Virulence factors of Klebsiella pneumonia as emerging infection on the food chain. Vet World. 2022;15(9):2172-2179.
Crossref - Holden I victoria. Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1a Stabilization during Pneumonia. mBio. 2016;7(5):01397-16
Crossref - Wang G, Zhao G, Chao X, Xie L, Wang H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health. 2020;17(17):1-17.
Crossref - Dai P, Hu D. The making of hypervirulent Klebsiella pneumoniae. J Clin Lab Anal. 2022;36(12):1-16.
Crossref - Chang UI, Kim HW, Wie SH. Comparison of second- and third-generation cephalosporin as initial therapy for women with community-onset uncomplicated acute pyelonephritis. Yonsei Med J. 2015;56(5):1266-1273.
Crossref - Alfitra KA, Saputro ID, Juniastuti, Budi ASA. Profile of Burn Patients with Cefuroxime Antibiotic Administration at Dr. Soetomo General Hospital in March 2021 – February 2022. Int J Res Publ. 2022;115(1):274-279.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.