ISSN: 0973-7510
E-ISSN: 2581-690X
Protein hydrolysates are composed of peptones and peptides, and their type depends on the biological source and manufacturing process. We studied the survival response of Salmonella enterica in the presence of casein, soy protein, and fish protein hydrolysates at refrigeration and freezing temperatures. Fish protein hydrolysate (FPH) was identified as the only capable hydrolysate of sustaining the viability of S. enterica for 60 days at refrigeration temperatures. None of the three hydrolysates were able to impart cryoprotection to S. enterica under freezing conditions. The survival rate of S. enterica in FPH was further enhanced by growing the inoculum on agar instead of broth. An optimization study using response surface methodology was also conducted to identify suitable concentrations of protein hydrolysates capable of maximizing the survival of S. enterica. The results of this study can be used to manage Salmonella-related food safety incidents by replacing animal-sourced with plant-sourced protein hydrolysates at the recipe development stage, as well as the use of FPH in microbiological growth media and the formulation of secondary reference materials. These results indicate that FPH produced by enzymatic hydrolysis of Stolephorus indicus at lower concentrations (5%) can serve as nutritive agents in culture media, contribute to long-term preservation, and can be used in the formulation of quantitative reference materials for Salmonella.
Protein Hydrolysate, Survival Study, Food Governance, Food Security
Peptones and peptides are common names used for protein hydrolysates.1 The preferred sources of protein hydrolysates are by-products and waste produced after processing plants and animal products. Protein hydrolysates are widely used for food fortification, feed supplements, and health foods.2 The types of peptones and peptides present in protein hydrolysates depend on the biological source and manufacturing process. The protein hydrolysate manufacturing process involves hydrolysis using acids, alkalis, heat, enzymes, or microbial fermentation. Hydrolysis generates different types of bioactive peptides with antimicrobial properties.3,4,5 Several studies have recommended the use of bioactive peptides derived from protein hydrolysates in food and food packaging to prevent microbial spoilage.6,7 The most expensive ingredient in microbiological culture media is nitrogen, and studies have been conducted to evaluate FPHs produced from fish or shrimp waste and peptones from casein, yeast, soy, and gelatin as cost-effective alternatives.8-13 Catfish protein hydrolysate has been used in the formulation of microbiological secondary reference materials by food-testing laboratories under ISO 17025:2017 to maintain data accuracy and prevent false-negative reporting.14 Peptones are classified based on their source, and the four major categories are meat (e.g., tryptose and gelatin), vegetable (e.g., soy and pea), milk-derived (e.g., casein and whey), and mycological (e.g., yeast and fungi).10 Casein hydrolysate (CH), fish protein hydrolysate (FPH), and soy protein hydrolysate (SPH) were selected for this study to represent the three major categories of peptones. The nitrogen content of plant- and animal-derived protein hydrolysates ranges from >7% to 15% and is mostly dependent on the source and method of hydrolysis employed (thermal, acidic, alkaline, or enzymatic).15,16,17 The total nitrogen content from proteins estimated with the Kjeldahl method based on commercial manufacturer data for casein and soy protein hydrolysates were ≥ 8% and ≥ 12%, respectively. Stolephorus indicus was used for the production of FPH, which has an average of 12±1% of total nitrogen content from protein.18 Salmonella enterica was selected as the target microorganism for assessing the effects of the selected protein hydrolysates, as Salmonella is among the top three bacteria responsible for foodborne illnesses worldwide.19 In 2022, the International Food Safety Authorities Network (INFOSAN) reported a total of 109 biological food safety incidents, where Salmonella contributed to 35% (38 incidents) of the total biological food safety incidents.20 All three protein hydrolysates were individually assessed for their potential to maintain long-term survival of S. enterica at 5±2°C and -18±3°C for 60 days. To enhance the survival of S. enterica, an agar-based inoculum, was tested to determine whether it imparts better survival properties than the broth-grown inoculum. Response surface methodology (RSM) was employed to optimize the concentration of the selected protein hydrolysates, and the combined effect of the different protein hydrolysates was determined. The key objectives of this study were to assess the survival of S. enterica in the presence of different types of protein hydrolysates and identify potential hydrolysates that can be used as nitrogen sources in microbiological culture media and as nutritive agents in the formulation of secondary reference materials. Owing to the presence of bioactive peptides in hydrolysates, the findings of this study may allow the development of better-suited hydrolysates for use in food formulations to avoid food safety incidents involving Salmonella.
Culture Preparation
The Microbial Type Culture Collection and Gene Bank (MTCC) strain of S. enterica subspp. Arizonae MTCC 660T (equivalent to NCTC8297 & ATCC13314) was obtained from the Institute of Microbial Technology (IMTech), Chandigarh, in a lyophilized state. Resuscitation was performed as per instructions from IMTech, which involved the use of nutrient broth as the growth media and incubation at 25°C for 24 h. After the revival of lyophilized cultures, 10% glycerol stocks were prepared for long-term usage and stored at -18±3°C. For the preparation of 2 log CFU culture, the culture of S. enterica was inoculated in tryptone soy broth (TSB) and incubated overnight at 37°C followed by serial dilution. The last three serial dilution tubes (10-7, 10-8, and 10-9) were used for estimating the culture density. After estimating the titer, the appropriate serial dilution tube was used for inoculation in protein hydrolysates.21,22 Further, the effect of inoculum type on the growth and survival of the test organism was studied by reviving the 10% glycerol stock on trypticase soy agar (TSA) instead of TSB. In the earlier method of inoculum preparation, the growth medium was TSB, and, after revival at 37±1°C for 18 – 24 h, the TSB inoculum was subjected to serial dilution in 0.85% saline and the desired serial dilution was used for further inoculation in protein hydrolysates. For agar-grown inoculum, TSA was used as the growth medium for the revival of S. enterica present in 10% glycerol stock solutions. The bacterial culture was streaked on TSA and incubated at 37±1°C for 18 – 24 h. A single colony was selected for serial dilution in 0.85% saline to estimate the titer, and the desired dilution tube was used for further inoculation in FPH. Introduction of agar instead of broth for inoculum preparation further acted as an environmental stress to already stressed S. enterica, which is preserved in glycerol at freezing conditions, to evaluate the survival of S. enterica owing to the inclusion of agar as growth media instead of broth.19
Protein hydrolysates used in the study
SPH obtained from Merck (Sigma catalogue no. S1674-100G) was a beige-colored powder derived from the hydrolysis of soybeans (Glycine max). SPH is soluble in water in the presence of a slight haze containing 4% moisture, 8% ash, 9% amino nitrogen, and 13% total nitrogen. Technical-grade CH obtained from Loba Chemie (Catalogue no. 02575-500G) was a light brown powder with an ash content of 20.80% and a total nitrogen content of 12.7%. FPH obtained from ICAR – Central Institute of Fisheries Technology (CIFT), Kochi (India), was used for conducting the experimental study. Notably, FPH were prepared from Indian anchovy (Stolephorus indicus) by enzymatic hydrolysis using papain at ICAR-CIFT. The liquid extract was spray-dried to produce a light brown powder containing <10% moisture and a total nitrogen content of 13.5±0.5%.
RSM software
Design Expert V 22.0.0.1 (US, Stat-Ease Inc.) software was used for optimization studies employing RSM. RSM, a multivariate optimization strategy, was used to reduce reagent or consumable spending, along with a reduction in laboratory work owing to the optimization of the required experimental runs.23 The RSM software used a quadratic model, and accuracy was determined using analysis of variance (ANOVA). The response from the RSM study was tested for a quadratic model utilizing the Box-Behnken Design methodology (BBD), as there were three variable factors involved: CS, SPH, and FPH. The lowest level factor for each of the protein hydrolysates was set at 0, the mid-level factor at 5%, and the highest factor at 10%; the coded value for the lowest level factor was -1, 0 for the mid-level factor value, and +1 for the highest factor value. Refrigeration (5±2°C) and freezing temperatures (-18±3°C) were used for storage, and, after regular intervals, the viability was assessed using TSA in duplicates. The two types of variables included in this multivariate study were responses and factors. The response was the dependent variable, and the factors had three levels. Based on individual studies on each protein hydrolysate, the factors were finalized at three concentration levels. In our study, more than one response was observed, as the response was measured in the form of survival/viability at consecutive intervals of 0, 7, 14, 21 and 28 days. Employing BBD for RSM was beneficial, as it allowed the estimation of parameters using a quadratic model, sequential design build-up, application of blocks, and model lack-of-fit detection.23 The correlations between the variables in the quadratic model and the three variables are represented by Eq. 1. The coefficient estimate represents the expected change in response per unit change in the factor value when all the remaining factors are held constant. The intercept in the orthogonal design is the overall average response of all runs. The coefficients are adjusted around that average based on the factor settings.
Y=Intercept+x1 (A)+x2 (B)+ x3 (C)+ x4 (AB)+ x5 (AC)+ x6 (BC)+ x7 (A)2+x8 (B)2+x9 (C)2 (1)
where Y = response value (survival rate), A = casein hydrolysate, B = soy protein hydrolysate, C = fish protein hydrolysate, and x1– x9 = coefficient estimates of the respective factors.
Protein hydrolysates were individually assessed using S. enterica at 5%, 10%, 15%, 20%, and 25% to identify their effects on the survival of S. enterica. A culture (2.16 log) of S. enterica used for the study was prepared using a serial dilution of a culture grown overnight in TSB.21,22 The individual study was followed by an optimization study using RSM to investigate the presence of synergistic or antagonistic effects among the three protein hydrolysates and to identify the best concentration of protein hydrolysates leading to the maximum survival of S. enterica.
Effects of CH on the survival of S. enterica
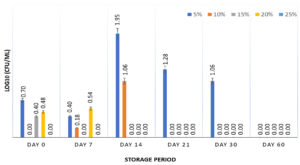
The survival of S. enterica was studied at 5, 10, 15, 20, and 25% of CH for 60 days at 5±2°C. The results (Figure 1) show that 5% CH supported the growth of the bacterium (~59% survivors) for up to 30 days, beyond which no survivor was observed. None of the other concentrations of CH supported the survival of S. enterica at 5±2°C. Generally, the major protein of milk is casein, which constitutes 80% of the total milk proteins and comprises α-, β-, g-, and κ-casein.24 CHs and peptides derived from casein have antioxidant and antimicrobial properties against foodborne pathogens, including S. enterica.25-29 The generation time and yield of Salmonella serovar Typhimurium were dependent on the source; peptones derived from casein and soy were unable to produce significant yields when used as constituents in buffered peptone water, as compared to peptones derived from yeast and gelatin.10 These results are well supported by another study in which UV-irradiated S. Typhimurium was resuscitated with CH in broth and minimal media. No survival enhancement was observed for lag phase cells in minimal media and stationary phase cells in broth and minimal media, suggesting the ineffectiveness of CH in promoting the survival of S. Typhimurium under stress conditions.30 Intermittent survival of S. enterica was observed, which could result from the presence of environmental stresses such as low temperature and the presence of CH as the only nutrient, leading to a viable but non-culturable state that can be resuscitated later.31 Furthermore, CH did not exhibit any cryoprotective effects under freezing conditions, with no survival of S. enterica observed at -18 ± 3°C after 7 days of storage.
Figure 1. Survival of S. enterica subspp. Arizonae MTCC 660T in presence of 5, 10, 15, 20 & 25% casein hydrolysate stored at 5±2°C for 60 days. Graph contains viability results obtained at fixed intervals of 0, 7, 14, 21, 30, and 60 days with error bars at 95% confidence interval and n=2 for each response
Effects of SPH on the survival of S. enterica
The survival of S. enterica was studied using 5, 10, 15, 20, and 25% SPH at 5±2°C, and -18±3°C for a 60-day storage period. All SPH concentrations failed to support the survival of S. enterica at refrigeration (Figure 2), as only 10% SPH showed 47.22% survival on Day 7, whereas 5% SPH resulted in 27.76% survival on Day 14, which reduced to no survival from Day 21 for all SPH concentrations. Similar to CH, SPH was unable to impart cryoprotection at -18±3°C, and no growth was observed from 7 days of storage period onwards. This pattern may result from the antimicrobial peptides present in the plant products.32 SPH is derived from soybeans (Glycine max) that are rich in bioactive peptides such as glycinin and β-conglycinin, which have prominent antibacterial and antioxidant effects against S. enterica.33 Hydrolysates from soy protein isolates inhibit the growth of S. Typhimurium and, in some instances, synergistic inhibitory effects can also be seen with hydrolysates from bovine whey protein and egg protein.34 CH and SPH at concentrations of 15%–25% were unable to support growth from Day 14 of storage, which can be attributed to the increase in the concentration of antibacterial peptides from 15% concentration onwards. This could result from an increase in a particular fraction of bioactive peptides present in CH and SPH with increasing concentrations.35,36
Figure 2. Survival of S. enterica subspp. Arizonae MTCC 660T in presence of 5, 10, 15, 20 & 25% soy protein hydrolysate stored at 5±2°C for 60 days. Graph contains viability results obtained at fixed intervals of 0, 7, 14, 21, 30, and 60 days with error bars at 95% confidence interval and n=2 for each response
Effects of FPH on the survival of S. enterica
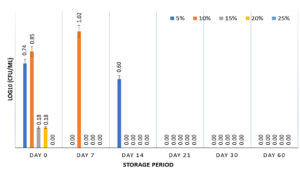
The survival of S. enterica was studied at 5, 10, 15, 20, and 25% of FPH at 5±2°C for a 60-day storage period. The FPH was the only hydrolysate capable of supporting S. enterica viability when stored at 5±2°C. At 5, 10, and15% FPH and a storage temperature of 5±2°C, the bacterium showed 102.11%, 113.29%, and 94.42% recovery of S. enterica, respectively, at the end of 60 days. However, FPH concentrations of 20, and 25% were ineffective in maintaining the viability of S. enterica at 5±2°C after 30 days of storage (Figure 3). In a similar study conducted with 14% tuna FPH, the highest viability of Salmonella after freeze-drying was 67.08%.37 FPH is the most effective protein hydrolysate for sustaining the growth of Salmonella according to a study conducted by Petrova et al.38 There have been studies in which FPH was used in matrices along with the freeze-drying technique to create a secondary reference material for Salmonella.14 The peptones obtained from cod FPH have demonstrated better growth of S. entereditis compared to the growth of Staphylococcus aureus when used in microbiological culture media.38 Higher concentrations of FPH (20% and 25%) were unsuitable for the prolonged survival of S. enterica . The results showed no growth of the bacterium at a concentration of 25% from Day 7 onwards, whereas a 20% concentration of FPH resulted in a decrease in survival from Day 7 onwards, with no growth on Day 14. The lack of survival of S. enterica at higher FPH concentrations can be attributed to an increase in the concentration of bioactive peptides, leading to an inhibitory effect on the test microorganisms.39,40 A pattern similar to that of CH and SPH was observed with FPH being unable to impart cryoprotection to S. enterica at -18±3°C with no growth observed from Day 7 onwards.
Figure 3. Survival of S. enterica subspp. Arizonae MTCC 660T in presence of 5, 10, 15, 20 & 25% fish protein hydrolysate stored at 5±2°C for 60 days. Graph contains viability results obtained at fixed intervals of 0, 7, 14, 21, 30, and 60 days with error bars at 95% confidence interval and n=2 for each response
Enhancement of survival using an agar-based inoculum of S. enterica in FPH
Adaptability to environmental stress can lead to prolonged survival, which can be induced if the inoculum is subjected to various stress conditions, including growth on an agar-based medium instead of broth.19 Hence, during inoculum preparation of S. enterica, TSA was used instead of broth. The inoculum grown on agar medium (2.74 log culture) was tested at 5, 10, 15, 20, and 25% of FPH and 5±2°C for a storage period of 60 days. When comparing the inoculum prepared from broth and agar, it was observed that the inoculum grown on agar offered a better survival rate, along with an increase in the number of S. enterica cells compared to the broth culture (Figure 4). The results showed recoveries of 121.29%, 120.07%, and 123.29% at FPH concentrations of 5, 10, and 15 %, respectively. Although an improvement in survival was observed at 20% and 25% FPH concentrations, no growth was observed after 60 days of storage. The inoculum prepared on agar plates survived after 60 days of storage at 5 to 15% FPH concentrations. This could result from an increase in the survival capacity of the inoculum grown on agar compared to broth, as the development of resistance in Salmonella in response to unfavorable environmental conditions can be achieved by growing the inoculum on agar-based media.19 Several studies on the survival of Salmonella in the presence of environmental stress have shown that inocula grown on agar are resistant to adverse conditions of drying, heat, desiccation, and cold storage, leading to an increase in survival rate compared to broth-grown inocula,41,42,43 which can be attributed to the expression of various stress response regulons upon the transition from a liquid to a solid medium.44 Therefore, the inoculum grown on agar medium was used for further optimization studies using RSM.
Figure 4. Survival of agar grown inoculum of S. enterica subspp. Arizonae MTCC 660T in presence of 5, 10, 15, 20 & 25% fish protein hydrolysate stored at 5±2°C for 60 days. Graph contains viability results obtained at fixed intervals of 0, 7, 14, 21, 30, and 60 days with error bars at 95% confidence interval and n=2 for each response
Optimization of protein hydrolysate concentration using RSM
To evaluate synergistic effects among the hydrolysates that could improve the survival of S. enterica at low temperatures (5±2°C and -18±3°C), RSM was performed using controllable variables (factors) of 0, 5, and 10%, concentrations of each protein hydrolysate (CH, SPH, and FPH) keyed in the software with the coded values are -1, 0, and -1, respectively. The experimental setup for the RSM involved three-level factor (0, 5%, and 10%) concentrations for all three protein hydrolysates, resulting in 17 experimental points or runs that resulted in a combination of different concentrations of the three protein hydrolysates (Table 1). The Box-Behnken experimental design (BBD) was used because the study design met the minimum three-factor requirements of BBD, and there was more than one response that had to be monitored over an interval of 7 days for a storage period of 28 days. All readings were taken in duplicate using blank and culture controls. The RSM study at the storage temperature of -18±3°C showed no survival from Day 7 onwards. Table 1 provides details of the BBD, along with the actual recovery and predictive values for S. enterica converted to log 10 values for storage temperature at 5±2°C. ANOVA of the proposed model for the optimization of protein hydrolysates was found to be significant, as the model F- values were higher (291.56 at Day 0, 403.13 at Day 7, 345.50 at Day 14, 210.35 at Day 21, and 403.15 at Day 28) and the model p-values were <0.0001, which needs to be <0.05 to confirm the significance of the model. Similarly, the predicted R² value of 0.9799 (Day 28) was in reasonable agreement with the adjusted R² value of 0.9956 (Day 28), that is, the difference was less than 0.2, demonstrating a strong correlation between the experimental and predicted values (Table 2). The coefficients of variance (C.V%) observed for the model were 3.50, 6.89, 4.74, 9.90, and 4.58 at 0, 7, 14, 21, and 28 days, respectively. The observed C.V% was <10%, indicating that the model was reproducible. Adequate precision determines the variation in the response in relation to the target under varying noise conditions, where a value of > 4 is desired. The adequate precision for the model was observed to be 60.18, 68.93, 62.60, 45.46, and 70.08 for 0, 7, 14, 21, and 28 days, respectively.
Table (1):
Box Behnken design for optimization of protein hydrolysates along with actual and predicted values of S. enterica subspp. Arizonae MTCC 660T recovery value converted to Log10 value
| Independent variables | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | A | B | C | A:CH(%) | B: SPH(%) | C: FSH(%) | Day 0 Log10(CFU/ml) | Day 7 Log10(CFU/ml) | Day 14 Log10(CFU/ml) | Day 21 Log10 (CFU/ml) | Day 28 Log10 (CFU/ml) |
||||||
| Coded levels | Actual levels | AV | PV | AV | PV | AV | PV | AV | PV | AV | PV | ||||||
| 1 | +1 | 0 | +1 | 10 | 5 | 10 | 1.18 | 1.24 | 0.78 | 0.83 | 1.18 | 1.22 | 1.18 | 1.17 | 1.20 | 1.19 | |
| 2 | 0 | 0 | 0 | 5 | 5 | 5 | 1.57 | 1.53 | 1.38 | 1.42 | 1.49 | 1.44 | 1.38 | 1.40 | 1.28 | 1.19 | |
| 3 | -1 | -1 | 0 | 0 | 0 | 5 | 2.78 | 2.81 | 3.02 | 3.06 | 2.52 | 2.48 | 2.85 | 2.94 | 3.03 | 2.99 | |
| 4 | +1 | -1 | 0 | 10 | 0 | 5 | 2.39 | 2.33 | 1.78 | 1.89 | 1.72 | 1.75 | 2.22 | 2.22 | 2.08 | 2.04 | |
| 5 | -1 | +1 | 0 | 0 | 10 | 5 | 1.11 | 1.17 | 0.90 | 0.95 | 1.20 | 1.14 | 0.60 | 0.59 | 0.30 | 0.35 | |
| 6 | 0 | 0 | 0 | 5 | 5 | 5 | 1.53 | 1.53 | 1.53 | 1.42 | 1.38 | 1.44 | 1.60 | 1.40 | 1.18 | 1.19 | |
| 7 | +1 | 0 | -1 | 10 | 5 | 0 | 1.18 | 1.21 | 0.78 | 0.85 | 0.90 | 0.93 | 0.90 | 0.92 | 0.70 | 0.72 | |
| 8 | 0 | 0 | 0 | 5 | 5 | 5 | 1.49 | 1.53 | 1.41 | 1.42 | 1.49 | 1.44 | 1.20 | 1.40 | 1.15 | 1.19 | |
| 9 | 0 | 0 | 0 | 5 | 5 | 5 | 1.54 | 1.53 | 1.40 | 1.42 | 1.41 | 1.44 | 1.20 | 1.40 | 1.18 | 1.19 | |
| 10 | 0 | +1 | -1 | 5 | 10 | 0 | 0.70 | 0.70 | 0.30 | 0.38 | 0.70 | 0.63 | 0.00 | 0.11 | 0.30 | 0.25 | |
| 11 | 0 | 0 | 0 | 5 | 5 | 5 | 1.51 | 1.53 | 1.36 | 1.42 | 1.36 | 1.44 | 1.51 | 1.40 | 1.18 | 1.19 | |
| 12 | 0 | -1 | -1 | 5 | 0 | 0 | 2.52 | 2.55 | 2.54 | 2.48 | 1.99 | 1.97 | 2.63 | 2.45 | 2.01 | 2.04 | |
| 13 | -1 | 0 | +1 | 0 | 5 | 10 | 1.80 | 1.77 | 1.99 | 2.00 | 1.93 | 1.96 | 2.08 | 1.89 | 2.09 | 2.07 | |
| 14 | 0 | -1 | +1 | 5 | 0 | 10 | 2.43 | 2.43 | 2.59 | 2.47 | 2.36 | 2.26 | 2.60 | 2.70 | 3.24 | 3.30 | |
| 15 | -1 | 0 | -1 | 0 | 5 | 0 | 2.10 | 2.04 | 2.11 | 2.02 | 1.57 | 1.67 | 1.63 | 1.64 | 1.08 | 1.09 | |
| 16 | 0 | +1 | +1 | 5 | 10 | 10 | 0.60 | 0.57 | 0.30 | 0.36 | 0.85 | 0.9179 | 0.30 | 0.36 | 0.48 | 0.45 | |
| 17 | +1 | +1 | 0 | 10 | 10 | 5 | 0.30 | 0.27 | 0.00 | -0.22 | 0.48 | 0.41 | 0.00 | -0.13 | 0.00 | 0.04 | |
Actual values (AV); predicted values (PV)
Table (2):
Statistical analysis (ANOVA) by RSM of the proposed model for survival of S. enterica subspp. Arizonae MTCC 660T leading to optimization of protein hydrolysates when stored at 5±2°C over a period of 28 days
| Model | Day 0 | Porb>F | Day 7 | Porb>F | Day 14 | Porb>F | Day 21 | Porb>F | Day 28 | Porb>F |
|---|---|---|---|---|---|---|---|---|---|---|
| 7.93 (Significant) | <0.0001 | 11.62 (Significant) | < 0.0001 | 4.84 (Significant) | < 0.0001 | 2.19 (Significant) | < 0.0001 | 13.31 (Significant) | < 0.0001 | |
| A | 0.9384 | < 0.0001 | 2.74 | < 0.0001 | 1.08 | < 0.0001 | 1.02 | < 0.0001 | 0.7938 | < 0.0001 |
| B | 6.86 | < 0.0001 | 8.88 | < 0.0001 | 3.59 | < 0.0001 | 11.04 | < 0.0001 | 10.76 | < 0.0001 |
| C | 0.0300 | 0.0161 | 0.0006 | 0.8046 | 0.1682 | < 0.0001 | 0.1250 | 0.0245 | 1.07 | < 0.0001 |
| A2 | 0.0150 | 0.0610 | – | – | – | – | – | – | 0.0070 | 0.2098 |
| B2 | 0.0138 | 0.0700 | – | – | – | – | – | – | 0.1672 | 0.0003 |
| C2 | 0.0022 | 0.4239 | – | – | – | – | – | – | 0.0550 | 0.0061 |
| AB | 0.0441 | 0.0065 | – | – | – | – | – | – | 0.1056 | 0.0010 |
| AC | 0.0225 | 0.0294 | – | – | – | – | – | – | 0.0650 | 0.0040 |
| BC | 0.0000 | 0.9301 | – | – | – | – | – | – | 0.2756 | < 0.0001 |
| R2 | 0.9973 | 0.9894 | 0.9876 | 0.9798 | 0.9981 | |||||
| Adj. R2 | 0.9939 | 0.9869 | 0.9848 | 0.9752 | 0.9956 | |||||
| Lack of fit | Not Significant | Not Significant | Not Significant | Not Significant | Not Significant |
Probability value of Fisher’s variance ratio (Prob>F), coefficient of determination (R2)
The concentration of CH, SPH and FSH is represented by independent variables A, B and C.
The effect of variables in squared terms (A2, B2, C2)
The overall linear model was satisfactory and highly statistically significant at Days 7, 14 & 21 (model p < 0.0001, leading to a confidence limit of >99%) for S. enterica. For Day 0 & Day 28, the quadratic model was found to be satisfactory & statistically highly significant (model p value < 0.0001 leading to a confidence limit of >99%). The relationship between the survival rates of S. enterica and the three protein hydrolysates observed on Day 28 are shown in the Eq. 2.
Y=1.91-0.32(A)-1.16(B)+0.37(C)+ 0.16(AB)-0.13(AC)-0.26(BC)-0.04(A)2+ 0.20(B)2+0.11 (C)2(2)
Throughout the study, variance inflation factors (VIFs) were observed to be 1.00, indicating their orthogonal nature. Multicollinearity between the independent variables was assessed using the VIFs, and because the VIFs were observed to be 1.00, no correlation was observed between the independent variables. Calculated deviation values (actual and predicted values of activities) for S. enterica at Days 0 (3.50%), 7 (6.89%), 14 (4.74%), 21 (9.90%), and 28 (4.58%) were observed within the allowed limits (±10%), therefore showing the model to be satisfactory and statistically significant. The signal-to-noise ratio for S. enterica at Days 0 (60.18), 7 (68.927), 14 (62.601), 21 (45.458), and 28 (70.083) were greater than the desired ratio (4.0), further indicating the accuracy of the proposed model. The effects of CH, SPH, and FPH on S. enterica survival was further elaborated by 3-D surface contour graphs (Figure 5), where the maximum survival response was achieved at 5% FPH in the absence of CH and SPH.
Protein hydrolysates have wider applications; apart from their nutritional properties; they exhibit biological properties, including antioxidant, antimicrobial, cholesterol-reducing, hypoglycemic, and anticancer effects. Bioactive peptides from protein hydrolysates have gained significant attention, and several studies have explored their antimicrobial properties. In this study, we evaluated the nutritive properties of animal- and plant-based protein hydrolysates for future application in microbiological culture media and the creation of a secondary reference material for Salmonella. The key findings were that FPH was the only hydrolysate that was effective in maintaining the long-term viability of S. enterica. Inoculum grown on agar further aided the survival of S. enterica with increased recovery and better viability in FPH. We further observed that treatment with 5% FPH from Stolephorus indicus resulted in better recovery and prolonged survival of S. enterica and can be used along with other preservation techniques, such as freeze-drying and encapsulation, for the homogeneous and stable preservation of Salmonella. Additionally, CH and SPH can be utilized to isolate bioactive peptides and as food additives for Salmonella control in food and food-contact packaging materials.
ACKNOWLEDGMENTS
The author (Rohit Rai) acknowledges the Science and Engineering Research Board (SERB), Department of Science & Technology, Government of India for providing financial assistance under the Start-up Research Grant (SRG/2021/001243) scheme, and also acknowledges the financial support received from the Research & Development Cell, Lovely Professional University, India, under the scheme of Matching Grant Seed Money (LPU/DRD/SEED/SAC/090922/25274).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SSN and RR conceptualized the study. SSN, SKP, KAS, RR and KE collected resources. KAS, RR and SSN applied methodology. KAS and RR performed software and formal analysis. KAS, RR and SSN performed validation. SSN and RR performed supervision. KAS performed investigation. RR funding acquisition. SKP, KAS and KE wrote the original draft. SKP, KAS, SSN, RR and KE wrote the manuscript. SSN and RR reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by Science and Engineering Research Board (SERB), Department of Science & Technology, Government of India through grant number SRG/2021/001243, and Research & Development Cell, Lovely Professional University, India, under the scheme of Matching Grant Seed Money (LPU/DRD/SEED/SAC/090922/25274).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Pasupuleti VK, Braun S. State of the art manufacturing of protein hydrolysates. Protein Hydrolysates in Biotechnology. 2008:11-32.
Crossref - Etemadian Y, Ghaemi V, Shaviklo AR, Pourashouri P, Mahoonak ARS, Rafipour F. Development of animal/ plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J Clean Prod. 2021;278:123219.
Crossref - Pasupuleti VK, Holmes C, Demain AL. Applications of protein hydrolysates in biotechnology. Protein Hydrolysates in Biotechnology. 2008 1-9.
Crossref - Jia L, Wang L, Liu C, Liang Y, Lin Q. Bioactive peptides from foods: production, function, and application. Food & Function. 2021;12:7108-7125.
Crossref - Nasri R, Abdelhedi O, Nasri M, Jridi M. Fermented protein hydrolysates: biological activities and applications. Curr Opin Food Sci. 2022;43:120-127.
Crossref - Li-Chan EC. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr Opin Food Sci. 2015;1:28-37.
Crossref - Tkaczewska J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci Technol. 2020;106:298-311.
Crossref - Dufosse L, De La Broise D, Guerard F. Evaluation of nitrogenous substrates such as peptones from fish: a new method based on Gompertz modeling of microbial growth. Curr Microbiol. 2001;42(1):32-38.
Crossref - Poernomo A, Buckle KA. Crude peptones from cowtail ray (Trygon sephen) viscera as microbial growth media. World J Microbiol Biotechnol. 2002;18:337-344.
Crossref - Gray VL, Muller CT, Watkins ID, Lloyd D. Peptones from diverse sources: pivotal determinants of bacterial growth dynamics. J Appl Microbiol. 2008;104(2):554- 565.
Crossref - Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem. 2009;115(1):238-242.
Crossref - Kandra P, Challa MM, Jyothi HKP. Efficient use of shrimp waste: present and future trends. Appl Microbiol Biotechnol. 2012;93(1):17-29.
Crossref - Najim SM, Al-Noor JM, Al-Waely WA. Extraction of crude peptone from fish wastes for use as a nitrogen source in microbiological media. Global Journal of Fisheries and Aquaculture Researches. 2015;2:29-37. https://www.researchgate.net/profile/SalahNajim/publication/309241783_Extraction_of_crude_peptone_from_fish_wastes_for_use_as_a_nitrogen_source_in_microbiological_media/links/582bbefb08aef19cb806c1de/Extraction-of-crude-peptone-from-fish-wastes-for-use-as-a-nitrogen-source-in-microbiological-media.pdf. Accessed March 11, 2024.
- Kurniawati E, Ibrahim B, Desniar D. Potency of catfish (Clarias sp.) protein hydrolysates as candidates matrices for microbiology reference material. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology. 2019;14(3):121-130.
Crossref - Colla G, Hoagland L, Ruzzi M, et al. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front Plant Sci. 2017;8.
Crossref - Ertani A, Cavani L, Pizzeghello D, et al. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J Plant Nutr Soil Sci. 2009;172(2):237-244.
Crossref - Ertani A, Schiavon M, Muscolo A, Nardi S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant and Soil. 2013;364(1):145-158.
Crossref - Ali SSR, Abdhakir ES, Muthukkaruppan R. Proximate and mineral composition of commercially important marine fin fishes from the Kasimedu fish landing centre Chennai. Journal of Fisheries and Life Sciences. 2020;5(1):20-25. https://www.researchgate.net/profile/Syed-Raffic-2/publication/342330465_Proximate_and_mineral_composition_of_commercially_important_marine_fin_fishes_from_the_Kasimedu_fish_landing_centre_Chennai_A_R_T_I_C_L_E_I_N_F_O_ K_E_Y_W_O_R_D_S_Body_ indices_Lipid_ Proximate_compositi/links/5eeddac0458515814a6eda4b/Proximate-and-mineral-composition-of-commercially-important-marine-fin-fishes-from-the-Kasimedu-fish-landing-centre-Chennai-A-R-T-I-C-L-E-I-N-F-O-K-E-Y-W-O-R-D-S-Body-indices-Lipid-Proximate-compositi.pdf. Accessed March 11, 2024.
- Singh KA, Nair SS, Rai R. Survival of Salmonella spp. and Pathogenic Escherichia coli in Food Matrixes and Its Relevance in the Development of Proficiency Testing Samples. Journal of AOAC International. 2023;106(4):956-969.
Crossref - WHO INFOSAN Quarterly Summary Report.2022. https://www.who.int/ home, Accessed 11 March, 2024
- Reynolds J. Serial dilution protocols. American Society for Microbiology. 2005:1-7. https://asm.org/ASM/media/Protocol-Images/Serial-Dilution-Protocols.pdf?ext=.pdf. Accessed March 11, 2024.
- Matsuoka H, Shigetomi T, Funabashi H, Saito M, Igimi S. Tryptic soy medium is feasible for the in situ preparation of standards containing small defined numbers of microbial cells. J Microbiol Methods. 2013;93(1):49-51.
Crossref - Ferreira SC, Bruns RE, Ferreira HS, et al. Box-Behnken design: An alternative for the optimization of analytical methods. Analytica Chimica Acta. 2007;597(2):179-186.
Crossref - Choi J, Horne DS, Lucey JA. Determination of molecular weight of a purified fraction of colloidal calcium phosphate derived from the casein micelles of bovine milk. J Dairy Sci. 2011;94(7):3250-3261.
Crossref - Meisel H. Biochemical properties of bioactive peptides derived from milk proteins: potential nutraceuticals for food and pharmaceutical applications. Livestock Production Science. 1997;50(1-2):125-138.
Crossref - Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73(2):239-244.
Crossref - Powers JPS, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24(11):1681-1691.
Crossref - Tang W, Yuan H, Zhang H, Wang L, Qian H, Qi X. An antimicrobial peptide screened from casein hydrolyzate by Saccharomyces cerevisiae cell membrane affinity method. Food Control. 2015;50:413-422.
Crossref - Kim DY, Yoo JS, Cho YA, Yoon HS, Kim CH. Biological Potential of Novel Specific Casein-Derived Peptides. J Dairy Sci Biotechnol. 2021;39(1):36-50.
Crossref - Williams PH, Clarke CH. Pre-and post-irradiation effects upon lethality and reversion in Salmonella typhimurium. Microbiology. 1971;68(2):199-205.
Crossref - Liu J, Yang L, Kjellerup BV, Xu Z. Viable but nonculturable (VBNC) state, an underestimated and controversial microbial survival strategy. Trends Microbiol. 2023;31(10):1013-1023.
Crossref - Kokoska L, Polesny Z, Rada V, Nepovim A, Vanek T. Screening of some Siberian medicinal plants for antimicrobial activity. J Ethnopharmacol. 2002;82(1):51-53.
Crossref - Vasconcellos FCS, Woiciechowski AL, Soccol VT, Mantovani D, Soccol CR. Antimicrobial and antioxidant properties of-conglycinin and glycinin from soy protein isolate. Int J Curr Microbiol Appl Sci. 2014;3(8):144-157. https://www.academia.edu/download/70778626/Original_Research_Article_Antimicrobial_20210930-16146-jgoixt.pdf. Accessed March 11, 2024.
- de Castro RJS, Sato HH. Simultaneous hydrolysis of proteins from different sources to enhance their antibacterial properties through the synergistic action of bioactive peptides. Biocatal Agric Biotechnol. 2016;8:209-212.
Crossref - Elbarbary HA, Abdou AM, Nakamura Y, Park EY, Mohamed HA, Sato K. Identification of novel antibacterial peptides isolated from a commercially available casein hydrolysate by autofocusing technique. Biofactors. 2012;38(4):309-315.
Crossref - Joo J H, Yi SD, Lee GH, Lee KT, Oh MJ. Antimicrobial activity of soy protein hydrolysate with Asp. saitoi protease. J Korean Soc Food Sci Nutr. 2004;33(2):229-235.
Crossref - Kurniawati E, Ibrahim B. Homogeneity and stability of a secondary microbiological reference material candidate for Salmonella in fish matrix. IOP Conf Ser: Earth Environ Sci. 2020;404(1):012036.
Crossref - Petrova I, Tolstorebrov I, Zhivlyantseva I, Eikevik TM.Utilization of fish protein hydrolysates as peptones for microbiological culture medias. Food Biosci. 2021;42:101063.
Crossref - Wald M, Schwarz K, Rehbein H, BuBmann B, Beermann C. Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by- products with trout pepsin. Food Chem. 2016;205:221-228.
Crossref - Baco N, Oslan SNH, Shapawi R, Mohhtar RAM, Noordin WNM, Huda N. Antibacterial activity of functional bioactive peptides derived from fish protein hydrolysate. IOP Conf Ser: Earth Environ Sci. 2022;967(1):012019.
Crossref - Uesugi AR, Danyluk MD, Harris LJ. Survival of Salmonella Enteritidis phage type 30 on inoculated almonds stored at− 20, 4, 23, and 35 C. J Food Protect.2006;69(8):1851-1857.
Crossref - Gruzdev N, Pinto R, Sela S. Persistence of Salmonella enterica during dehydration and subsequent cold storage. Food Microbiology. 2012;32(2):415-22.
Crossref - Wang X, Devlieghere F, Geeraerd A, Uyttendaele M. Thermal inactivation and sublethal injury kinetics of Salmonella enterica and Listeria monocytogenes in broth versus agar surface. Int J Food Microbiol. 2017;243:70-77.
Crossref - Cuny C, Lesbats M, Dukan S. Induction of a global stress response during the first step of Escherichia coli plate growth. Appl Environ Microbiol. 2007;73(3):885-889.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.