ISSN: 0973-7510
E-ISSN: 2581-690X
The application of actinomycetes for bio-fabrication of silver nanoparticles as a rapid, eco-friendly and promising approach is desired for its non-toxicity and simplicity advantages. From sixteen actinomycetes were isolated and checked for their ability to produce nano-Ag, Streptomyces rectiviolaceus strain SMWN3.2 showed more effective nano-Ag (surface plasmon resonance peak at 420 nm and its size 10nm) as an antimicrobial agent. Comparing with the biological process microbial nano –Ag synthesis have advantageous because of its natural abundance, easy culture and its potential to scale up for large scale synthesis, By using Plackett-Burman and Box-Behnken experimental designs the optimized medium components recorded the larger biomass production (16g/l) than the basal conditions (3.8 folds). Also, the optimal nano-Ag bio-fabrication conditions were 0.5M silver nitrate and 5v/v cell filtrate at 45°C. Kinetic conversion rates in submerged batch cultivation in 7L stirred bioreactor was: YX =30.5, Pmax =85.5g/l and YP = 42.6 at 30hr. The best nano-Ag concentrations that formed large inhibition zones were 35- 60µg/ml and the MIC/ MBC and MIC/MFC measured as 25µg/ml/50µg/ml, 50µg/ml/60µg/ml which showed against Streptococcus pneumoniae and Aspergillus fumigatus respectively. This work is focuses on large-scale production of nano-Ag as an antimicrobial agent against hospital-acquired infectious pathogens.
Large-scale strategy, Nano-Ag bio-fabrication, Plackett-Burman designs, Box-Behnken experimental designs, Streptomyces rectiviolaceus.
Nanotechnology deals with the biosynthesis, characterization, exploration and application of nanoparticles ranging from approximately 1-100 nm in one dimension for the development of science22, 39. Silver nanoparticles have widely medicinal applications. Bio nanotechnology employs nanoparticles synthesized on biological platforms such as algae, fungi, yeast, bacteria, actinomycetes, and plants 13, 23. Biological systems as eco-friendly nano-factories provide a wide range of environmentally acceptable procedures, clean, nontoxic and low-cost production with a minimum production time 19, 23. Pathogens such as bacteria, molds, yeasts, and viruses in the living environment are often caused severe human infections. Infectious diseases caused by human systemic pathogens are life threatening and cause leading health problems in the developing countries. Repeated use of antibacterial drugs had resulted in multiple drug resistance which is a major problem. There is a critical need to search for new antimicrobial agents from natural substances required to overcome this multi-drug resistance 23, 37. The advantage of adopting synthesis of metal nanoparticles from microorganisms is mainly due to the simplicity of the methods of the biogenesis and the ease of downstream processing. Previously the studies reported that metal nanoparticles showed the proficient bactericidal activity against Gram-positive as well as Gram-negative bacteria, including multidrug resistant strains which produced by interacting with their enzymes, proteins, or DNA to inhibit cell proliferation38. Statistical optimization of the process parameters has been used by applying the experimental designs to improve the yield with cost effectiveness and scaling-up of the industrial process 23. The most important factors that affect the production of the bioactive compound are mainly carbon, nitrogen sources, growth factors, metal ions, aeration, agitation and dissolved oxygen tension (DOT) in the fermentation medium 12, 23. So, optimization of nutritional and physical factors is of prime importance for reaching the cost-effective and economically viable industrial process 10, 23. Statistical and mathematical methods are compiled in response surface methodology (RSM) based on the Box–Behnken design matrix to determine the effect of multiple variables and optimize several biotechnological procedure 33, 34. This paper encompasses the applying the Plackett-Burman and Box–Behnken designs to optimize the culture medium composition followed by RSM to optimize the reaction conditions for nano-Ag bio-fabrication by using extracellular fraction from Streptomyces rectiviolaceus strain SMWN3.2. This work focuses on the optimization for nano-Ag bio-fabrication reaction by Box-Behnken statistical experimental design for large-scale production of nano-Ag from Streptomyces rectiviolaceus as a novel antimicrobial agent against hospital-acquired infectious pathogens. The method is quiet economically and efficient with respect to any other response surface designs.
Samples collection and isolation of Actinomycetes
Samples were collected from different localities in Egypt; Salt marsh sediments, Nile sediment and cultivated soil samples at El-Sharkia, Alexandria, and Kafr El-Sheikh Governorates. The collected samples were transferred into sterilized container to Bioprocess development Laboratory and stored in the refrigerator at 4°C until further processing. Actinomycetes. were isolated from samples by spread plate method using starch nitrate agar medium of the following composition (g/l): starch, 20.0; KNO3, 2.0; K2HPO4, 1.0; MgSO4Å”7H2O, 0.5; NaCl, 0.5; CaCO3, 3.0; FeSO4Å”7H2O, 0.01; agar, 20.0; in sterile distilled water supplemented with antibiotic (Nalidixic acid 20.0µg/ml) and antifungals (Nystatin 25.0µg/ml and Cycloheximide 100.0µg/ml) to minimize the gram-negative bacteria, fungal and yeast contaminants respectively since Actinomycetes are filamentous gram-positive bacteria. The inoculated agar plates were incubated at 30oC for 7 days. After incubation period morphologically different typical powdery colony (16 isolates) was picked and sub cultured on starch nitrate agar slants and stored at 4oC for further studies.
Screening of different Actinomycetes isolates for Bio-fabrication of nano-Ag
The sixteen isolates were freshly inoculated in Malt Yeast Extract Glucose Peptone (MYGP) broth of the following composition (g/l): (Malt extract 3.0, Yeast extract 3.0 , Glucose 10.0 , Peptone 5.0, Distilled water 1000 ml, pH-7.2) and incubated at 28oC±2 for 3-5 days. After the incubation period, the aqueous extract was centrifuged at 14000 rpm for 15 min. This supernatant extract was further filtered through Whatman No. 1 filter paper (6 mm diameter) and employed for the bio-fabrication of nano-Ag or stored in the deep freezer for further analysis. Fifty ml of 0.5M AgNO3 solution was mixed with 50 ml of the cell filtrate in a 500 ml Erlenmeyer flask and agitated at 50°C in darker condition.
A negative control (silver nitrate solution only) was also run along with the experimental flask. The bio-reduction of Ag+ ions was monitored by changes in color which noted at specific intervals (12 hours and 72 hours) and characterized by UV-visible spectroscopy analysis. After that, the reaction mixture containing nano-Ag was centrifuged at 10000 rpm for 25 min, for disposing of any impurities. For better separation particles was repeatedly centrifugated and re-dispersed in sterile double distilled water, this particles was maintained in a dark place at room temperature for further antimicrobial spectrum.
Antagonistic effect of nano-Ago in vitro
All bio-fabricated nano-Ag were tested to determine the antimicrobial activity against the selected human pathogens (bacteria and fungi) which were kindly provided by Dr. Shahira H. EL-Moslamy (Department of Bioprocess Development, Genetic Engineering and Biotechnology Research Institute, City for Scientific Research and Technological Applications, Alexandria, Egypt) by using the following steps;
Preparation of media
Mueller Hinton Agar (MHA) was employed for bacterial pathogens and Potato Dextrose Agar (PDA) for fungal pathogens. MHA medium containing (g/l): Beef (dehydrated infusion from 300) 2, Casein hydro lysate 17.5, Starch 1.5, agar 17 and final pH 7.3 ± 0.1 at 25°C and PDA containing (g/l): Potato extract; 4.0 (equivalent to 200.0g of potato infusion), Dextrose 20.0 and agar 15.0 dissolved in 1L distilled water and sterilized by autoclaving at (121°C) for 15 minutes for sterilization.
Preparation of inoculums
Pure bacterial and fungal pathogen cultures were maintained on sterile MHA or PDA slants respectively by frequent sub culturing each isolate in fresh media prior to testing. Loopful of pathogens was inoculated into 5 ml of sterile broth media and incubated at 37°C or 30°C respectively for 24hr or 96hr. These cultures were used as inoculums in all our experiments.
Antimicrobial assay
Antimicrobial activity of bio fabricated nano-Ag was analyzed by using the agar well diffusion method. The wells were made by using sterile cork borer (5mm) wells was created into the each Petri-plates. A constant concentration of nano-Ag (200µg/ml) was used to assess it as antimicrobial efficiency. Fungal plates were swabbed with 100µl of the mature spore suspension of each individual pathogen. The inoculated plates were incubated at 37oC (24 hr) for bacteria and at 30oC (96 hr) for fungal pathogens. After incubation, all plates were examined for the different level of the inhibition zones which measured and recorded in millimeter (mm). Triplicates were maintained for each test pathogen to calculate the mean standard deviation.
Statistical analysis
The grouped data were statistically evaluated using ANOVA with SPSS/14 software. Values are presented as the mean ± SD of the three replicates of each experiment. According to the fast reduction of AgNO3 into nano-Ag and its antimicrobial efficacy, a proficient Streptomyces isolate was selected and used for further experiments.
Molecular identification of the most potent isolate
The selected Actinomycetes cells which grown at 30°C for 24 hr were harvested by centrifugation (10000 rpm for 10 min) and washed once with TE buffer (pH 7.0) then re-suspended in 10ml TE buffer (pH 7.0). This mixture was heated in boiling water bath for 10min and centrifuged (10000 rpm for 3min). The final supernatant was transferred to a clean tube and genomic DNA was extracted by using Bacterial genomic (miniprep) kit. PCR was carried out in 50µl volumes by using PCR master mix with Universal 16S rDNA primers; F (5’CAGCAGCCGCGGTAATAC3’) and R (5’CCGTCAATTCCTTTGAGTTT3’) primer. In this work the used PCR program was set up as the following: an initial denaturation (95°C for 5 min), 30 cycles of denaturation (94°C for 1 min), annealing (56°C for 1 min) and extension (72°C for 2 min), and a final extension (72°C for 10 min). The PCR fragments were purified by a PCR purification kit which purchased from Invitrogen. Sequencing of the purified PCR fragment was carried out bi-directionally using the dideoxy chain termination method. The similarity and homology of the16S rDNA partial gene sequence were analyzed with the similar existing sequences available in BLAST network services at the NCBI. Finally, multiple sequence alignment and molecular phylogeny were performed using BioEdit software (2006). The phylogenetic tree was displayed using the MEGA6 program (2013).
Screening of different fractions from Actinomycetes for Bio-fabrication of nano-Ag
The cellular bioactive metabolites were used for bio-fabrication of nano-Ag from cultures was determined by measuring the nano-Ag at different localities as extracellular, periplasmic and cytoplasmic fractions was prepared 5, 6, 8, 9.
Extracellular fraction
The grown cultures were filtered using suction filtration system so extracellular fraction collected. The residual pellets (grown cell biomass) were further used for preparing the periplasmic and cytoplasmic fractions.
Periplasmic fraction
The grown cell biomass were washed extensively using Milli-Q deionized water to remove any medium component then 10g (wet weight) of cells were added to 100ml distilled water in an Erlenmeyer flask and agitated again at 200rpm for 48 hr at 30°C. Then, the cell filtrate (periplasmic fraction) was obtained by filtering through Whatman filter paper No. 1.
Cytoplasmic fraction
The grown cell biomass (10g fresh weight) was re-suspended in 5ml cold 50mM PBS at pH 7.0, grinded by using mortar & pestle and mixed with 200ml of Millipore water deionized water in a 500 ml Erlenmeyer flask and agitated at 200rpm for 72hr at 37oC. The entire lysate was centrifuged; the remaining supernatant (cytoplasmic fraction) was collected and saved for bio- fabrication of nano-Ag.
Bio-fabrication of nano-Ag by using different fractions
Ten milliliters of different fractions were mixed with 10 m1 of 1 mM silver nitrate in flasks; this mixture incubated at 50oC and agitated on orbital shaker at 200 rpm in dark condition. Silver nitrate solution with no cell filtrate was used as controls under similar experimental conditions. After 24hr the formations of nano-Ag were screened by visual observation of color that changes to dark brown. Change in color was visually observed over a period of time. Then it was further confirmed by subjecting the reaction mixture to UV– Visible spectrophotometer analysis. The spectrum was scanned at the resolution of 1 nm, between 300–1000 nm for each sample. For all fractions; nano-Ag dry mass weight (g/l) was determined after 24hr.
Characterization of Bio-fabricated nano-Ag
The UV–visible spectrometry measurements (City of Scientific Research and Technological Applications (STRA-CITY), Egypt) were carried out in ELICO double beam equipment (Model Lambda 35) in the wavelength range of 100–600 nm range. FTIR analysis was performed with SHIMADZU FTIR 8400S at STRA-CITY, Egypt. The wavelength intervals of 400-4000cm-1 wave were used to record the spectra. XRD measurements of the bio-fabricated nano-Ag were carried out on X-ray diffractometer (XRD-600, SHIMADZU, Japan) instrument operating at a voltage of 40 kV and current of 30 mA with Cu K (±) radiation to determine the crystalline phase. EDX analysis was performed on a scanning electron microscope (JEOL JEM 6110 Japan–Japan University of Science and technology (E-JUST), Egypt ), attached with an EDX detector (OXFORD X-MAX instrument –E-JUST, Egypt), to confirm the bio-fabricated nano-Ag. EDX spectrum was measured at 20 kV accelerating voltage. To determine the size and shape of the nano-Ag, TEM measurements were performed on an instrument (JEOL-JEM 2100F –JAPAN –E-JUST- Egypt), operating at accelerating voltages of 120 keV at 500 nm scales.
Optimization for Actinomycetes biomass production by using statistical experimental designs (Plackett-Burman and Box-Behnken design)
A single cell is considered as a microscopic biochemical factory. Materials such as carbon, nitrogen, and others are absorbed by the cell and processed in the cell via hundreds of reactions to the various constituents 2. Also, biochemical products may be retained or transported back into the environment outside the cell. Metabolic activities inside the cell are regulated at various levels 3 .In this work; the bioprocessing strategies like comparison of biomass production among different industrial media, Plackett-Burman and Box-Behnken experimental design as well as Excel solver were applied in the optimization of the nutritional condition for the biomass production of the identified Streptomyces strain
Biomass production at different industrial media
Four published formulated media was chosen in this experiment 4,15,41 Medium No. 1(ISP 1);(g/l)Yeast Extract 3.0 and Tryptone 5.0, (pH 7.0), Medium No. 2; Modified Bennett’s Broth medium(ISP2);(g/l) Yeast Extract 4.0; Malt Extract10.0; Dextrose 4.0 (pH 7.3), Medium No.3; industrial medium for Streptomyces,(g/l) Glucose 20.0, Yeast extract 10.0, Peptone 10.0, K2HPO4 0.05, MgSO4 .7H2O 0.5, NaCl 4.0, CaCO3 5.0 (pH 6.8), and finally medium No.4;(g/l) Dextrose 20.0, K2HPO4 1.0, MgSO4 0.5 (pH 7.0). All media dissolved in distilled water up to 1 L and sterilized for 15 min at 121º C, inoculated by Streptomyces strain until 0.8 as initial O.D600 (zero time for cultivation) and finally, the flasks incubated at 30°C, 200rpm. The culture growth was monitored and the biomass weight (g/l) was determined after 24, 48, 72, and 96 hr.
Identifying the Significant Variables Using Plackett-Burman Design
Screening process was carried out by conducting the experiments to determine which variables significantly affected microbial biomass production. The seven independent variables tested in this application and their settings are recorded in (Table 1).
Table (1):
Values of the variables randomized in Plackett-Berman experimental design for biomass production from the selected Actinomycetes strain.
Trails |
Medium component |
Low level (-1) |
High level (+1) |
|---|---|---|---|
X1 |
Yeast extract |
7 |
15 |
X2 |
Peptone |
7 |
15 |
X3 |
Glucose |
16 |
25 |
X4 |
NaCl |
2 |
8 |
X5 |
K2HPO4 |
0.05 |
0.2 |
X6 |
MgSO4 |
0.4 |
1 |
X7 |
CaCO3 |
4 |
10 |
Based on the biomass production, the factorial experiment was analyzed using regression analysis and ANOVA. The model created for the analysis of Plackett-Burman design using multiple regression analysis is based on the 1st order model (Eq. 1):
Y= βo + ∑βi xi …(1)
Where: Y is the response (biomass production), ²o is the model intercept, ²i is the variable estimate; and Xi represents the variable.
The predicted optimum levels of the independent variables were carried out and compared with the basal conditions and the averages of biomass production were calculated.
Table (2):
Values of the biomass production variables randomized in Box-Behnken design.
g/L |
High level (1) |
Medium level (0) |
Low level (-1) |
|---|---|---|---|
Yeast extract |
20.0 |
15.0 |
10.0 |
Peptone |
20.0 |
15.0 |
10.0 |
K2HPO4 |
0.5 |
0.2 |
0.1 |
Box-Behnken Design (Response surface methodology)
Three variables Box-Behnken design for response surface methodology was used to study the combined effect of Yeast extract, Peptone, and K2HPO4 on biomass production over three levels. In this study, the experimental plan consisted of 15 trials and the independent variables were studied at three different levels, low (“1), medium (0), and high (+1) as shown in (Table 2). This kind of optimization design involves three main steps: performing the statistically designed experiments, estimating the coefficients of the structured mathematical model & predicting the response and checking the adequacy of the model 4.The experimental results of RSM were fitted via the response surface regression procedure using the following second-order polynomial equation (Eq. 2):
𝑌 = 𝛽0 + ∑𝑖𝛽𝑖𝑋𝑖 + ∑𝑖𝑖𝛽𝑖𝑖𝑋𝑖2 + ∑𝑖𝑗𝛽𝑖𝑗𝑋𝑖𝑋𝑗 …(2)
In the equation above Y is the predicted response, b0 is the regression coefficients, bi is the linear coefficient, bii is the quadratic coefficients, bij is the interaction coefficients, and X1 is the coded levels of independent variables. However, in this study, the independent variables were coded as X1, X2, and X3. Thus, the second-order polynomial equation can be presented as Eq. 3:
𝑌 = 𝛽0 + 𝛽1𝑥1 + 𝛽2𝑥2 + 𝛽3𝑥3 + 𝛽12𝑥1𝑥2 + 𝛽13𝑥1𝑥3+ 𝛽23𝑥2𝑥3 + 𝛽11𝑥1 2 + 𝛽22𝑥2 2 + 𝛽33𝑥3 …(3)
Optimization for nano-Ag reaction by Box-Behnken statistical experimental design
To establish the best condition for biofabrication of nano-Ag to get the large mass weight of nano-silver, the biofabrication reactions set up were prepared by using the different concentration of the precursor (0.5, 1.0, 2.0M) which prepared and added to the different reductant concentration (0.5, 5.0, 10.0 % v/v), then these mixtures were incubated at different temperature (30, 40 and 50°C) in ranges of pH (5.5-6) in light or dark conditions (Table 3), Box-Behnken modeling and the final results analysis were carried out using the Microsoft Excel 2007 and essential experimental design software by multiple regression analysis to evaluate the analysis of variance (ANOVA).
Table (3):
The coded and actual values of the studied variables at various levels.
Code |
Variables |
Low level (-1) |
Medium level (0) |
High level (1) |
|---|---|---|---|---|
F1 |
Precursor concentration (M) |
0.5 |
1 |
2 |
F2 |
Reductant concentration (v/v) |
0.5% |
5% |
10% |
F3 |
Temperature (ᵒC) |
30 |
40 |
50 |
Large scale batch fermentation for biomass production
The most common fermentation system is the batch fermentation, due to its simplicity and low cost. This is a closed system in which there is no input or output of materials, and the microbial population cell density increased constantly until exhaustion of some nutrient components of the culture medium which decreased over time and the produced bioactive compounds during growth increased in the culture medium 21, 23. The scale-up process was automated through a computer aided data bioprocessing system Bio Command ((BIOFLO® 310) multi-process management program. The bioreactor was initially contained 4.6 Liter of optimized medium and autoclaved for 20 min at 121°C. There are several mathematical relationships of specific growth rate coefficient to the concentration of growth-limiting nutrient 23.
Application of nano-Ag against multidrug-resistant human pathogens in vitro
The antimicrobial activity of the pure nano-Ag (µg/ml) was evaluated using the well diffusion method 14, 32 against tested multidrug-resistant human pathogens. Briefly, PDA plates and MHA ager plates were prepared and, subsequently, 100µl of fungal inoculums (1-5 x 106 CFU/ml) and bacterial inoculums of 1×108 CFU/ml were uniformly spread onto the plates. Then, a 50µl aliquot of nano-Ag solution was loaded into wells. The plates were then incubated at 30ºC for 24-72hr. Finally, the inhibition halo was measured.
Minimal inhibitory concentration (MIC)
Antimicrobial activity of nano-Ag was examined using the standard broth dilution method 43 according to the Clinical and Laboratory Standards Institute. The MIC was determined in LB broth using serial two-fold dilutions of nano-Ag in concentrations ranging from 200 to 10µg/ml; initial bacterial inoculums were (1×108CFU/ml,0.5 McFarland’s standard) and the time and temperature of incubation being 24hr at 37°C, respectively. The MIC is the lowest concentration of antimicrobial agents that completely visually inhibits 99% growth of the microorganisms. The MIC measurement was done in triplicate to confirm the value of MIC for each tested bacteria 1.
Minimal bactericidal concentration (MBC)
After MIC determination of the nano-Ag tested, aliquots of 100µl from all tubes in which no visible bacterial growth was observed were seeded in LB agar plates not supplemented with nano-Ag and were incubated for 24hr at 37°C. The MBC endpoint is defined as the lowest concentration of antimicrobial agent that kills 100% of the bacterial population 36.
Minimum fungicidal concentration (MFC)
In this case, MIC was the lowest concentration of nano-Ag that resulted in visual inhibition of fungal growth. The determination of the minimum fungicidal concentration (MFC) was performed after 48hr of treatment with the inhibitory concentrations used in the broth micro dilution assay. An aliquot of all treatments was transferred onto PDA plates. The plates were incubated at 30ºC for 72hr and the MFC was determined. MFC means the lower concentration which showed no fungal growth18.
Statistical analysis
MIC, MFC and MBC tests were performed in triplicate, and the results were expressed as the mean. Student’s test was used to compare these results. P values lower than 0.05 were considered significant
Isolation and screening of Actinomycetes for the bio-fabrication of nano-Ag and its efficiency as an antimicrobial agent
A total of different 16 Actinomyces were isolated from collected sediments and soil samples, coded and checked for their ability to produce silver nanoparticles. Among the tested isolates, only E3.2 isolate showed the ability to fabricate more effective nano-Ag as an antimicrobial agent against human pathogenic bacteria and fungi (Table 4, 5). The Ag+ ions reduction was evidently noticeable when AgNO3 was added to the E3.2 supernatant, and the color changed from yellow to dark brown compared to no color development for the control. Due to the reduction of Ag+ ions and formation of surface plasmon resonance (SPR) in the reaction mixture, while no color change appeared in E3.2 culture filtrates without silver nitrate. Synthesis of nano-silver was confirmed as indicated by UV-Visible spectra (at a range of 300nm-600nm) of the reaction mixture after 24hr. The UV-Vis spectrum showed SPR peak of silver nanoparticles at 420nm (Fig.1). Other studies reported same color change during the extracellular biosynthesis of nano-Ag 18, 26. The synthesis of nanoparticles by Actinomycetes has many advantages as they are safe to handle, easily available, and possess variable metabolites that may help in the reduction. Moreover, these particles have innumerable applications 24.
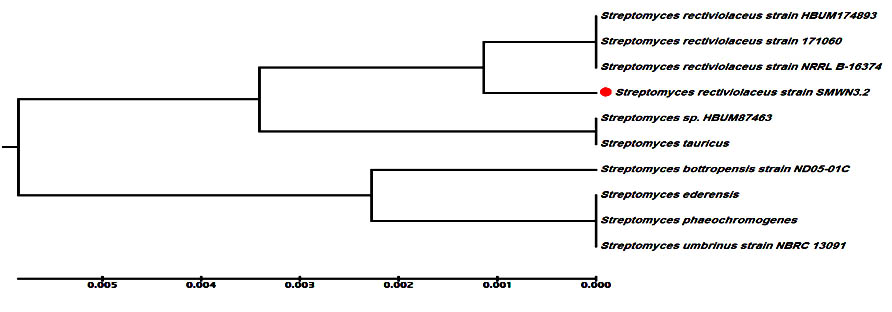
Fig. 1. The most potent isolate (E3.2) which showed ability to fabricate more affective nano-Ag as antimicrobial agent against human pathogenic bacteria and fungi A; UV-visible spectroscopy analysis showed SPR peak of nano-Ag at 420 nm: nano-Ag compared with bioactive supernatant fraction as a control B; Digital photographs indicates biosynthesis nano-Ag reaction by using bioactive supernatant fraction as reducing agent and silver nitrate as a precursor: nano-Ag compared with bioactive supernatant fraction as a control C; Digital photographs indicates color of the aerial mycelium of the selected isolates grown on ISP2 medium for 7days at 30 °C D; Scanning electron micrograph showing the spore-chain morphology and spore-surface ornamentation at constant magnification
Table (4):
Zone of inhibition of bio-fabricated nano-Ag against different human pathogenic bacteria.
| Actinomycetes isolates | Bio-fabricated nano-Ag Average inhibition Zone(diameter mm ) |
|||||
|---|---|---|---|---|---|---|
| Staphylococcus aureus | Klebsiella pneumonia | Pseudomonas aeruginosa | Salmonella typhi | Vibrio cholera | E.coli | |
| E 58 | 8±0.01 | 8±0.01 | 10±0.01 | 8±0.01 | 8±0.02 | 2±0.01 |
| G 45 | 10± 0.001 | 3±0.02 | 5±0.01 | 3±0.05 | 3±0.03 | 3±0.02 |
| E 3 | 15±0.01 | 7±0.01 | 11±0.01 | 7±0.04 | 7±0.01 | 5±0.02 |
| H 8 | 3±0.02 | 4±0.02 | 3±0.01 | 4±0.05 | 4±0.01 | 4±0.01 |
| G 40 | 3±0.001 | 8±0.02 | 7±0.01 | 8±0.02 | 8±0.20 | 6±0.03 |
| G 49 | 14±0.10 | 10±0.01 | 0 | 10±0.01 | 10±0.02 | 10±0.01 |
| E 59 | 6±0.20 | 2±0.01 | 6±0.01 | 2±0.02 | 2±0.01 | 2±0.01 |
| E 3.1 | 8±0.20 | 5±0.01 | 7±0.01 | 5±0.01 | 5±0.02 | 5±0.04 |
| G 50 | 13±0.04 | 6±0.01 | 6±0.01 | 6±0.01 | 6±0.02 | 6±0.03 |
| E 3.2 | 16±0.01 | 14±0.04 | 15±0.02 | 19±0.01 | 19±0.02 | 15±0.02 |
| E 56 | 3±0.01 | 4±0.01 | 2±0.03 | 2±0.01 | 4±0.02 | 3±0.01 |
| G 46 | 6±0.01 | 3±0.01 | 4±0.04 | 4±0.01 | 3±0.02 | 6±0.01 |
| G 51 | 4±0.02 | 4±0.04 | 5±0.01 | 5±0.01 | 4±0.01 | 4±0.01 |
| G 41 | 5±0.01 | 4±0.04 | 7±0.01 | 7±0.02 | 4±0.01 | 5±0.04 |
| E 56 | 3±0.10 | 3±0.10 | 3±0.01 | 3±0.02 | 3±0.01 | 3±0.01 |
| H 58 | 4±0.01 | 6±0.12 | 7±0.01 | 7±0.01 | 6±0.02 | 4±0.02 |
The data represents mean values of three independent experiments± SD.
Table (5):
Zone of inhibition of bio-fabricated nano-Ag against different human pathogenic fungi.
| Actinomycetes isolates |
Bio-fabricated nano-Ag Zone of inhibition (diameter mm ) |
|||
|---|---|---|---|---|
| Fusarium sp. | Aspergillus niger | Aspergillus fumigates | Candida albicans | |
| E 58 | 2±0.01 | 8±0.01 | 10±0.01 | 3±0.03 |
| G 45 | 1±0.01 | 3±0.01 | 5±0.01 | 5±0.03 |
| E 3 | 10±0.02 | 7±0.01 | 13±0.01 | 9±0.01 |
| H 8 | 3±0.01 | 4±0.02 | 3±0.01 | 3±0.01 |
| G 40 | 3±0.04 | 8±0.01 | 7±0.01 | 7±0.01 |
| G 49 | 5±0.02 | 6±0.02 | 2±0.01 | 2±0.01 |
| E 59 | 2±0.01 | 4±0.01 | 4±0.04 | 4±0.01 |
| E 3.1 | 4±0.01 | 5±0.01 | 7±0.01 | 7±0.01 |
| G 50 | 3±0.05 | 6±0.01 | 6±0.01 | 6±0.01 |
| E 3.2 | 10±0.01 | 12±0.03 | 13±0.01 | 11±0.01 |
| E 56 | 3±0.01 | 3±0.01 | 2±0.02 | 2±0.01 |
| G 46 | 0 | 3±0.02 | 4±0.01 | 4±0.01 |
| G 51 | 4±0.01 | 4±0.01 | 5±0.01 | 5±0.01 |
| G 41 | 5±0.02 | 4±0.01 | 7±0.02 | 7±0.05 |
| E 56 | 3±0.01 | 3±0.08 | 3±0.01 | 3±0.01 |
| H 58 | 0 | 6±0.04 | 7±0.01 | 7±0.01 |
The data represents mean values of three independent experiments± SD.
Molecular identification of the most potent isolate
Actinomycete (isolate E3.2) that produced the highly effective nano-Ag capable of inhibiting the growth of all tested human pathogens (bacteria and fungi) was selected. This isolate was identified by using molecular phylogenetic analysis. Comparisons of the partial 16S rDNA sequence of this isolate with other 16S rRNA sequences available in GenBank using BLAST searches were used to select related sequences for constructing a multiple alignment. The 16S rDNA gene sequence showed that the actinomycete isolate was similar to Streptomyces rectiviolaceus with an identity of 99%. Neighbor-joining method with the software package MEGA6 was used to construct the phylogenetic tree based on 16S rDNA gene sequences of members of the genus Streptomyces (Fig.2). The sequence of nucleotide was deposited into the database of GenBank as Streptomyces rectiviolaceus strain SMWN3.2 with accession number KX077914 https://www.ncbi.nlm.nih.gov/nuccore/1036392264.
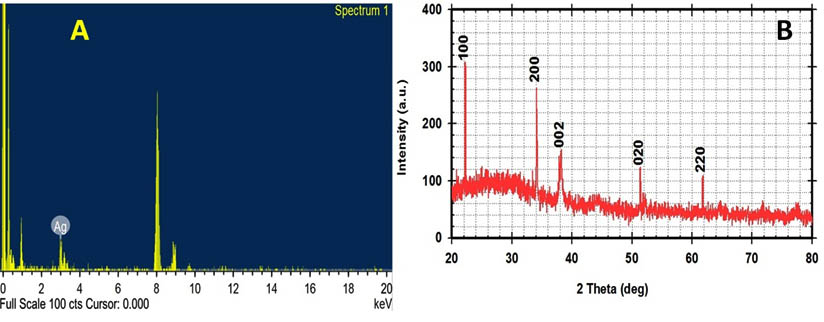
Fig. 2. The phylogenetic tree of S.rectiviolaceus strain SMWN3.2 was constructed using the neighbour-joining method with aid of MEGA 6.0 program
Bio-fabrication of nano-Ag by using different fractions
Previously, nanoparticles biosynthesis with different size and shapes depends on the microorganisms optimized by using the concentration of the bioactive compounds, the concentration of used metal ions and the reaction incubation period. Through this study, to facilitate verification, of extracting cell protein and bioactive compounds that founded in the extracellular, periplasmic and cytoplasmic fractions was observed. All these fractions produced nano-Ag but the high nano-Ag dry weight was recorded by using the extracellular fraction as a reducing agent (Fig.3).
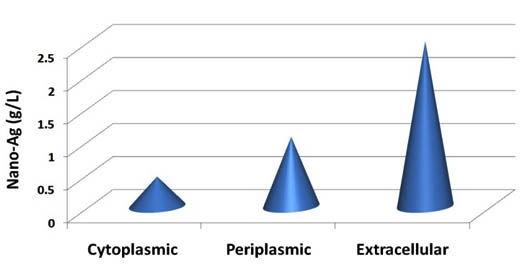
Fig. 3. Dry mass weight of nano-Ag powder bio-fabricated by using the extracellular, periplasmic, and cytoplasmic fractions from S.rectiviolaceus strain SMWN3.2
Characterization of bio fabricated nano-Ag from Streptomyces rectiviolaceus strain SMWN3.2
Electron microscope analysis
The electron microscopes are a powerful analysis to determine the morphology and particle distribution of nanostructures, so in this work, the electron microscopy micrographs of the bio fabricated nano-Ag revealed the formation of extracellular spherical and elongated nanoparticles with a size range of 8.9–11.4nm as shown in (Fig.4). The sizes of the nanoparticles which recorded from the previous reports were ranged from 25-80nm by using a microbial biosynthesis method 7, 39. The smaller size of nano-Ag is very important to medical and pharmacological purposed because they could easily entrance into the microbial cell through the cell membrane to destroy it 7.
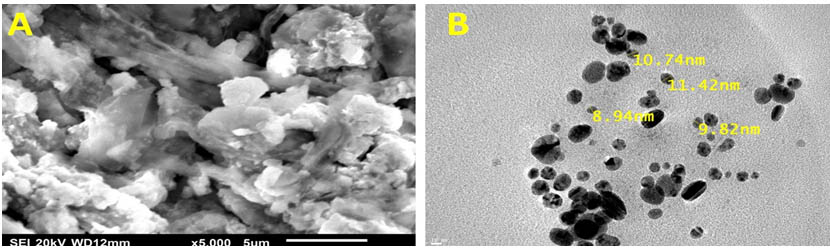
Fig. 4. SEM (A) and TEM (B) Image of nano-Ag bio-fabricated by the reaction of 1mM AgNO3 and the cell-free culture supernatant of S.rectiviolaceus strain SMWN3.2. Scale bar of 5μm and 10nm respectively
XRD and EDX analysis
X-ray analysis and EDX analytical technique used for analysis the elemental composition of metal nanoparticles and confirmed the presence of the silver ions as the major constituent element. The spectrum at 3keV indicates a strong signal for silver (Fig.5A), which is characteristic to nano-sized metallic silver 39, 42. In this study, the next method used to improve nano-Ag formation was x-ray diffractometry (XRD) to determine the chemical composition of its surrounded thin layer by X Ray diffraction diagram 16, 17. The obtained XRD spectrum was matched with JCPDS card No.010893722 that exhibits the silver peaks observed at 2¸ values of 22.71o, 32.16o, 38.28°, 52.70o and 64.64° as shown in (Fig.5B). Other workers have been confirmed the biosynthesized Ag NPs by using XRD and JCPDS card 89-3722, with similar results to those obtained in the current study 18, 24, 26.
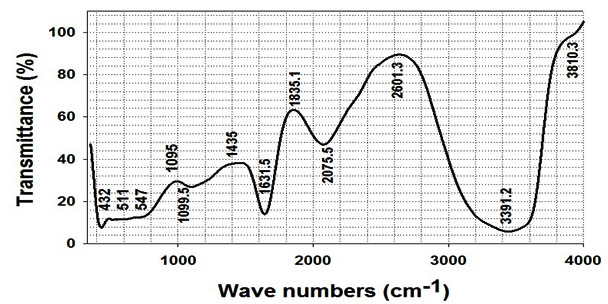
Fig. 5. EDX spectrum (A) showing a peak between 3keV and XRD pattern (B) of nano-Ag bio-fabricated using cell free supernatant of S.rectiviolaceus strain SMWN3.2 confirming the presence of silver
Identifications of functional group using FT-IR
Fourier transformed infrared spectroscopy used for determining the chemical composition of the nanoparticles surface which used as a stabilizing agent that prevent the reduced silver particles agglomeration17,28 and identifying the possible biomolecules which responsible for the reduction of the Ag+ ions into Ag° as shown in (Fig.6). Some other workers have reported that functional groups such as -C-O-C-, -C-O-, and -C=C- are derived from heterocyclic compounds like proteins which present as the capping ligands of the nanoparticles 29,34. Our findings were confirmed by other studies who reported the stabilization of the nano-Ag by proteins and bioactive compounds, which surround or bind to nanoparticles through free amine groups and residues in the proteins and through the electrostatic attraction of negatively charged carboxylate groups in the cell-free supernatant 29, 31, 34. Finally, we can conclude that the actinomycetes, which secrete the higher proportions of the bioactive substances, may make it more suitable for the production of nano-Ag. The precise reaction mechanism leading to the bio fabricated nanoparticles is not definitely realized yet 28. In this regard, the results obtained in this work open several avenues of further studies.
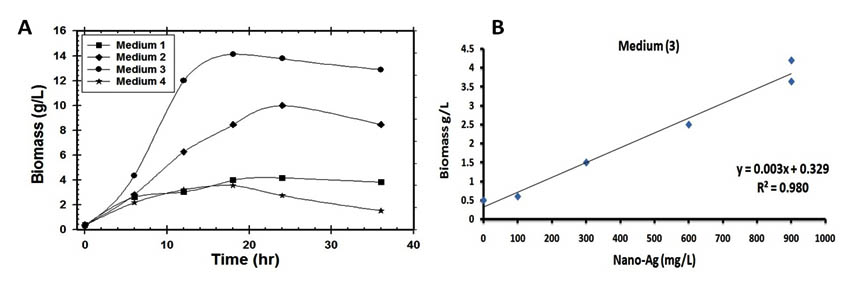
Fig. 6. FTIR spectrum recorded from a drop-coated film of nano-Ag bio-fabricated by S.rectiviolaceus strain SMWN3.2
Statistical evaluation of the medium components for the production of high biomass using Plackett-Burman and Box-Behnken experimental designs
The aim of these examinations was to optimize the best medium formulation for the cultivation of Streptomyces rectiviolaceus strain SMWN3.2 through the following steps:
Biomass production at different industrial media
As a general the success of industrial production for biological control agents depending not only the isolation, characterization & pathogenicity but also on the successful mass production of the microbial cells in laboratory23. The selection of a carbon source to enhance microbial growth without catabolic repression is highly desirable because a high concentration of microbial biomass is required in order to maximize bioactive compounds & proteins productivity 10, 23. The relation between bioactive compound, protein secretion and the growth profile of the microorganism is a key consideration for increased nanoparticles production. Hence, the growth medium could be one of the main factors affecting cells and protein production during a fermentation process 11, 23. Therefore, the use of high cell density cultivation is required in order to improve nano-Ag biosynthesis 13, 23. So, in the present study several substrates of both organic, inorganic and mixed media were tested for mass multiplication of Streptomyces rectiviolaceus strain SMWN3.2 to select the best medium to achieve high cell growth. Among the tested media, medium 3 produced significantly higher 4.2 g/l of biomass production (Fig.7) and 900 mg/l of nano-Ag dry weight was recorded.
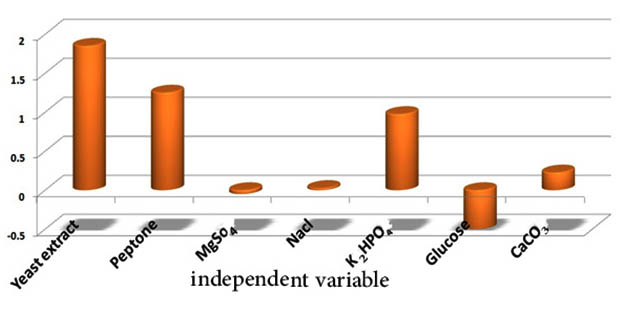
Fig. 7. (A) Time course of biomass production of S.rectiviolaceus strain SMWN3.2 cultivated in various industrial media and (B) The correlation between produced nano-Ag (mg/L) and biomass mass weight (g/L) for S.rectiviolaceus strain SMWN3.2 which grown in medium (3)
Optimization of the culture medium formulation by application of Plackett–Burman design
This design has been applied to evaluate the significant effect of selected medium components according to a design matrix (Table 6). The main effects of each variable were calculated and the t-values were estimated for each variable to identify the statistical significance of the measured response and determine the main effects (Fig.8). This experiment was carried out to verify the optimization conditions to validate the obtained optimized medium. Streptomyces rectiviolaceus strain SMWN3.2 was grown on the optimized medium of the following components (g/l): 15 Yeast extract, 15 Peptone, 25 Glucose, 8 NaCl, 0.2 K2HPO4, 1 MgSO4 and 10 CaCO3, recorded the larger biomass production (10g/l) than that in the basal conditions by 2.38 fold increase. The present result confirms the validity of the optimized conditions.
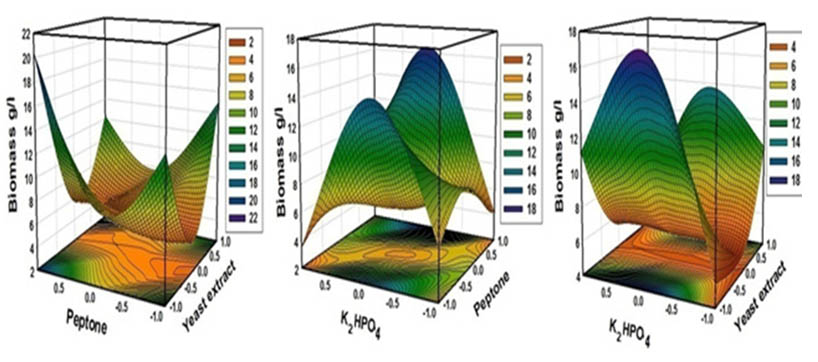
Fig. 8. Column chart shown the main of culture variables influencing the S.rectiviolaceus strain SMWN3.2 biomass production
Table (6):
Independent variables matrix and the experimental results of Plackett–Burman design
for seven variables affecting S.rectiviolaceus strain SMWN3.2 biomass production.
Trail |
Yeast extract |
Peptone |
MgSO4 |
NaCl |
K2HPO4 |
Glucose |
CaCO3 |
Biomass g/l |
Predicted biomass g/l |
|---|---|---|---|---|---|---|---|---|---|
1 |
1 |
-1 |
-1 |
1 |
-1 |
1 |
1 |
4.50 |
4.64 |
2 |
1 |
1 |
-1 |
-1 |
1 |
-1 |
1 |
9.90 |
10.04 |
3 |
1 |
1 |
1 |
-1 |
-1 |
1 |
-1 |
6.40 |
6.54 |
4 |
-1 |
1 |
1 |
1 |
-1 |
-1 |
1 |
4.20 |
4.34 |
5 |
1 |
-1 |
1 |
1 |
1 |
-1 |
-1 |
6.90 |
7.04 |
6 |
-1 |
1 |
-1 |
1 |
1 |
1 |
-1 |
4.80 |
4.94 |
7 |
-1 |
-1 |
1 |
-1 |
1 |
1 |
1 |
2.60 |
2.74 |
8 |
-1 |
-1 |
-1 |
-1 |
-1 |
-1 |
-1 |
1.29 |
1.43 |
Statistical optimization of Streptomyces rectiviolaceus strain SMWN3.2 biomass production by using Response surface methodology (Box-Behnken Design)
In order to determine the maximum S. rectiviolaceus SMWN3.2 biomass production (Table7) and to calculate the optimal levels of the tested variables (yeast extract, peptone, and K2HPO4) the second-order polynomial model was proposed in this work and the independent variables was obtained as shown in Eq.4.
Y = 5.46 -0.82*F1 + 0.59*F2 + 0.22*F3 + 6.21*F1*F1 + 3.36*F2*F2 -3.67*F3*F3 -1.93*F1*F2 -2.75*F1*F3 + 1.44*F2*F3 …(4)
A multiple regression analysis of the experimental data was applied and a second-order polynomial model indicates the role of each variable and their second order interactions, which affected on biomass production. An ANOVA analysis was employed to detect the significance and the adequacy of this model, which carried by Fisher’s statistical test for the analysis of variance and the regression model results, demonstrated that the model is highly significant as was evident from the Fisher’s F-test (67.06) at a very low probability value (0.000112). Optimal concentrations of the tested variables were experimentally verified and compared with the theoretically predicted data and the predictive accuracy of the model was 96%. The interaction effects of the variables and biomass production were determined (Fig.9). Finally, a verification experiment was carried out to validate the obtained optimized medium which composed of (g/l): 10 yeast extract, 20 peptone, 0.37K2HPO4, 1 MgSO4 ,10 CaCO3 , 25 Glucose and 8 NaCl, that recorded the larger biomass production (16g/l) than that in the basal medium (4.2) by 3.8 fold.
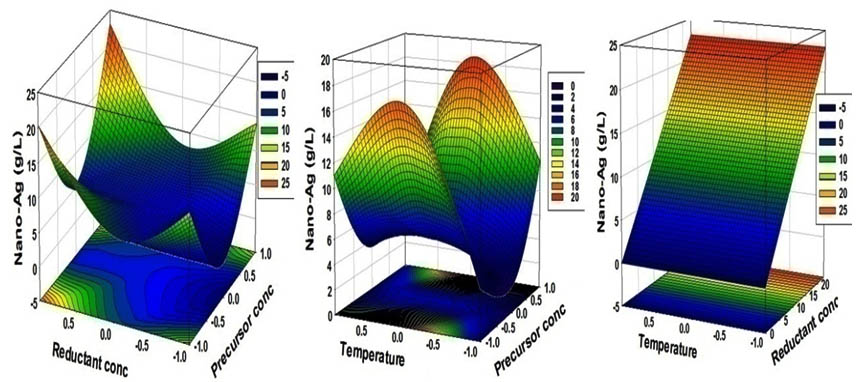
Fig. 9. Three-dimensional response surfaces plot representing S.rectiviolaceus strain SMWN3.2 biomass production yields (g/l)
Table (7):
Independent variables matrix and the experimental results of Box-Behnken design for variables affecting S.rectiviolaceus strain SMWN3.2 biomass production.
Exp # |
Yeast extract |
Peptone |
K2HPO4 |
Biomass (g/l) |
Predicted biomass (g/l) |
|---|---|---|---|---|---|
1 |
0 |
0 |
0 |
5.6 |
5.47 |
2 |
-1 |
-1 |
0 |
13.9 |
13.33 |
3 |
0 |
1 |
1 |
7.3 |
7.41 |
4 |
0 |
-1 |
1 |
3.5 |
3.34 |
5 |
0 |
0 |
0 |
5.2 |
5.47 |
6 |
1 |
1 |
0 |
12.3 |
12.88 |
7 |
1 |
0 |
1 |
5.2 |
4.66 |
8 |
1 |
0 |
-1 |
10.3 |
9.71 |
9 |
-1 |
1 |
0 |
20.4 |
19.84 |
10 |
-1 |
0 |
1 |
11.2 |
11.79 |
11 |
0 |
1 |
-1 |
4.2 |
4.08 |
12 |
0 |
-1 |
-1 |
5.6 |
5.77 |
13 |
-1 |
0 |
-1 |
5.3 |
5.84 |
14 |
0 |
0 |
0 |
5.6 |
5.47 |
15 |
1 |
-1 |
0 |
15 |
15.56 |
Statistical evaluation of the bio-fabrication nano-Ag reaction by Box-Behnken experimental design
Silver nanoparticles can be induced to have different forms depends on filtrate volume, salt concentration and physical conditions: pH, and light intensity that affects the maximum yield, the rate of synthesis and its size. There are many previous studies connected the stability and antimicrobial activity of nano-Ag bio-fabricated from different actinomycetes with pH and reported 18, 26 the optimum pH was ranged from 5 to 9. In this work by using survey experiments on nano-Ag bio-fabrication reaction at different physical conditions (pH, and light intensity), we observed the narrow ranges of pH (5.5-6) in light or dark conditions (these results unpublished). So these physical conditions used constantly in all work experiments. The results obtained from the experimental runs carried out according to the Box-Behnken design is summarized in (Table 8). In this study, a total of 15 experiments with the different combination of precursor conc. (AgNO3), reductant conc. (cell filtrate) and the temperature were performed and the results of experiments for studying the effects of three independent variables on bio-fabrication of nano-Ag are presented.
Table (8):
Independent variables matrix and the experimental results of Box-Behnken design for variables affecting on extracellular bio-fabrication of nano-Ag from S.rectiviolaceus strain SMWN3.2.
Trails |
Precursor concentration |
Reductant concentration |
Temperature |
Nano-Ag (g/l) |
Predicted nano-Ag (g/l) |
|---|---|---|---|---|---|
1 |
0 |
0 |
0 |
5 |
5.0 |
2 |
-1 |
-1 |
0 |
14 |
14.8 |
3 |
0 |
1 |
1 |
7 |
7.3 |
4 |
0 |
-1 |
1 |
0 |
-0.6 |
5 |
0 |
0 |
0 |
5 |
5.0 |
6 |
1 |
1 |
0 |
23 |
22.3 |
7 |
1 |
0 |
1 |
5.5 |
5.9 |
8 |
1 |
0 |
-1 |
10.5 |
10.7 |
9 |
-1 |
1 |
0 |
20.3 |
20.2 |
10 |
-1 |
0 |
1 |
11 |
10.8 |
11 |
0 |
1 |
-1 |
4 |
4.5 |
12 |
0 |
-1 |
-1 |
0 |
-0.3 |
13 |
-1 |
0 |
-1 |
4 |
3.6 |
14 |
0 |
0 |
0 |
5 |
5.0 |
15 |
1 |
-1 |
0 |
14 |
14.1 |
Statistical Analysis, ANOVA, and Model Fitting
The coefficient of determination (R2) of the model indicated that the model adequately represented the real relationship between the variables under consideration. The R2 value of 0.99 means that 99 % of the variability was explained by the model and only 1% of the total variance could not be explained by the model. Therefore, the present R2 value reflected a very good fit between the observed and predicted responses and implied that the model is reliable for nano-Ag bio-fabricated in this study. All values of model coefficients were calculated by multiple regression analysis. The significance of each coefficient was determined by Student’s t-test and P values. The P values were used as a tool to check the significance of each of the coefficients which, in turn, are necessary to understand the pattern of the mutual interactions between the test variables. The results of the second-order response surface model fitting in the form of analysis of variance (ANOVA) which required testing the significance and the adequacy of the model. The analysis of variance (ANOVA) of the regression model demonstrates that the model is highly significant, as is evident from Fisher’s F-test (147.79) and a very low probability value (0.0000158). In order to evaluate the relationship between dependent and independent variables and to determine the maximum dry mass weight of nano-Ag corresponding to the optimum levels of precursor conc., (X1), reductant conc. (X2), and temperature (X3), a second-order polynomial model was proposed to calculate the optimum levels of these variables. By applying the multiple regression analysis to experimental data, the second-order polynomial equation that defines predicted response (Y= dry mass weight of nano-Ag) in terms of the independent variables (X1, X2, and X3) was obtained:
Y = 5 + 0.56𝑋1 + 3.18𝑋2+ 0.62𝑋3 + 0.87𝑋1𝑋2− 3𝑋1𝑋3 + 0.78𝑋2𝑋3+ 8.81 (𝑋1)2 + 3.81 (𝑋2)2− 6.06(𝑋3)2
The three-dimensional response surface curves which are shown in Fig. (10) Were plotted to understand the interaction of the variables and the optimal levels of each variable required for the optimal production. Each figure presents the effect of two factors on nano-Ag production, while the third factor was held at the constant level.
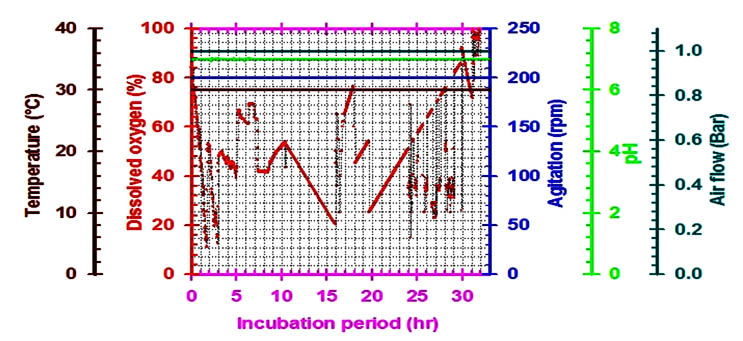
Fig. 10. Three-dimensional response surfaces plot representing nano-Ag dry mass weight yield (g/l) bio-fabricated by using the supernatant of S.rectiviolaceus strain SMWN3.2
Verification of the Model
The validity of the estimated results indicated from the regression model was confirmed by carrying out repeated experiments under optimal reaction conditions (precursor conc. (0.5M), reductant conc. (5v/v) and temperature (45°C). The results obtained from three replications indicated that the average of the maximum dry mass weight of nano-Ag obtained was close to the predicted result. The verification revealed a high degree of accuracy of the model (more than 98.9%), indicating the model validation under the tested conditions. The excellent correlation between the expected and measured values from these experiments indicates the validity of response model. The optimal levels of the process variables nano-Ag production recorded the larger yield production (20.4g/l) than that in the basal conditions (0.9 g/l) by 22.3 folds increase.
High cell density cultivation of Streptomyces rectiviolaceus strain SMWN3.2 in 7 L Stirred tank bioreactor
For industrial applications, actinomycetes should have certain properties which include a high production of a specific metabolite, high growth rate, easy handling in large-scale production and a low-cost requirement for production procedures(El-moslamy et al. 2016) . Many reports have appeared in a bioactive compound and nitrate reductase enzymes produced by the submerged cultivation of Streptomyces spp. The present investigation was carried out to evaluate available cheaper medium for mass multiplication of Streptomyces rectiviolaceus strain SMWN3.2 for bio-fabrication of nano-Ag as an antimicrobial agent by using submerged batch fermentation mode. The pre-culture of Streptomyces rectiviolaceus strain SMWN3.2 was inculcated in 7L stirred tank bioreactor (working volume 5L) to reach the initial biomass of 0.5g/l. Then the bioreactor was incubated and agitated at 30°C, 200rpm. The aeration, agitation, pH, temperature and composition of nutrients are considering the principle factors that have an effect on growth yield. The culture was maintained at higher value of dissolved oxygen, which decreased gradually indicating cell growth and glucose consumption from the culture broth. To ensure a sufficient oxygen supply, oxygen was kept above 20% through raising the agitation speed (Fig.11).In this experiment, by using kinetics measurements we conclude that; YX (30.5), P max was 85.5g/l; YP was 42.6 and the incubation period was 30hr. Fig. (12) Shows the growth patterns, kinetics in the batch culture, the concentration of consuming glucose, biomass and nano-Ag dry mass weight plotted against the time. Nano-Ag dry mass weight was increased near the end of the stationary phase. The biomass Xmax achieved was 67.5 g/l. Cells were grown directly after the lag phase which lasted for about 9hr. Cell mass was then increased exponentially over time with a specific growth rate, µmax (0.85 hr-1) within the exponential phase. Batch model prediction for biomass and nano-Ag production was higher than the shake flask experimental data at different stages of cell growth. The possible reason was that the fermentation conditions in stirred tank bioreactor (agitation, airflow, and pH) were controlled.
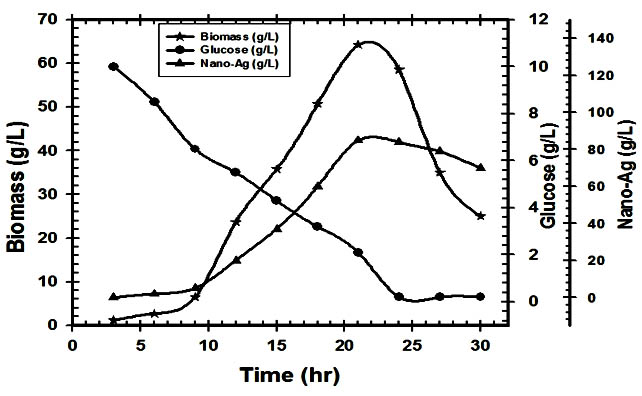
Fig. 11. On-line data (temperature, dissolved oxygen, pH, air flow rate and agitation) as a function of time during batch cultivation of S.rectiviolaceus strain SMWN3.2 in bioreactor
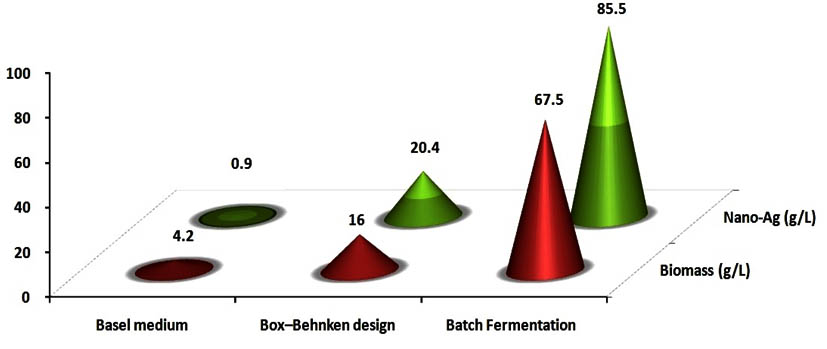
Fig. 12. Variation of growth kinetic parameters, biomass, glucose concentration and nano-Ag bio-fabricated from supernatant as a function of time for batch cultivation of S.rectiviolaceus strain SMWN3.2.
Application of bio-fabricated nano-Ag against multidrug-resistant human pathogens in vitro
Streptomycetes have received much attention because of their potential to produce bioactive compounds and useful enzymes for industrial applications. Nowadays many researchers focused on the biosynthesis of nano-Ag which is used as bioactive compounds against various human pathogens 20, 22, 25 with a broad spectrum against Gram-positive and Gram-negative bacteria as well as multi-resistant strains 27. In the present study, the antagonistic activity of nano-Ag bio-fabricated from Streptomyces rectiviolaceus strain SMWN3.2 with different concentrations was investigated against selected human pathogens (Table 9, 10). The maximum zones of inhibition ranged from 50 to 65mm by using 30 and 35µg/ml of nano-Ag, 35–54 mm by using 30 and 60µg/ml of nano-Ag for pathogenic bacteria and fungi, respectively. Generally, nano-Ag showed higher activities against the tested pathogenic bacteria rather than tested pathogenic fungi, and the highest activity was recoded against Streptococcus pneumonia (65mm) and Aspergillus fumigatus (54mm) respectively. MIC, MFC, and MBC of nano-Ag was evaluated against tested human pathogenic bacteria and fungi. The nano-Ag exhibited MIC against Streptococcus pneumoniae at 255µg/ml, & MBC at 505µg/ml and MIC against Aspergillus fumigates at 505µg/ml & MFC 605ßg/ml. Similarly, many other researchers have been demonstrated the reactive antimicrobial activity of silver nanoparticles 20, 22, 27. Also, it has been reported that silver nanoparticles synthesized by Streptomyces sp. have reactive antibacterial activity against test pathogens including Staphylococcus aureus, Proteus vulgaris, Escherichia coli, Shigella dysenteriae, Klebsiella pneumoniae and Salmonella typhi and the antimicrobial efficiency of Ag NPs was shape and size dependent. Ag NPs was reported to attach to the cell membrane surface and significantly damage its permeability and respiratory function 20.This work focuses on large-scale production of nano-Ag from high-cell-density filtrate of Streptomyces rectiviolaceus strain SMWN3.2 (Fig.13) as a novel antimicrobial agent against hospital-acquired infectious pathogens.
Table (9):
Antibacterial activity, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of nano-Ag against multidrug-resistant human pathogens.
| Human pathogenic bacteria | Pure bio-fabricated nano-Ag (μg/ml) | MIC (µg/ml) | MBC (µg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone of inhibition (mm) | ||||||||||||||
| 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 60 | 70 | |||
| Klebsiella pneumoniae | 12 | 15 | 15 | 2 | 35 | 53 | 48 | 41 | 35 | 12 | 10 | 5 | 30 | 40 |
| Pseudomonas aeruginosa | 21 | 15 | 29 | 31 | 34 | 45 | 50 | 35 | 25 | 2 | 5 | 1 | 25 | 30 |
| Salmonella typhimurium | 24 | 18 | 25 | 29 | 34 | 42 | 55 | 45 | 24 | 19 | 15 | 1 | 30 | 40 |
| Shigella flexneri | 15 | 23 | 23 | 28 | 35 | 46 | 55 | 36 | 24 | 14 | 5 | 2 | 20 | 35 |
| Staphylococcus aureus | 13 | 12 | 26 | 32 | 38 | 58 | 52 | 42 | 32 | 14 | 5 | 4 | 25 | 40 |
| Streptococcus pneumoniae | 19 | 26 | 27 | 35 | 41 | 65 | 58 | 52 | 45 | 19 | 4 | 1 | 25 | 50 |
Table (10):
Antifungal activity, minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of nano-Ag against multidrug-resistant human pathogens.
| Human pathogenic Fungi | Pure bio-fabricated nano-Ag (μg/ml) | MIC (µg/ml) | MFC (µg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone of inhibition (mm) | ||||||||||||||
| 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 60 | 70 | |||
| Campylobacter jejuni | 17 | 16 | 19 | 25 | 36 | 45 | 41 | 35 | 23 | 21 | 20 | 15 | 40 | 60 |
| Fusarium sp | 1 | 2 | 5 | 12 | 18 | 21 | 25 | 29 | 32 | 37 | 45 | 34 | 40 | 65 |
| Aspergillus niger | 2 | 4 | 7 | 15 | 19 | 25 | 28 | 32 | 32 | 34 | 35 | 33 | 60 | 75 |
| Aspergillus fumigates | 5 | 2 | 5 | 9 | 14 | 19 | 21 | 28 | 34 | 45 | 54 | 44 | 50 | 60 |
| Candida albicans | 1 | 3 | 6 | 11 | 16 | 50 | 45 | 36 | 30 | 25 | 20 | 15 | 50 | 80 |
In conclusion, the present work could successfully potentiated the biogenesis of nano-Ag mediated by Streptomyces rectiviolaceus strain SMWN3.2 with a remarkable antimicrobial activity against bacterial and fungal human pathogens with a successful mass production of the microbial cells in the laboratory. Optimization approaches have been successfully designed for bio fabrication of nano-Ag reaction. Actinomycete isolate has reached the high production of the specific metabolite with a high growth rate; easy handling in large-scale production and the low-cost requirement for production procedures at both the shake flask and stirred tank bioreactor levels. Finally, this current work could be applied on the large-scale production of nano-Ag as a novel antimicrobial agent against hospital-acquired infectious pathogens.
ACKNOWLEDGMENTS
The authors acknowledge the Bioprocess Development and FTR Departments, Genetic Engineering and Biotechnology Research Institute (GEBRI), City of Scientific Research and Technological Applications (SRTA-City), New Borg El-Arab, Alexandria, Egypt for providing the facilities for this work to be achieved.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Abadi FDM, Mehrabian S, Asghari B, Namvar E, Ezzatifar F, Lari R A.Silver nanoparticles as active ingredient used for alcohol-free mouth wash . GMS Hygiene and Infection Control, 2013; 8(1).
- Abdel-Fattah R Y, El-Enshasy AH, Soliman A N, El-Gendi H. Bioprocess Development for Production of Alkaline Protease by Bacillus pseudofirmus Mn6 Through Statistical Experimental Designs. J Microbiol Biotechnol, 2009; 19(4):378–386.
- Abdel-Fattah R Y, EL-Helow R E, Ghanem M K, and Lotfy A W. application of factorial designs for optimization of avicelase production by a thermophilic Geobacillus isolate. Research journal of microbiology, 2007a; 2(1):13-23.
- Abdel-Fattah R Y, Soliman A N, Yousef M S, and EL-Helow R E. Application of experimental designs to optimize medium composition for production of thermostable lipase/esterase by Geobacillus thermodenitrificans AZ1. Journal of Genetic Engineering and Biotechnology, 2012; 10: 193–200.
- Abdel-Hafez I I S, Nafady A N, Abdel-Rahim R I, Shaltout M A, and Mohamed A M. Biogenesis and Optimisation of Silver Nanoparticles by the Endophytic Fungus Cladosporium sphaerospermum. Int J Nano Chem, 2016a; 2(1):11-19.
- Abdel-Hafez S I I, Nafady N A, Abdel-Rahim R I, Shaltout M A, Daro ‘s J A, M A M . Assessment of protein silver nanoparticles toxicity against pathogenic Alternaria solan Biotech, 2016b; 6:199.
- Abd-Elnaby M H, Abo-Elala M G, Abdel-Raouf M U, and Hamed M M . Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine Streptomyces rochei MHM13. Egyptian Journal of Aquatic Research, 2016; 42(3):301-312.
- Abu-Elsaoud A M, and Hassan H M. Evaluation of chlorophyll meter method in Egyptian wheat cultivars after Photobiostimulation with Red Polarized Light. Catrina, 2015b; 11: 67–74.
- Abu-Elsaoud M A, Abdel-Azeem M A, Mousa A S, and Hassan SM S. Biosynthesis optimization and Photostimulation of ±-NADPH-Dependent Nitrate Reductase-Mediated Silver Nanoparticles by Egyptian Endophytic Fungi. Adv Environ Biol, 2015a; 9(24), 259-269 .
- Ahamed M E H, Marjanovic L, and Mbianda X Y. Statistical Optimization, Kinetic and Isotherm Studies on Selective Adsorption of Silver and Gold Cyanocomplexes Using Aminoguanidyl-Chitosan Imprinted Polymer. J Adv Chem Eng, 2016; 6:149.
- Al-Sarrani A Q and El-Naggar M Y. Application of Plackett-Burman factorial design to improve citrinin production in Monascus ruber batch cultures. Bot Sci (Formerly BotBull Acad Sin), 2006; 47(2):167-174.
- Banerjee A, Banerjee S, and Sarkar P. Statistical design of experiments for optimization of arsenate reductase production by Kocuria palustris (RJB-6) and immobilization parameters in polymer beads. RSC Adv 6, 2016; 55: 49289-49297.
- Basavaraj U., Praveenkumar N., Sabiha T. S., Rupali S., and Samprita B. “Synthesis and characterization of silver nanoparticles”. Int J Pharm Bio Sci, 2012; 2(3):10–14.
- Bashardoust B, Mohammadi R S, Roudbary M, Nikoomanesh F . Susceptibility Evaluation of Aspergillus fumigatus to Silver Nanoparticles Compared with Voriconazole. Infect Epidemiol Med, 2016; 2(3): 20-23.
- Bhasin S and Modi A H. Optimization of Fermentation Medium for the Production of Glucose Isomerase Using Streptomyces sp. SB-P1. Biotechnology Research International, 2012; Article ID 874152, 10 pages.
- Bhosale SR, Hajare Y K, Mulay B, Mujumdar S, and Kothawade M. Biosynthesis, Characterization and Study of Antimicrobial Effect of Silver Nanoparticles by Actinomycetes spp. Int. J. Curr. Microbiol. App. Sci, 2015; 2: 144-151.
- Buszewski B, Railean-Plugaru V, Pomastowski P, Rafinska K, Szultka-Mlynska M, Golinska P, Wypij M, Laskowski D, and Dahm H . Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. Journal of Microbiology, Immunology and Infection, 2018; 51(1): pp.45-54. .
- Chen X, Schluesener H J. Nanosilver: a nanoproduct in medical application. Toxicol Lett, 2008; 176: 1–12.
- Das V L, Thomas R, Varghese RT, Soniya E V, Mathew J, Radhakrishnan E K . Extracellular synthesis of silver nanoparticles by the Bacillus strain CS11 isolated from industrialized area. Biotech, 2014; 4: 121-6.
- Desai P P, Prabhurajeshwar, Hudge VB, Krishnaraj P U, Kelmani CR . Characterizations and optimization study on influence of different parameters on fabricated spherical nanoparticles from Streptomyces sp. GUT 21 (KU500633). IJRET, 2016; 5(2): 161-172.
- El-Gendi H, Azab S M, Soliman A N, and Abdel-Fattah R Y. Application of Plackett-Burman Design for Optimization of Alkaline Protease and ±-amylase Production by the Marine Bacterium Bacillus methylotrophicus SCJ4. RJPBCS7, 2016; 4: 899-909.
- Elgorban M A, Al-Rahmah N A, Sayed R S, Hirad A, Mostafa A A, and Bahkali H A. Antimicrobial activity and green synthesis of silver nanoparticles using Trichoderma viride. Biotechnology & Biotechnological equipment, 2016; 30(2): 299-304.
- EL-Moslamy H S, Kabeil S A S and Hafez E E. Bioprocess development for Chlorella vulgaris cultivation and biosynthesis of anti-phytopathogens silver nanoparticles. J Nanomater Mol Nanotechnol, 2016; 5:1. Of, 9, p.2.
- El-Naggar E N, Abdelwahed A M N, and Darwesh M M O. Fabrication of Biogenic Antimicrobial Silver Nanoparticles by Streptomyces aegyptia NEAE 102 as Eco-Friendly Nanofactory. J Microbiol Biotechnol, 2014; 24(4): 453–464.
- El-Naggar E N, Mohamedin A, Hamza S S, and Sherief A. Extracellular Biofabrication, Characterization, and Antimicrobial Efficacy of Silver Nanoparticles Loaded on Cotton Fabrics Using Newly Isolated Streptomyces sp. SSHH-1E. Journal of Nanomaterials, 2016; Article ID 3257359, 1-18.
- El-Naggar M Y, Yousry M Gohar, Khouloud M Barakat, Nowara S Aly . Physiological response, anti bacterial activity, and cinnamaldehyde production by a marine Streptomyces chartreusis. Journal of Pure & Applied Microbiology, 2016; 10 3): 1797-1808.
- El-Naggar M Y, El-Aassar S A, Amany S Y, Nermeen A E and Beltagy I A. ²-Mannanase production by the immobilization of the locally isolated Aspergillus niger. Int J Agri Biol, 2006; 8(1): 57-62.
- Govarthanan M, Selvankumar T, Manoharan K, Rathika R, shanthi K, Lee K-J, Cho M, Kamala-Kannan S, Oh B.-T. Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int J of Nanomedicine, 2014; 9: 1593–1599.
- Kamel Z, Saleh M, and El Namoury N. Research Journal of Pharmaceutical, Biological and Chemical Sciences Biosynthesis, Characterization, and Antimicrobial Activity of Silver Nanoparticles from Actinomycetes. RJPBCS, 2016; 7(1):119-127.
- Kumar S, Malarkodi C, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G. Algae-mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. International Journal of Metals, 2014; 692-643.
- Manivasagan P, Venkatesan J, Senthilkumar K, Sivakumar K, and Kim1S.-K. Biosynthesis, Antimicrobial and Cytotoxic Effect of Silver Nanoparticles Using a Novel Nocardiopsis sp. MBRC-1. BioMed Research International, 2013; Volume 2013: Article ID 287638, 1-9 pages.
- McFarland J: the nephelometer: an instrument for estimating the numbers of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc, 1907; 49:1176–1178.
- Mohammed N M, Yusoh B K, and Shariffuddin H B H J. Methodized depiction of design of experiment for parameters optimization in synthesis of poly (N vinyl capro lactam) thermo responsive polymers. Materials Research Express, 2016; 3:12.
- Naser Y, Mohammad P, Alikhani H, and Mehdi A F. Statistical Evaluation of the Pertinent Parameters in Biosynthesis of Ag/MWf-CNT Composites Using Plackett-Burman Design and Response Surface Methodology. Iran J Chem Chem Eng, 2016; 35:2.
- Nejad S M, Khatami M, and Bonjar H S G .Streptomyces somaliensis mediated green synthesis of silver nanoparticles. Nanomed J, 2015; 2(3): 217-222.
- Paredes D, Ortiz C, Torres R. Synthesis, characterization, and evaluation of antibacterial effect of ag- nanoparticles against Escherichia coli O157:h7 and methicillin resistant Staphylococcus aureus (Mrsa). International Journal of Nano medicine, 2014; 9: 1717–1729.
- Prabhu S, and Poulose E K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. International Nano Letters, 2012; 2:32.
- Prakasham R S, Kumar B S, Kumar Y S, Kumar K P .Production and characterization of protein encapsulated silver nanoparticles by marine isolate Streptomyces parvulus SSNP11. Indian J Microbiol, 2014; 54:329-336.
- Priyaragini S, Sathishkumar S R, and Bhaskararao K V. Biosynthesis of silver nanoparticles using Actinobacteria and evaluating its antimicrobial and cytotoxicity activity. Int J Pharm Pharm Sci, 2013; 5(2): 709-712.
- Singh D, Rathod V, Fatima L, Kausar A, Vidyashree, Anjum N Priyanka B.Biologically Reduced Silver Nanoparticles from Streptomyces sp. VDP-5 and its Antibacterial Efficacy. Int J of Phar and Pharm Sci Res, 2014; 4(2): 31-36.
- Srih-Belghith K and Bejar S .A thermostable glucose isomerase having a relatively low optimum pH study of activity and molecular cloning of the corresponding gene. Biotechnology Letters, 1998; 20(6):553–556.
- Subashini G, Bhuvaneswari S, and Karthiga R. Biosynthesis of silver nanoparticles from Streptomyces and its antimicrobial, anticancerous activity. Int J of Recent Sci Re, 2016; 7(9):13255-13258.
- Yan J, Abdelgawad M A, El-Naggar E M, Rojas J O. Antibacterial activity of silver nanoparticles synthesized In-situ by solution spraying onto cellulose. Carbohydrate Polymers, 2016; 147: 500–508.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


