ISSN: 0973-7510
E-ISSN: 2581-690X
Fish is an indispensable source of protein, omega-3 fatty acids, and critical nutrients. However, its quick perishable characteristics demand a proper storage strategy to extend shelf life. Refrigeration is one of the earliest methods of storage where temperature is maintained between 2 °C and 5 °C. Temperatures below 5 °C significantly regulate microbial growth and spoilage in most food products, but psychrotrophs thrive at these temperatures. Thus, it is essential to thoroughly identify and understand the development patterns of specific spoilage bacteria (SSB). The study aims to evaluate the impact of SSB activity on rohu quality assessed under refrigeration over 11 days. The study observations identified Aeromonas hydrophila as SSB. Also, observed bacterial load was found to correlate directly with spoilage parameters such as TMA, TVBN, PV, FFA, and TBARS, confirming the relationship between bacterial load and the progression of spoilage. Next, the role of SSB in fish spoilage was validated through controlled SSB inoculation experiments. Here, refrigerated rohu spoiled faster than the control groups, indicating the bacteria responsible for the deterioration of refrigerated rohu. Furthermore, the predominance of A. hydrophila over other microbes confirms its role as the primary SSB. Thus, monitoring and controlling SSB is crucial for understanding its growth dynamics, which assists in developing targeted strategies and effective control measures to preserve the organoleptic qualities of refrigerated products.
Aeromonas hydrophila, Refrigeration, Rohu, Spoilage, SSB
Fish is a vital source of protein, omega-3 fatty acids, and essential elements. However, fish are highly perishable and demand quick storage for shelf-life extension. Additionally, appropriate storage is crucial to maintain its freshness, safety, and nutritional integrity for an extended period. Refrigeration is one of the earliest methods of storage where temperature is maintained between 0 °C and 5 °C. Its origins can be traced to ancient traditions involving the utilization of ice and snow. In the 18th century, mechanical refrigeration developed, progressing into contemporary systems by the 20th century. Today, it is a widely accessible system that has transformed food preservation worldwide. Temperatures below 5 °C significantly regulate microbial growth and spoilage in most food products.1,2 Owing to the impact of refrigeration on fresh food in extending shelf-life, the US Food and Drug Administration (FDA) and the European Union (EU) strongly recommend maintaining a temperature range of 0 °C to 4 °C to prevent the growth of microbes that influence the product’s integrity.3,4 Rohu (Labeo rohita), is a popular freshwater fish in India known for its flavor and nutritional value. However, given its high moisture and nutrition levels, it is quite perishable, rendering it vulnerable to microbial growth and subsequent deterioration. Generally, refrigeration at 0 °C to 5 °C can extend its shelf life up to 15 days,5 while freezing at -18 °C or lower can preserve it for up to 30 days.6 Further, modern techniques like vacuum packaging and modified atmosphere packaging enhance storage efficiency beyond 30 days. Despite the effectiveness of modern techniques and freezing systems, their high equipment costs present significant challenges for many users. Consequently, refrigeration remains a more economically viable option for small and medium-scale entrepreneurs, offering a practical balance between cost and preservation efficiency.
Microbial growth is a major concern during refrigeration and is influenced by multiple factors, including harvesting conditions, handling practices, duration, and moisture content of the fish. Refrigerated stored fish are susceptible to deterioration due to microbial activity, enzymatic reactions, and oxidation, which influence the organoleptic characteristics of the stored product. Previous studies indicated that refrigeration, even at low temperatures, facilitates the growth of bacteria, molds, and yeasts.7,8 Therefore, understanding the characteristics of fish deterioration during refrigeration is crucial, particularly for freshwater fish with high moisture content, as they are more prone to rapid spoilage and microbial growth. Microbial growth during refrigeration includes specific spoilage bacteria (SSBs), which are initially a small fraction of the microbiota. The term “specific” highlights the unique adaption of the microbe to thrive and cause spoilage. Unlike general microbes, SSBs exhibit selectivity in their ability to proliferate at low temperatures and tend to act as the major drivers of spoilage in refrigerated food.9,10 SSB dominates the degradation process as their metabolites promote the breakdown of proteins, lipids, and carbohydrates, resulting in texture, flavor, and odor alterations. SSBs were reported as early as the 1990s, with bacteria such as Pseudomonas and Shewanella identified as dominant spoilage bacteria in refrigerated fish, particularly under anaerobic conditions.11 Later, further investigations laid the foundation for SSB microbial growth rates and spoilage patterns at refrigeration temperatures. However, most of the investigations were inconclusive. Therefore, it is essential to thoroughly identify and understand the development patterns of SSB, particularly in developing countries like India. This understanding is crucial for preparing strategies and implementing effective control measures that ensure the organoleptic characteristics of the stored products.
Refrigeration retains freshness but does not kill bacteria or stop enzymatic activity.12 For instance, hydrolysis occurs 3-4 times faster at room temperature compared to 0 °C. While lower temperatures significantly slow microbial growth, psychrotrophic bacteria can still survive and eventually cause spoilage. Previous reports suggest that fresh fish stored at lower temperatures were appear to be dominated by Pseudomonas spp. and Shewanella spp.13 Similarly, multiple investigations demonstrated the presence of Yersinia spp., Aeromonas spp., and Bacillus cereus in various fish products stored at 4 °C.14-17 These observations highlight the ability of psychrotrophic pathogens to persist and potentially proliferate under chilled storage conditions. Despite these challenges, in this age of increasing urbanization and global food trade, refrigeration ensures that consumers have access to fresh, high-quality fish, even in regions far from the source of catch. Furthermore, Advances in refrigeration technology, packaging innovations, and natural preservatives can help enhance storage efficiency and safety. Specifically, the development of biodegradable packaging films embedded with nanoencapsulated natural antimicrobials specifically targeting SSB. These cutting-edge materials contribute to extending shelf life and reducing spoilage during extended storage period. Thus, investigation on SSB will help us to overcome the challenges posed by psychrotrophs. Furthermore, continued research is essential to address emerging pathogens and improve the efficiency and reliability of cold chain logistics. Therefore, the study aims to identify SSB in refrigerated Rohu (2 to 5 °C), one of the widely distributed Indian Major Carps (IMC) and a major aquaculture species, over a storage period of 11 days.
Statement of ethics
All procedures involving the handling and sampling of live Labeo rohita (rohu) were performed in strict accordance with institutional guidelines for the care and use of aquatic animals in research. The experimental protocol was reviewed and approved by the Central Institute of Fisheries Education, Mumbai. Efforts were made to minimize stress and discomfort to the fish throughout the study, and humane endpoints were employed, as necessary.
Experimental design
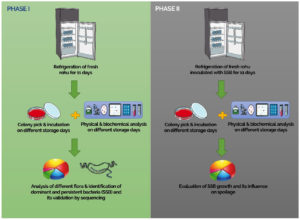
The study comprised two independent segments, each intended to achieve specific goals. The first phase of the study focused on identifying SSB by systematically observing the alterations in the bacterial population linked to spoiling in fresh rohu over 11 days under refrigeration storage conditions. The objective of the first phase was achieved by analyzing microbial growth and characterizing the bacteria sampled at different storage intervals, along with biochemical evaluations to assess the spoilage characteristics. Here, bacteria that showed a steady increase in their population during the storage period and subsequently emerged as the dominant species by the end of the storage duration were identified as SSB. In the next phase, the role of the identified SSB in fish spoilage was validated through targeted experiments aimed at confirming their contribution to the deterioration of fish quality under refrigerated storage conditions. Specifically, identified SSB was inoculated into refrigerated stored fish samples. Then, the SSB growth was assessed compared to other microbial populations in the inoculated group and to a control group, where fish were stored under identical conditions without the introduction of SSB. The study design is schematically illustrated in Figure 1.
Figure 1. Schematic representation of study design. The first phase identified specific spoilage bacteria (SSB) by monitoring bacterial changes in refrigerated rohu over 11 days. Bacteria that were consistently observed and dominated by the end of storage were classified as SSB. The second phase validated SSB role in spoilage by inoculating SSB into refrigerated fish samples and comparing their growth to other microbes and a control group without SSB
Sample collection and storage conditions
Live rohu were purchased from a local fish market in Nalasopara, Mumbai, India. All necessary precautions were taken to ensure the purchase of high-quality fish with minimal handling to preserve their freshness and integrity. Upon arrival at the laboratory, the fish were stunned, washed, packed in a thermal box, and refrigerated for up to 11 days. Samples were collected on days 0 (day of purchase), 1, 2, 4, 7, 9, and 11 for sensory, chemical, and microbiological analysis to assess changes over time. In the second phase, fish were stored in similar conditions with 103 CFU/gm of identified SSB. Later, samples were collected on days 0, 1, 2, 4, 7, 9, and 11 to evaluate the impact of SSB on microbial growth and spoilage pattern by measuring the Trimethylamine (TMA), Total volatile basic nitrogen (TVBN), Peroxide value (PV), Free fatty acid (FFA), and Thiobarbituric acid reactive substances (TBARS).
Identification of SSB
As reported earlier, microorganisms were isolated from samples collected at predetermined intervals with minor modifications.18,19 Tissue samples from stored fish were homogenized with a physiological saline solution. The resultant suspension was serially diluted, spread on agar plates, and incubated at 37 °C for 24 h. Total plate count (TPC) was performed as outlined in earlier reports.20 All the counts were expressed as colony-forming units (CFU) per gram. A total of 35 representative colonies were selected over the 11 days of refrigerated storage, with the following distribution: 8 colonies on day 0, 7 colonies on day 1, 6 colonies on day 2, 5 colonies each on days 4 and 7, 3 colonies on day 9, and 1 colony on day 11. These colonies were thoroughly evaluated for shape, color, size, transparency, and other morphological characteristics. Additionally, their contribution to the overall bacterial population was calculated. Besides, selected colonies were subjected to biochemical testing, including Gram staining, catalase test, oxidation test, urease test, oxidative-fermentative (OF) test, triple sugar iron (TSI) test, amino acid (AA) decarboxylation test, and sugar fermentation tests, to facilitate bacterial classification. All biochemical tests were conducted as described in prior reports.21-24 Additionally, proteolytic and lipolytic assays were conducted to further elucidate the properties observed in microbes.
Measurement of spoilage characteristics
Sensory evaluation was performed using a 9-point scale of hedonic sensory scores to understand consumer acceptance and preferences. Here, scores range from 1 to 9 to measure the degree of likability of a product. A score of 9 represents the highest level of preference, while 8 reflects higher preference, and 7 corresponds to moderate preference. On the other hand, the lowest scores, like 2, indicate dislike very much, and 1 indicates extreme dislike, suggesting the lowest level of preference. The biochemical characteristics, including pH, Trimethylamine (TMA),25,26 Total Volatile Basic Nitrogen (TVB-N),27 Peroxide value (PV),28 Free fatty acids (FFA),29 and thiobarbituric acid (TBA)30 were assessed to measure the spoilage process.
Extraction of genomic DNA and PCR amplification
Genomic DNA from the bacterial cultures was extracted using the lysate method using TE buffer with minor modifications.31 Here, a loopful of the culture was mixed with TE buffer, vortexed thoroughly, and boiled at 98 °C for 10 min. The resulting mixture was centrifuged at 12000 rpm for 10 min using a refrigerated centrifuge to separate the supernatant containing DNA fragments and stored at -80 °C. Next, DNA was amplified with indicated primers. Primers used in the investigation are as follows: Forward 5’-AGAGTTTGATCCTGGCTCAG-3′ and Reverse 5’-TACGGTTACCTTGTTACGACTT-3′. PCR conditions for amplification included an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 sec, annealing at 56 °C for 45 sec, elongation at 72 °C for 1 min, and a final elongation at 72 °C for 10 min. Amplification was performed with a thermal cycler (Applied Biosystems, USA). The amplified products were visualized by adding 1 µl of tracking dye and captured using a Bio-Rad Gel Documentation System (Bio-Rad Gel Documentation System, USA).
DNA purification and sequencing
The PCR product was purified using the Gene JET Gel Extraction Kit (Thermo Fisher Scientific, India) per the manufacturer’s instructions. Briefly, 90 µl of DNA sample was added to the purification columns provided in the Kit, followed by the addition of binding buffer to facilitate DNA binding to the column matrix, and the mixture was centrifuged with SLM-MCF-14K centrifuge (GeNei, India). Next, the reaction column was washed and DNA was eluted using the elution buffer. This purified DNA was sent to Eurofins Laboratory, Bangalore, India, for sequencing. The obtained DNA sequences (FASTA format) were processed by trimming low-quality regions and aligning them using bioinformatics tools to ensure accuracy. The processed sequences were compared against reference databases, such as NCBI BLAST, to identify closely related species. The highest similarity between the obtained sequence and the reference sequence was considered the correct identification of the bacteria.
Statistical analysis
All statistical calculations and graphical illustrations were done with Origin application (OriginLab, MA, USA). Data are shown as the mean ± SD. One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used for multiple comparisons. The threshold for statistical significance was set at p < 0.05.
Phase I – Bacterial profiling, identification of SSB and spoilage indicators
Identification SSB
Screening SSB during refrigeration is essential for food safety and quality. Identification facilitates the optimization of storage conditions and the prediction of deterioration rates, thereby determining the maximum storage time while maintaining quality and safety. In this study, representative colonies were screened at various intervals during refrigeration. Observations revealed a steady increase in bacterial counts during the initial refrigeration storage phase, which was followed by a marked acceleration in microbial proliferation as storage time progressed. This trend highlights the dynamic nature of bacterial growth under refrigerated conditions and emphasizes the importance of monitoring for spoilage.
Bacterial load and bacterial profile during refrigeration
The alterations in total plate count (TPC) in refrigerated rohu are shown in Table 1. On the day of collection (Day 0), the bacterial load was recorded at 168 × 10³ CFU/g, indicating that the live fish obtained from the market was already contaminated despite the careful handling. This suggests poor handling practices during the fish journey from farm to market. By the end of storage (Day 11), the bacterial count had increased considerably to 2.25 × 109 CFU/g, signifying an extensive microbial load even under refrigeration conditions. Specifically, the bacterial load increased significantly by Day 7, reaching 2.25 × 109 CFU/g. Thereafter, the growth rate plateaued, likely due to the accumulation of metabolic waste, including organic acids, ammonia and sulfur compounds, which may have inhibited further bacterial proliferation between Day 7 and Day 11. The gradual increase in microbial count indicates the acclimatization of psychrotrophic microbes during refrigeration, marking the onset of spoilage after Day 4. Based on morphological characteristics and biochemical analysis, the study identified the presence of multiple bacterial species during refrigeration. On Day 0, we observed Aeromonas spp., Shigella spp., Bacillus spp., Shewanella spp., and Yersinia spp. Interestingly, by Day 4, the study identified seven species, including Aeromonas spp., Shigella spp., Bacillus spp., Shewanella spp., Yersinia spp., Pseudomonas spp., and Acinetobacter spp. However, after Day 9, only Aeromonas spp., Shigella spp., and Bacillus spp. were observed, with Aeromonas spp. appearing to dominate during refrigeration. Further, approximately 59% of the colonies were identified as Aeromonas spp. on day 7. Their contribution to the TPC increased to 93% by Day 11. These observations strongly suggest that microbial growth aligns with the rapid spoilage period occurring between Day 4 and Day 7.
Table (1):
Microbial growth in phase I of refrigerated Labeo rohita (rohu)
| Sampling day | TPC count CFU/g | Dilution factor | Biochemically identified genus | Contribution (%) |
|---|---|---|---|---|
| 0 | 168 × 103 | -1 | Aeromonas spp. | 25 |
| Shigella spp. | 13.69 | |||
| Bacillus spp. | 20.83 | |||
| Shewanella spp. | 23.80 | |||
| Yersinia spp. | 16.66 | |||
| 1 | 275 × 103 | -1 | Aeromonas spp. | 23.63 |
| Shigella spp. | 13.81 | |||
| Bacillus spp. | 17.45 | |||
| Shewanella spp. | 16.72 | |||
| Yersinia spp. | 15.27 | |||
| Pseudomonas spp. | 8.36 | |||
| Acinetobacter spp. | 4.72 | |||
| 2 | 27.5 × 105 | -2 | Aeromonas spp. | 28 |
| Shigella spp. | 12.72 | |||
| Bacillus spp. | 17.81 | |||
| Shewanella spp. | 16.36 | |||
| Yersinia spp. | 12.36 | |||
| Pseudomonas spp. | 9.09 | |||
| Acinetobacter spp. | 3.63 | |||
| 4 | 21.9 × 105 | -3 | Aeromonas spp. | 38.35 |
| Shigella spp. | 13.2 | |||
| Bacillus spp. | 20.54 | |||
| Shewanella spp. | 5.93 | |||
| Yersinia spp. | 10.04 | |||
| Pseudomonas spp. | 10.04 | |||
| Acinetobacter spp. | 1.82 | |||
| 7 | 2.07 × 109 | -4 | Aeromonas spp. | 58.45 |
| Shigella spp. | 11.11 | |||
| Bacillus spp. | 20.77 | |||
| Yersinia spp. | 6.28 | |||
| Pseudomonas spp. | 3.38 | |||
| 9 | 2.39 × 109 | -4 | Aeromonas spp. | 89.53 |
| Shigella spp. | 5.85 | |||
| Bacillus spp. | 4.60 | |||
| 11 | 2.25 × 109 | -5 | Aeromonas spp. | 92.88 |
| Shigella spp. | 3.11 | |||
| Bacillus spp. | 4 |
Dominance-based selection and validation of SSB
Bacterial profiling of refrigerated rohu samples demonstrated the presence of multiple bacterial species during storage (Table 1). The data convincingly suggests that Aeromonas spp. steadily established itself and eventually dominated the bacterial population. By Day 9, Aeromonas completely dominated the microbial flora. Other species, including Pseudomonas, Yersinia, Shewanella, Shigella, and Bacillus, which were initially present, showed a steady decline in their contributions over the 11-day storage period. By Day 9, only Bacillus and Shigella were observed alongside Aeromonas, but their contributions were negligible. Thus, Aeromonas, which prevailed during the spoilage phase, emerges as the most likely candidate to be identified as the SSB. To investigate the observed further, BLAST analysis of 16S rRNA sequences was conducted to accurately identify the species corresponding to the observed microbes. Analysis revealed that the microbes observed during refrigeration included Aeromonas hydrophila, Shigella sonnei, Bacillus firmicutes, Yersinia ruckeri, Shewanella putrefaciens, Acinetobacter baumannii, and Pseudomonas putida. Among these, Aeromonas hydrophila dominated the microbial flora during refrigeration and was therefore identified as the SSB. All relevant details regarding the sequence alignment and matching results are presented in Table 2.
Table (2):
Identification of microbial species through DNA sequencing during Phase I of the study
| Sampling day | Collected colony samples | Biochemically identified genus | Molecularly identified spp. (% Seq. match) | Accession No. (Gen Bank) |
|---|---|---|---|---|
| 1 | 65 | Aeromonas spp. | Aeromonas hydrophila (99.16) | CP143514.1 |
| 38 | Shigella spp. | Shigella sonne (95.33) | CP049177.1 | |
| 48 | Bacillus spp. | Bacillus firmicutes (87.10) | KY849501.1 | |
| 46 | Shewanella spp. | Shewanella putrefaciens (95.85) | MK447627.1 | |
| 42 | Yersinia spp. | Yersinia ruckeri (91.16) | KX388238.1 | |
| 23 | Pseudomonas spp. | Pseudomonas putida (96.15) | KT429603.1 | |
| 13 | Acinetobacter spp. | Acinetobacter baumannii (98.69) | KT156752.1 | |
| 2 | 77 | Aeromonas spp. | Aeromonas hydrophila (98.72) | CP143514.1 |
| 35 | Shigella spp. | Shigella sonne (92.01) | CP049177.1 | |
| 49 | Bacillus spp. | Bacillus firmicutes (87.10) | KY849501.1 | |
| 45 | Shewanella spp. | Shewanella putrefaciens (94.69) | MK447627.1 | |
| 34 | Yersinia spp. | Yersinia ruckeri (90.06) | KX388238.1 | |
| 25 | Pseudomonas spp. | Pseudomonas putida (94.32) | KP192772.1 | |
| 10 | Acinetobacter spp. | Acinetobacter baumannii (97.01) | KT156752.1 | |
| 4 | 84 | Aeromonas spp. | Aeromonas hydrophila (98.64) | CP143514.1 |
| 29 | Shigella spp. | Shigella sonne (92.35) | CP049177.1 | |
| 45 | Bacillus spp. | Bacillus firmicutes (77.54) | KY849501.1 | |
| 13 | Shewanella spp. | Shewanella putrefaciens (92.58) | MK447627.1 | |
| 22 | Yersinia spp. | Yersinia ruckeri (88.03) | KX388238.1 | |
| 22 | Pseudomonas spp. | Pseudomonas putida (94.22) | KP192772.1 | |
| 4 | Acinetobacter spp. | Acinetobacter baumannii (94.24) | KT156752.1 | |
| 7 | 121 | Aeromonas spp. | Aeromonas hydrophila (98.62) | CP143514.1 |
| 23 | Shigella spp. | Shigella sonne (94.69) | CP049177.1 | |
| 43 | Bacillus spp. | Bacillus firmicutes (81.51) | KY849501.1 | |
| 13 | Yersinia spp. | Yersinia ruckeri (89.17) | KX388238.1 | |
| 07 | Pseudomonas spp. | Pseudomonas putida (97.69) | KT429603.1 | |
| 9 | 214 | Aeromonas spp. | Aeromonas hydrophila (98.30) | CP143514.1 |
| 14 | Shigella spp. | Shigella sonne (91.18) | CP049177.1 | |
| 11 | Bacillus spp. | Bacillus firmicutes (82.10) | KY849501.1 | |
| 11 | 209 | Aeromonas spp. | Aeromonas hydrophila (99.16) | CP143514.1 |
| 07 | Shigella spp. | Shigella sonne (88.96) | CP049177.1 | |
| 9 | Bacillus spp. | Bacillus firmicutes (81.48) | KY849501.1 |
Spoilage indicators in refrigerated rohu
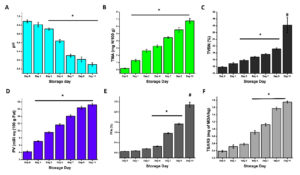
In this Phase I study, the initial pH of the sample was measured at 6.88 ± 0.03 on Day 0. By Day 1, the pH slightly decreased to 6.81 ± 0.05. The pH continued to decline progressively over the storage period, reaching 5.90 ± 0.04 by Day 11, indicating extensive biochemical changes. This consistent decrease in pH reflects the accumulation of acidic metabolites, likely resulting from microbial activity and spoilage processes during refrigeration. Moreover, a comprehensive analysis of biochemical spoilage indicators like TMA, TVBN, PV, FFA, and TBARS levels revealed a clear pattern of progressive deterioration in the quality of rohu during refrigerated storage. Specifically, a significant elevation in these parameters was observed starting from Day 4 of storage, marking the initial stages of spoilage. By Day 11, the levels of all these indicators had surpassed commonly accepted freshness thresholds, clearly signifying advanced spoilage and a substantial decline in the quality and safety of the refrigerated rohu (Figure 2A-F). These observations suggest protein breakdown and a corresponding decline in freshness during refrigeration, which align with the increasing bacterial population detailed in Table 1.
Figure 2. Spoilage indicators during refrigeration of Rohu. (A) pH, (B) Levels of TMA, (C) Lev-els of TVBN, (D) PV, (E) FFA, and (F) TBARS. Data are presented as mean ± SEM (n = 3, * p < 0.05 vs. Day 0; # p < 0.05 vs. Day 2,4,7, 9). TMA, Trimethylamine; TVBN, Total Volatile Basic Nitrogen; PV, Peroxide value; FFA, Free fatty acids; TBARS, Thiobarbituric Acid Reactive Substances
Sensory analysis
The study observed that the sensory attributes of smell, taste, and odor began to deteriorate after day 5 of storage. These changes were indicative of the onset of spoilage, with declines in the freshness of the fish. The study observed that smell, taste, and odor began appeared to be deteriorating after day 5. The sensory rejection point was reached by the 11th day of refrigeration storage, as the samples exhibited noticeable spoilage characteristics. These included darkened gills, a decayed muddy odor, significant yellowing of the belly region, and soft, deteriorated muscle texture. These changes significantly reduced the product’s visual appeal and overall acceptability.
Phase II – Evaluation of SSB influence during refrigeration
Microbial profile in refrigerated rohu inoculated with SSB
SSB (Aeromonas hydrophila) microbial inoculation examinations are vital for evaluating the shelf life of refrigerated products. They replicate realistic storage conditions with prevalent bacteria, facilitating an understanding of microbial proliferation and its influence on decomposition during storage. In this investigation, fresh rohu purchased from the market were inoculated with 103 CFU/g SSB prior to refrigeration, and rohu was observed for up to 10 days. Here, the bacterial count on day 1 was 3 × 103 CFU/g and this count increased to 1.08 × 108 CFU/g by day 10. As expected, SSB dominated the microbial flora throughout the storage period, demonstrating its ability to thrive at refrigerated temperatures while inhibiting the growth of other microorganisms (Table 3).
Table (3):
Growth dynamics of Aeromonas hydrophila during refrigeration following controlled inoculation
| Storage Day | Species | Count (CFU/g) | ~% to TC |
|---|---|---|---|
| Day 0 | A. hydrophila | 1 × 103 | 100 |
| Other spp. | 0 | 0 | |
| Day 1 | A. hydrophila | 2 × 103 | 66.66 |
| Other spp. | 1 × 103 | 33.33 | |
| Day 4 | A. hydrophila | 2.9 × 105 | 76.31 |
| Other spp. | 9 × 104 | 23.7 | |
| Day 7 | A. hydrophila | 8.8 × 108 | 90.7 |
| Other spp. | 9 × 107 | 9.3 | |
| Day 10 | A. hydrophila | 1.01 × 108 | 93.51 |
| Other spp. | 7 × 103 | 6.49 |
TC: Total count on respective day; Other species include Shigella spp., Bacillus spp., Shewanella spp., Yersinia spp., Pseudomonas spp., and Acinetobacter spp. However, their exact counts were not provided, as the Phase II investigation focused on the growth dynamics of the SSB
Progression of spoilage in presence of SSB
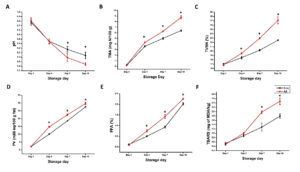
In this study, the pH of the control group was 6.77 ± 0.07 on Day 1 but decreased significantly to 6.03 ± 0.07 after 10 days of refrigeration. Similarly, rohu stored with A. hydrophila showed a declining trend, with the pH dropping to 5.84 ± 0.03 by Day 10, potentially facilitating protein denaturation. Additionally, all spoilage-related parameters, including TMA, TVBN, PV, FFA, and TBA, exhibited substantial degradation (Figure 3A-F), reflecting key biochemical and microbial processes. Specifically, increased TMA signifies microbial activity, while higher levels of TVBN, and FFA suggest extensive protein and enzymatic lipid degradation in refrigerated rohu.
Fresh fish is highly perishable and requires proper handling and storage to maintain safety and extend its shelf life. Health-conscious consumers increasingly prefer minimally processed, nutrient-rich foods. Concerns about preservatives further emphasize the importance of effective fish storage methods. Refrigeration is a modern system for extending the shelf life of food products. It is now widely recognized as the standard storage method among both entrepreneurs and households. It offers consistency and control over low temperatures, ensuring quality by slowing microbial growth and enzymatic activity. Additionally, refrigeration avoids direct water contact, preventing texture loss and flavor dilution. However, the growth of psychrotrophs presents a significant challenge to refrigeration storage. These cold-tolerant microorganisms thrive at temperatures between 0 °C and 4 °C. The increase in bacterial load during extended storage32 is particularly concerning, as psychrotrophs produce extracellular enzymes that degrade proteins and lipids,33-35 ultimately compromising food quality. Research has shown that combining low-temperature storage with specific additives can significantly extend the shelf life of fish and fishery products.4,36,37 To address these challenges, identifying the primary spoilage organisms (SSB) is crucial for developing effective control strategies to mitigate the risks associated with psychrotrophic growth in refrigerated foods. Thus, the study aims to identify SSB in refrigerated Rohu, a widely distributed Indian Major Carp (IMC) and a key species in aquaculture in India. This study examines the impact of SSB activity on fish quality under refrigeration (2-5 °C) over 11 days, providing insights into control measures to extend the shelf life of fresh fish.
Following the death of fish, various sensory, physicochemical, and microbiological changes occur. These changes include muscle browning or darkening, often attributed to Maillard reactions, enzymatic activity, and the secretion of mucus.38 In this study, the sensory rejection point was reached by the 11th day of refrigeration storage, which clearly suggests that spoilage began much earlier during storage. Specifically, when fresh fish are stored under refrigeration, they undergo a pattern of deterioration in which autolytic processes, marked by changes in smell, taste, and odor, dominate the initial stages. In the later stages, bacterial activity becomes the primary factor contributing to spoilage.39 Consistent with previous reports, the study observed that sensory evaluation showed smell, taste, and odor deterioration by day 5, coinciding with a spike in bacterial load (Table 1). Furthermore, controlled inoculation tests produced the same results (Table 2). The spike in microbial growth induces the production of amines, organic acids, ketones, aldehydes, and alcohols, which impart unpleasant odors and tastes.40 Interestingly, study results show that Aeromonas hydrophila, confirmed by sequence analysis, dominated the microbial flora during refrigeration. Additionally, controlled inoculation of Aeromonas hydrophila in refrigerated rohu supports this observation, as the presence of Aeromonas hydrophila inhibits the growth of other microorganisms throughout the storage period (Table 2). These observations are supported by previous reports, which indicate that Aeromonas spp. are commonly present bacteria that produce ammonia-like and fishy off-flavors.41-43 Furthermore, A. hydrophila is known to exhibit both proteolytic and lipolytic activities,44-46 which potentially degrade proteins. For instance, Aeromonas rivipollensis, one of the Aeromonas species known to degrade myofibrillar proteins (MFPs) through its proteolytic and lipolytic activities.47 Besides, approximately 36 species of Aeromonas have been identified in reports as being associated with fish spoilage.48 Likewise, prior investigations indicated that approximately 33% of raw, processed, and ready-to-eat food samples contained Aeromonas colonies.49 Moreover, the dominance of Aeromonas hydrophila generate metabolic byproducts like ROS.50 ROS production may lead to hydrogen peroxide, which may cause oxidative damage to fish proteins, lipids, and nucleic acids.51,52 These events trigger a cascade of reactions that lead to tissue breakdown and spoilage. Collectively, these observations highlight the role of A. hydrophila in spoilage and validate the identification of A. hydrophila as SSB in refrigerated rohu. The detection of SSB in preserved food products is essential for developing effective food storage solutions. The identification of SSB facilitates better management of storage conditions, including temperature and packaging materials. The study observations provided a fair understanding of the growth rate of A. hydrophila under refrigeration temperatures, which will allow us to predict spoilage patterns. Also, further investigations should emphasize the importance of understanding the environmental adaptability of A. hydrophila, which will help us develop targeted interventions to extend the shelf life of fresh fish under refrigerated conditions.
Additionally, post-harvest management and transportation of freshly caught fish are crucial factors influencing its shelf life and quality. If contamination occurs at any point prior to refrigeration, the potential for extending its shelf life is significantly compromised.53,54 Efficient handling protocols, such as prompt cleaning and immediate chilling, are crucial to reduce microbial contamination and enzymatic activity. The time interval between fish capture and initial chilling is a critical factor influencing product quality. Delays or inadequate temperature control during this period can facilitate the rapid proliferation of spoilage microorganisms and activate endogenous enzymes, leading to accelerated tissue degradation and the development of off-odors.55,56 It is well established that rapid cooling effectively suppresses microbial metabolism and enzymatic activity, thereby preserving both the organoleptic qualities and nutritional integrity of the fish. In the present study, rohu specimens were sourced from a local fish market, where post-harvest handling conditions prior to sample acquisition were beyond our control. This lack of oversight may have introduced external microbial contamination, potentially explaining the notable bacterial load observed upon arrival at the laboratory (Day 0). Thus, ensuring proper hygiene and temperature management during post-harvest processing and transportation is essential to minimize the risk of spoilage, including contamination by specific spoilage bacteria (SSB). Notwithstanding this methodological limitation, the identification of A. hydrophila as the dominant microbial species remains a key contribution of the study. Interestingly, Nanoencapsulation technology allows for the controlled release of antimicrobial compounds,57,58 enhancing their stability, solubility, and bioavailability. Further, natural antimicrobials, such as essential oils, plant extracts, or bacteriocins, when encapsulated in nanocarriers such as liposomes, nanofibers, or polymeric nanoparticles, can retain their efficacy over extended periods and respond to environmental triggers such as moisture or pH changes.59 These nanoformulations can be incorporated to develop biodegradable packaging films specifically formulated to target A. hydrophila, representing a promising future innovation. Incorporating these nanoformulations into biodegradable film matrices actively inhibits microbial growth while degrading naturally without contributing to long-term plastic pollution. Moreover, the use of biodegradable active packaging aligns with current sustainability goals in the food industry by reducing reliance on conventional petroleum-based plastics and minimizing environmental impact. Like this identification of SSB could lead to the development of multifaceted solutions to prevent SSB’s growth, enhance product safety, extend shelf life, and contribute to environmental sustainability.
Furthermore, identifying A. hydrophila as a SSB under refrigerated conditions may be considered presumptive, given that the conclusion is based on a single fish species. Still, the consistent dominance of A. hydrophila across multiple sampling points, supported by both phenotypic and molecular identification methods, indicates its significant contribution to spoilage within the parameters of this study. Thus, further research involving diverse fish species and storage conditions is necessary to establish the broader relevance of these findings. The current investigation provides meaningful insight into the spoilage potential of A. hydrophila in L. rohita during refrigerated storage. As such, it serves as a valuable reference study for future investigations on species-specific spoilage microbiota.
The study identifies A. hydrophila as the SSB primarily responsible for the deterioration of refrigerated rohu. A. hydrophila survives and proliferates efficiently between 0-5 °C, facilitating spoilage. The dominance of A. hydrophila, evidenced by its ability to inhibit the growth of other microbes, confirms its role as the SSB. Further, monitoring and controlling SSB is crucial for understanding its growth dynamics, which assist in developing targeted strategies and effective control measures. Therefore, identifying SSB is key to preserving the organoleptic qualities of refrigerated products.
ACKNOWLEDGMENTS
The authors are thankful to the Indian Council of Agricultural Research (Ministry of Agriculture, Government of India) and the Director, ICAR-Central Institute Fisheries Education, Mumbai, for supporting the present study. Authors are also thankful to their colleagues at the Department of Post-Harvest Technology for their unwavering support and invaluable help in preparing this original article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
BBN conceptualized and designed the study. SAJ performed data acquisition and experiments. HSK performed data analysis. HSK and BNN performed supervision. SAJ wrote the manuscript. AKB and LM reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the ICAR – Central Institute of Fisheries Education, Mumbai, India.
- Sherif AH, Farag EAH, Mahmoud AE. Temperature fluctuation alters immuno-antioxidant response and enhances the susceptibility of Oreochromis niloticus to Aeromonas hydrophila challenge. Aquacult Int. 2024;32(2):2171-2184.
Crossref - Tavares J, Martins A, Fidalgo LG, et al. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods. 2021;10(4):780.
Crossref - FDA. Guidance for industry (Food Drug Administration). 2017. https://www.fda.gov/consumers/consumer-updates/are-you-storing-food-safely
- Banerjee R, Maheswarappa NB. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit Rev Food Sci Nutr. 2019;59(8):1256-1263.
Crossref - Abouel-Yazeed AM. Maintaining quality and extending shelf-life of tilapia Oreochromis niloticus fish during storage at 4°C. 2013;8(2):293-305
- Hu Ym, Zhang Nh, Wang H, Yang Yf, Tu Zc. Effects of pre freezing methods and storage temperatures on the qualities of crucian carp (Carassius auratus var. pengze) during frozen storage. J Food Process Preserv. 2021;45(2):e15139.
Crossref - Tongbram T, Bora J, Makroo HA. Fresh and Refrigerated Foods: Science, Shelf Life, and Quality. Shelf Life and Food Safety. CRC Press. 2022:113-140.
Crossref - Tuan PQ. Refrigeration in food preservation and processing. Conventional and Advanced Food Processing Technologies. 2014:357-386.
Crossref - Boziaris IS, Parlapani FF. Specific spoilage organisms (SSOs) in fish. The Microbiological Quality of Food. 2017:61-98.
Crossref - Johansson P, Jaaskelainen E, Nieminen T, Hultman J, Auvinen P, Bjorkroth KJ. Microbiomes in the context of refrigerated raw meat spoilage. Meat and Muscle Biology. 2020;4(2).
Crossref - Stenström I-M, Molin G. Classification of the spoilage flora of fish, with special reference to Shewanella putrefaciens. J Appl Bacteriol. 1990;68(6):601-618.
Crossref - Sampels S. The effects of storage and preservation technologies on the quality of fish products: A review. J Food Process Preserv. 2015;39(6):1206-1215.
Crossref - Hussain MA, Sumon TA, Mazumder SK, et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review. Food Control. 2021;129:108244.
Crossref - Hassanien FS, Hassan MA, El-Hariri MD, Sayed E. Incidence and toxigenic profile of Bacillus cereus in some fishes. Benha Veterinary Medical Journal. 2018;34(1):420-429.
Crossref - Jarvis KG, Hsu C-K, Pettengill JB, et al. Microbiome population dynamics of cold-smoked Sockeye salmon during refrigerated storage and after culture enrichment. J Food Prot. 2022;85(2):238-253.
Crossref - Novoslavskij A, Terentjeva M, Eizenberga I, Valcina O, Bartkevics V, Berzins A. Major foodborne pathogens in fish and fish products: a review. Ann Microbiol. 2016;66(1):1-15.
Crossref - Hoel S, Lerfall J, Jakobsen AN. Growth and spoilage potential of an Aeromonas salmonicida strain in refrigerated Atlantic cod (Gadus morhua) stored under various modified atmospheres. Foods. 2022;11(18):2757.
Crossref - Mehta NK, Rout B, Balange AK, Nayak BB. Dynamic viscoelastic behaviour, gelling properties of myofibrillar proteins and histological changes in shrimp (L. vannamei) muscles during ice storage. Aquac Fish. 2023;8(2):180-189.
Crossref - Saklani P, Lekshmi M, Nayak BB, Kumar S. Survival of methicillin-resistant Staphylococcus aureus in fish and shrimp under different storage conditions. J Food Prot. 2020;83(5):844-848.
Crossref - Emire S, Gebremariam MM. Influence of frozen period on the proximate composition and microbiological quality of Nile Tilapia (Oreochromis niloticus). J Food Process Preserv. 2009;34(4):743-757.
Crossref - Acharjee M, Ahmed E, Munshi SK, Noor R, Science F. Validation of g-irradiation in controlling microorganisms in fish. Nutr Food Sci. 2014;44(3):258-266.
Crossref - Ahmed T, Baidya S, Acharjee M, Rahman T. Qualitative analysis of drinking water through the most probable number (MPN) method. S J Microbiol. 2013;3(1):9-16.
Crossref - Cappuccino JG, Sherman N. Microbiology: a laboratory manual. Pearson Higher Ed. 2013.
- Koneman E, Winn Jr W, Allen S, et al. Microbiological Diagnosis: Text and Color Atlas. In Microbiological Diagnosis: Text and Color Atlas. 2012: xxxv, 1565.
- Huss HH, Boerresen T, Dalgaard P, Gram L, Jensen BJF, Documento Tecnico de Pesca. Quality and quality changes in fresh fish. 1998. https://www.fao.org/4/v7180e/v7180e00.htm
- Malle P, Tao SH. Rapid Quantitative Determination of Trimethylamine using Steam Distillation. J Food Prot. 1987;50(9):756-760.
Crossref - Beatty SA, Gibbons NE. The Measurement of Spoilage in Fish. Journal of the Biological Board of Canada. 2011;3(1):77-91.
Crossref - Andina L, Riyanto S, Rohman AJIFRJ. Determination of peroxide value of red fruit oil by FTIR spectroscopy and multivariate calibration. Int Food Res J. 2017;24(6):2312 – 2316.
- Pagarkar AU, Rathod NB. Biochemical and sensory quality changes of fish cutlets, made from pangasius fish (Pangasianodon hypophthalmus), during storage in refrigerated display unit at-15 to-18 °C. Int J Food Agric Vet Sci. 2013;3(1):1-8.
- Tarladgis BG, Watts BM, Younathan MT, Dugan Jr L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37(1):44-48.
Crossref - Shin SK, Lee Y, Kwon H, Rhee JS, Kim JK. Validation of direct boiling method for simple and efficient genomic DNA extraction and PCR based macroalgal species determination. J Phycol. 2021;57(4):1368-1372.
Crossref - Yeasmin T, Reza MS, Shikha FH, Khan MNA, Kamal M. Quality changes in formalin treated rohu fish (Labeo rohita, Hamilton) during ice storage condition. Asian J Agric Sci. 2010;2(4):158-163.
- Brasca M, Decimo M, Morandi S, Machado SG, Bagliniere F, Vanetti MCD. Psychrotrophic bacteria. Microbiology in Dairy Processing: Challenges and Opportunities. 2017:37-61.
Crossref - Kanekar PP, Kanekar SP. Psychrophilic, Psychrotrophic, and Psychrotolerant Microorganisms. In: Diversity and Biotechnology of Extremophilic Microorganisms from India. Microorganisms for Sustainability. Springer, Singapore. 2022:215-249.
Crossref - Wei Q, Wang X, Sun D-W, Pu H. Rapid detection and control of psychrotrophic microorganisms in cold storage foods: A review. Trends Food Sci Technol. 2019;86:453-464.
Crossref - Afrin F, Rasul MG, Khan M, Akter T, Yuan C, Shah AKMA. Optimization of Chitosan Concentration on the Quality and Shelf Life of Frozen Rohu (Labeo rohita) Fillets. SQUALEN Bulletin of Marine and Fisheries Postharvest and Biotechnology. 2021;16(1).
Crossref - Manimaran U, Shakila RJ, Shalini R, et al. Effect of additives in the shelflife extension of chilled and frozen stored Indian octopus (Cistopus indicus). J Food Sci Technol. 2016;53(2):1348-1354.
Crossref - Gil MM, Barbosa AL. Microorganisms and safety. In: Cruz RMS, Editor. Practical Food and Research. 3rd ed. 2011;195–217.
- Duarte AM, Silva F, Pinto FR, Barroso S, Gil MM. Quality Assessment of Chilled and Frozen Fish-Mini Review. Foods. 2020;9(12):1739.
Crossref - Gram L, Dalgaard P. Fish spoilage bacteria-problems and solutions. Curr Opin Biotechnol. 2002;13(3):262-266.
Crossref - Qiu L, Zhao Y, Ma H, Tian X, Bai C, Liao T. The quality and bacterial community changes in freshwater crawfish stored at 4 °C in vacuum packaging. Molecules. 2022;27(23):8618.
Crossref - Hickey ME, Accumanno GM, McIntosh DM, Blank GS, Lee JL. Comparison of extracellular DNase and protease producing spoilage bacteria isolated from Delaware pond sourced and retail channel catfish (Ictalurus punctatus). J Sci Food Agric. 2015;95(5):1024-1030.
Crossref - Stratev D, Vashin I, Rusev V. Prevalence and survival of Aeromonas spp. in foods – a review. Rev Med Vet. 2012;163(10):486-494.
- Albarral V, Sanglas A, Palau M, Minana-Galbis D, Fuste MC. Potential pathogenicity of Aeromonas hydrophila complex strains isolated from clinical, food, and environmental sources. Can J Microbiol. 2016;62(4):296-306.
Crossref - Elgendy MY, Soliman WS, Abbas WT, Ibrahim TB, Younes AM, Omara ST. Investigation of some virulence determents in Aeromonas hydrophila strains obtained from different polluted aquatic environments. Jordan J Biol Sci. 2017;10(4):265-272.
- Lee HK, Ahn MJ, Kwak SH, Song WH, Jeong BC. Purification and characterization of cold active lipase from psychrotrophic Aeromonas sp. LPB 4. 2003;41(1):22-27.
- Zhuang S, Tan Y, Hong H, Li D, Zhang L, Luo Y. Exploration of the roles of spoilage bacteria in degrading grass carp proteins during chilled storage: A combined metagenomic and metabolomic approach. Food Res Int. 2022;152:110926.
Crossref - Fernandez-Bravo A, Figueras MJ. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms. 2020;8(1):129.
Crossref - Vivekanandhan G, Savithamani K, Hatha AAM, Lakshmanaperumalsamy P. Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. Int J Food Microbiol. 2002;76(1-2):165-168.
Crossref - Zhang C, Jiang D, Shi H, et al. Protective effect of fructooligosaccharide against oxidative stress and apoptosis induced by Aeromonas hydrophila in Megalobrama amblycephala. BMC Genomics. 2024;25(1):975.
Crossref - Banh S, Wiens L, Sotiri E, Treberg JR. Mitochondrial reactive oxygen species production by fish muscle mitochondria: potential role in acute heat-induced oxidative stress. Comp Biochem Physiol B Biochem Mol Biol. 2016;191:99-107.
Crossref - Li X, Liu P, Zhao Y, Zhang L, Zhang J. Oxidative stress contributes to cytoskeletal protein degradation of Esox lucius through activation of mitochondrial apoptosis during postmortem storage. Foods. 2022;11(9):1308.
Crossref - Getu A, Misganaw K, Bazezew M. Post-harvesting and major related problems of fish production. Fish Aquac J. 2015;6(4):1000154.
Crossref - Sone I, Skara T, Olsen SH. Factors influencing post-mortem quality, safety and storage stability of mackerel species: a review. Eur Food Res Technol. 2019;245(4):775-791.
Crossref - Nie X, Zhang R, Cheng L, Zhu W, Li S, Chen X. Mechanisms underlying the deterioration of fish quality after harvest and methods of preservation. Food Control. 2022;135:108805.
Crossref - Zhang Z, Wu R, Gui M, Jiang Z, Li P. Identification of the specific spoilage organism in farmed sturgeon (Acipenser baerii) fillets and its associated quality and flavour change during ice storage. Foods. 2021;10(9):2021.
Crossref - Chadha S. Recent advances in nano-encapsulation technologies for controlled release of biostimulants and antimicrobial agents. Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture. 2021:29-55.
Crossref - Delshadi R, Bahrami A, Assadpour E, Williams L, Jafari SM. Nano/microencapsulated natural antimicrobials to control the spoilage microorganisms and pathogens in different food products. Food Control. 2021;128:108180.
Crossref - Deshmukh RK, Gaikwad KK. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Conv Bioref. 2024;14(4):4419-4440.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.