ISSN: 0973-7510
E-ISSN: 2581-690X
Phosphate solubilizing bacterial strains (Arthrobacter luteolus S4C7, Klebsiella pneumoniae S4C9, K. pneumoniae S4C10, Enterobacter asburiae S5C7, K. pneumoniae S6C1 and K. quasipneumoniae S6C2) were isolated from the rhizospheric soil of Allium hookeri Thwaites. All the isolates were proved to be positive for rock phosphate (RP) solubilization at different concentration (0.5%, 1.0% and 1.5%). K. pneumoniae S4C10 was found to be most efficient as 81.6 µg/ml of soluble phosphate when amended with 1% of RP, followed by K. quasipneumoniae S6C2 where soluble phosphate release was 76.8 µg/ml in 0.5% RP amended medium. Also, maximum solubilization was noted to correlate with decrease in pH of the medium. Strain A. luteolus S4C7 liberated small amount of P as compared to other strains. The process of phosphate solubilization was optimized for different carbon sources. Fructose was preferred as best carbon source by K. pneumoniae S4C10 with 85.6 µg/ml of solubilized P in NBRIP broth medium. However, after fructose, glucose also proved to be best carbon source by K. quasipneumoniae S6C2 (83.2 µg/ml) and K. pneumoniae S6C1 (78.4 µg/ml) in the NBRIP medium. Among different nitrogen sources, di-ammonium sulphate was found to be best for phosphate solubilization by the strain K. pneumoniae S6C1 (151.2 µg/ml).
Phosphate solubilizing bacteria; rock phosphate; carbon; nitrogen.
Phosphorus (P) is an essential plant nutrient and plays a key role in plant growth and development, energy transport, signal transduction, macromolecular biosynthesis, photosynthesis, respiration, nutrient uptake, and nitrogen fixation1. It is the world’s most second largest nutritional supplement for crops after nitrogen. Although, most agricultural soil have large amount of inorganic and organic P, these are immobilized and mostly become unavailable. Hence, very limited concentration of P is available to plants due to P deficient in soil2. P deficiency decreased agricultural productivity on more than 2 billion hectares worldwide and therefore, agronomic treatments to improve P availability in the soil and to increase P utilization in agriculture are of special importance3. But, the concentration of soluble P in soil solution is in the range of 100- 400 g P/ha4. Chemical fertilizers are extensively used in traditional agriculture, but their use increases the production costs and environmental risks5. Excessive use of chemical fertilizers leads to the harmful damage to soil structure, composition, microflora and other properties of soil. They can cause environment hazard and expensive as well6,7.
Natural rock phosphates are the raw materials for production of phosphate fertilizers in sustainable agriculture system through microbial solubilization. Rock phosphate could play a significant role as cheaper P source to plants. However, it cannot be used in field because most of P in RP is present as non-exchangeable form, which is not directly available to plant uptake. One approach for solubilization in field condition is the application of rock phosphate as phosphate fertilizer along with activity of soil microorganisms (PSB) can be effective8.
There are several genera of bacteria reported as Phosphate solubilizing bacteria (PSB) involved in the conversion of insoluble phosphate to soluble e.g. Pseudomonas, Mycobacterium, Micrococcus, Bacillus, Flavobacterium, Rhizobium, Mesorhizobium, Sinorhizobium, Klebsiella, Arthrobacter, Enterobacter, Erwinia, etc. and have been reported to modify P nutrition and increase its solubilization in soil through many process, such as decrease the pH of the soil by producing organic and mineral acids, phytohormones, chelation and siderophores production which promote P solubilization in soil9,10,11,12.
Allium hookeri Thwaites is a member of family Alliaceae subgenus Amerallium, known to content its flavour content and therapeutic properties13,14,15. In this context, this study was designed to evaluate the P solubilization of insoluble RP of different concentration by the indigenous phosphate solubilizing bacteria from the rhizosphere of A. hookeri Th. and utilization of different carbon sources by PSB strains.
Bacterial strains
A. luteolus (S4C7), Enterobacter asburiae (S5C7), Klebsiella pneumoniae (S4C9, S4C10, S6C1) and Klebsiella quasipneumoniae (S6C2) isolated from the rhizospheric soil of Allium hookeri Thwaites growing as wild herbs in Manipur, India at latitude of 23°83´N – 25°68´N longitude of 93°03´E – 94°78´E, were used in this study and identified through a comparison of the 16S rDNA sequences with GeneBank accession number KX603401 (S4C7), KX603398 (S4C9), KX603397 (S4C10), KX603399 (S5C7), KX603402 (S6C1) and KX603400 (S6C2). These PSB strains showed solubilizing ability of tricalcium phosphate by forming clear halo zone around the colony16. All these phosphate solubilizing bacterial species were maintained in Pikovskaya agar. All the cultures were revived and sub-cultured before use in the present study.
Quantitative estimation of RP solubilization under In vitro conditions
Strains of 24- 48 hours old cultures grown in NBRIP (National Botanical Research Institute of Phosphate) broth shaken (128 rpm) at 30±1°C were used for quantitative estimation of RP. Rock phosphate was supplemented at different concentration (0.5%, 1.0% and 1.5% to 100 ml of NBRIP broth) as a phosphorus source instead of TCP. Quantitative estimation of phosphorus in supernatant was measured by vanado-molybdate-yellow color method in NBRIP broth [10g C6H12O6, 0.1g (NH4)2SO4, 0.25g MgSO4.7H2O, 0.2g KCl, 5.0g MgCl2.6H2O, 5g Ca3(PO4)2 in 1L distilled water]17. To a 0.5 ml aliquot of the supernatant, 2.5 ml Barton’s reagent was added and volume was made to 50 ml with de-ionized water. The absorbance of the resultant colour was read after 10 min at 430 nm in UV/Visible Spectrophotometer. The total soluble phosphorus was calculated comparing with the standard curve. The values of soluble phosphate liberated were expressed as ìg/mL over control. The pH of culture supernatants was measured in each case.
Effect of carbon sources on solubilization of TCP in NBRIP broth
To study the effect of carbon sources on the growth and phosphate solubilizing activity of bacterial isolates, glucose was replaced with an equal amount (10 g/L) of sucrose, maltose, fructose, galactose or mannitol added to the NBRIP broth. The flasks were incubated at 30°C shaken at 120 rpm for 24- 48 hours. The media was analysed for soluble P and pH reduction.
Effect of nitrogen sources on solubilization of TCP in NBRIP broth
To study the effect of nitrogen sources on the growth and phosphate solubilizing activity of bacterial isolates, (NH4)2S04 was replaced with an equal amount of (10g/L) of Sodium nitrate (NaNO3) and urea added to the NBRIP broth. The flasks were kept on the shaker incubator at 120 rpm for 24-48 hours. The media was analysed for soluble P and pH reduction by centrifugation the sample at 10000 rpm for 20 minutes and to 0.5 ml of supernatent, add 2.5 ml of Barton’s reagent, volume was made to 50 ml with de-ionized water. The absorbance of the resultant colour was read after 10 min at 430 nm in UV/Visible Spectrophotometer
Rock phosphates have been considered as a valuable alternative source for soluble P fertilizers especially for acid soil. But, unfortunately direct application of RP into the soils under alkaline condition is not always agronomically effective due to its low reactivity18. Research efforts are in progress to manipulate these rocks and convert them to value-added product by using chemico-physical means partially acidulating RP by using phosphate-solubilizing microorganisms19.In this present study, the PSB strains has the ability to solubilize the inorganic phosphate with indication of showing clear halo zone around the colony in Pikovskaya agar plate. Phosphate solubilization index (PSI) was calculated by the formula as described by Sharma et al.20 (Fig.1).
PSI= Solubilization diameter× 100
Colony diameter
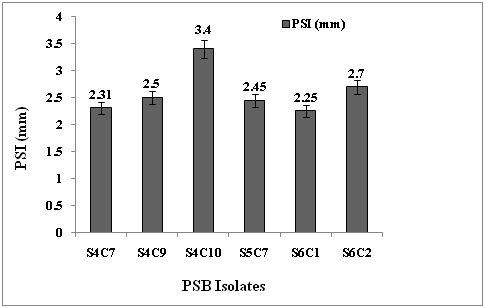
Fig.1: Solubilization effciency of PSB isolates obtained from the rhizosphere of A. hookeri Thwaites plant.
It is well known that many microorganisms isolated from the soil are able to dissolve different kinds of rock phosphate in liquid culture21,22,23.The PSB isolates were able to solubilize insoluble RP in different concentration 0.5%, 1.0% and 1.5% in NBRIP broth and the maximum amount of P was recorded 24 hours with 81.6µg/ml at 1% of RP and 79.2µg/ml at 0.5% of RP by K. pneumoniae S4C10 (Table. 1), followed by K. quasipneumoniae S6C2 released solubilized P of 76.8% at 0.5% of RP (Table.2), then K. pneumoniae S6C1 (72.8µg/ml of P at 0.5%) (Table.3)> E. asburiae S5C7 (56.2µg/ml of P at 1.5%) (Table.4)> K. pneumoniae S4C9 (44.8µg/ml of P at 0.5%) (Table.5)> A. luteolus S4C7 (33.6µg/ml of P at1%) (Table. 6) compared with uninoculated broth served as control. The pH reduction also observed corresponds to the respective increase in soluble P concentration. Kaur and Reddy24showed the isolates Pantoea cypripedii PSB-3 and Pseudomonas plecoglossicida PSB-5 are efficient for mineralizing inorganic phosphate with the accompaniment of decrease in the pH of the culture. A significant relationship between quantities of phosphate solubilized and drop in pH of culture filtrate in RP amended medium25. The combined of PSB (MRS 18 and MRS 34) and RP produced significant of amount of solubilized P release capacity (P mineralization) with reduction of pH in the broth. It also substantially increased microbial abundance in rhizosphere compared to all individual treatments (T0– T8) confirmed the stimulating effect of PSB on increasing microbial population in the rhizosphere that improved P solubilization and enhanced plant growth promotion26.The solubilization of inorganic phosphate by PSB was accompanied by a significant drop in pH in the liquid medium and it is correlated that the decrease in pH with the high phosphate solubilization27. Reddy et al28.,investigated that the PSMs Aspergillus niger and Aspergillus tubingensis capable of solubilizing of poorly soluble rock phosphates when grown in the presence of 2% of rock phosphate and can provide an efficient large scale biosolubilization of rock phosphates intented for P fertilizers. The arbuscular mycorrhizal fungi Glomus intraradices utilized more soluble phosphorus from soil mineral phosphate than non-inoculated plants. With the amendment of RP, the inoculated G. intraradices significantly stimulated plant growth and P content and affects the microbial activity in hyphosphere of Acacia holosericeaplant9. The selected PSB strains (genera Burkholderia and Bacillus) isolated from the rhizosphere of maize are able to solubilize RP(Araxá and Itafós phosphate) extracted from the RP mine, Brazil with the reduction of pH which suggests that the acidification of the culture medium can be one of the mechanisms involved in the solubilization of P29.
Table (1):
Soluble P and pH reduction by K. pneumoniae S4C10 in NBRIP broth having rock phosphate (equivalent to 100 mg P2O5/100 ml) as the sole phosphate.
| Concentration of RP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | aUninoculated broth | pH | b0.5% | pH | c1.0% | pH | d1.5% | pH |
| 24 | 28.8±3.0 | 5.89±0.1 | 52.00±2.0 | 5.87±0.5 | 68.00±2.0 | 5.8±0.2 | 49.5±4.0 | 5.92±0.3 |
| 48 | 23.4±2.0 | 5.85±0.4 | 79.2±4.0 | 5.72±0.4 | 81.6±1.0 | 5.61±2.0 | 64.8±2.0 | 5.73±0.5 |
| 72 | 15.2±5.0 | 6.19±0.3 | 44.8±4.0 | 5.96±0.3 | 32.8±2.0 | 6.08±0.4 | 30.4±2.0 | 6.12±0.5 |
| 96 | 20.8±4.0 | 6.10±0.5 | 50.4±2.0 | 5.91±0.4 | 33.6±2.0 | 6.11±0.5 | 34.4±5.0 | 6.14±0.4 |
| 120 | 17.6±2.0 | 6.13±0.3 | 47.2±1.0 | 5.88±0.1 | 31.2±1.0 | 6.05±2.0 | 31.2±2.0 | 6.12±1.0 |
| 144 | 15.2±5.0 | 6.23±0.5 | 32.8±1.0 | 6.08±0.5 | 19.2±1.0 | 6.24±1.0 | 24.0±4.0 | 6.44±0.5 |
| 168 | 26.4±4.0 | 6.00±0.1 | 53.6±2.0 | 5.83±0.2 | 36.00±3.5 | 6.01±0.3 | 40±1.0 | 6.37±0.3 |
Values are mean±SD, n=3.
a, b, c, d indicates the value of solubilized P in µg/ml.
Table (2):
Soluble P and pH reduction by K. quasipneumoniae S6C2 in NBRIP broth having rock phosphate (equivalent to 100 mg P2O5/100 ml) as the sole phosphate.
| Concentration of RP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | aUninoculated broth | pH | b0.5% | pH | c1.0% | pH | d1.5% | pH |
| 24 | 13.6±4.0 | 6.24±1.0 | 57.6±2.0 | 5.81±0.1 | 61.2±2.0 | 5.83±0.1 | 45.6±4.0 | 6.09±2.0 |
| 48 | 16.8±3.0 | 6.2±1.0 | 76.8±4.0 | 5.54±0.3 | 70.4±2.0 | 5.62±2.0 | 52.8±2.0 | 5.88±0.1 |
| 72 | 25.6±4.0 | 6.03±0.3 | 45.2±4.0 | 6.09±0.6 | 32.8±2.0 | 6.06±2.0 | 20.8±4.0 | 6.22±1.0 |
| 96 | 23.2±4.0 | 6.05±0.4 | 49.2±1.0 | 6.03±0.5 | 37.6±3.4 | 6.02±0.3 | 23.2±4.0 | 6.24±0.2 |
| 120 | 32.4±5.0 | 6.06±0.2 | 44.8±4.0 | 6.11±0.5 | 34.4±2.0 | 6.05±2.0 | 16.8±5.0 | 6.32±0.4 |
| 144 | 16.0±3.0 | 6.25±0.5 | 29.6±4.0 | 6.28±0.5 | 24.0±4.0 | 6.22±0.5 | 13.6±4.0 | 6.27±0.7 |
| 168 | 20.0±3.0 | 6.12±0.5 | 49.6±3.0 | 6.06±0.5 | 40.0±1.0 | 6.01±0.3 | 26.0±3.0 | 6.08±0.4 |
Values are mean±SD, n=3.
a, b, c, d indicates the value of solubilized P in µg/ml.
Table (3):
Soluble P and pH reduction by K. pneumoniae S6C1 in NBRIP broth having rock phosphate (equivalent to 100 mg P2O5/100 ml) as the sole phosphate.
| Concentration of RP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | aUninoculated broth | pH | b0.5% | pH | c1.0% | pH | d1.5% | pH |
| 24 | 13.6±4.0 | 6.24±1.0 | 61.6±5.0 | 5.77±0.5 | 60.8±1.0 | 5.76±0.5 | 44.8±3.0 | 6.01±0.4 |
| 48 | 16.8±3.0 | 6.2±1.0 | 72.8±4.0 | 5.7±0.5 | 68.4±2.0 | 5.68±0.5 | 55.2±2.0 | 5.85±0.5 |
| 72 | 25.6±4.0 | 6.03±0.3 | 38.4±2.0 | 6.09±0.3 | 34.4±5.0 | 6.11±1.0 | 17.6±2.0 | 6.21±0.5 |
| 96 | 23.2±4.0 | 6.05±0.4 | 46.4±4.0 | 5.99±0.3 | 40.0±1.0 | 6.07±0.8 | 22.4±4.0 | 6.11±0.4 |
| 120 | 32.4±5.0 | 6.06±0.2 | 28.4±4.0 | 6.21±1.0 | 38.0±2.0 | 6.08±0.5 | 34.0±3.5 | 6.11±0.4 |
| 144 | 16.0±3.0 | 6.25±0.5 | 32.0±2.0 | 6.05±0.5 | 26.0±4.0 | 6.15±0.5 | 18.4±1.0 | 6.19±0.6 |
| 168 | 20.0±3.0 | 6.12±0.5 | 41.6±1.0 | 6.0±0.3 | 42.4±4.0 | 6.03±0.3 | 22.4±2.0 | 6.18±0.4 |
Values are mean±SD, n=3.
a, b, c, d indicates the value of solubilized P in µg/ml.
Table (4):
Soluble P and pH reduction by Enterobacter asburiae S5C7 in NBRIP broth having rock phosphate (equivalent to 100 mg P2O5/100 ml) as the sole phosphate.
| Concentration of RP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | aUninoculated broth | pH | b0.5% | pH | c1.0% | pH | d1.5% | pH |
| 24 | 13.6±4.0 | 6.24±1.0 | 44.8±2.0 | 5.89±0.1 | 45.6±2.0 | 5.81±0.4 | 52.0±2.0 | 5.87±0.3 |
| 48 | 16.8±3.0 | 6.2±1.0 | 51.2±4.0 | 5.88±0.3 | 55.2±3.0 | 5.95±0.3 | 56.8±2.0 | 5.83±0.3 |
| 72 | 25.6±4.0 | 6.03±0.3 | 17.2±1.0 | 6.18±0.4 | 19.2±3.0 | 6.24±0.3 | 23.2±1.0 | 6.05±0.4 |
| 96 | 23.2±4.0 | 6.05±0.4 | 20.8±3.0 | 6.12±0.5 | 25.2±4.0 | 6.04±0.5 | 29.6±4.0 | 6.1±0.5 |
| 120 | 32.4±5.0 | 6.06±0.2 | 16.8±5.0 | 6.25±0.5 | 22.4±4.0 | 6.11±1.0 | 23.2±3.6 | 6.05±0.2 |
| 144 | 16.0±3.0 | 6.25±0.5 | 24.8±4.0 | 6.09±0.2 | 28.8±2.0 | 6.02±0.4 | 30.4±2.0 | 6.12±0.3 |
| 168 | 20.0±3.0 | 6.12±0.5 | 19.2±4.0 | 6.22±0.5 | 23.2±4.0 | 6.09±0.4 | 26.4±4.0 | 6.22±0.4 |
Values are mean±SD, n=3.
a, b, c, d indicates the value of solubilized P in µg/ml.
Table (5):
Soluble P and pH reduction by K. pneumoniae S4C9 in NBRIP broth having rock phosphate (equivalent to 100 mg P2O5/100 ml) as the sole phosphate.
| Concentration of RP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | aUninoculated broth | pH | b0.5% | pH | c1.0% | pH | d1.5% | pH |
| 24 | 13.0±4.0 | 6.23±0.2 | 23.2±2.0 | 6.05±0.4 | 14.4±4.0 | 6.21±0.4 | 16.8±2.0 | 6.2±0.2 |
| 48 | 13.6±4.0 | 6.24±0.2 | 26.4±2.0 | 6.0±0.2 | 12.8±3.0 | 6.2±0.4 | 14.4±5.0 | 6.21±1.0 |
| 72 | 20.8±3.0 | 6.1±0.3 | 44.8±4.0 | 5.96±0.1 | 23.3±4.0 | 6.05±0.4 | 23.2±4.0 | 6.05±0.5 |
| 96 | 19.2±3.0 | 6.24±0.4 | 32.0±1.0 | 6.06±0.4 | 31.6±2.0 | 6.09±0.3 | 32.8±2.0 | 6.08±0.4 |
| 120 | 20.8±3.0 | 6.11±0.5 | 32.8±5.0 | 6.08±0.9 | 20.8±4.0 | 6.1±0.3 | 26.0±4.0 | 6.02±0.4 |
| 144 | 13.6±4.0 | 6.22±0.5 | 36.8±1.0 | 6.01±0.2 | 26.4±2.0 | 6.0±0.3 | 28.8±4.0 | 6.18±0.2 |
| 168 | 15.2±5.0 | 6.23±0.2 | 22.4±2.0 | 6.05±0.2 | 18.4±2.0 | 6.19±0.5 | 20.0±3.0 | 6.11±0.4 |
Values are mean±SD, n=3.
a, b, c, d indicates the value of solubilized P in µg/ml.
Table (6):
Soluble P and pH reduction by Arthrobacterluteolus S4C7 in NBRIP broth having rock phosphate (equivalent to 100 mg P2O5/100 ml) as the sole phosphate.
| Concentration of RP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time intervals | aUninoculated broth | pH | b0.5% | pH | c1.0% | pH | d1.5% | pH |
| 24 | 13.0±4.0 | 6.23±0.2 | 13.6±5.0 | 6.25±0.2 | 13.2±5.0 | 6.24±0.2 | 13.6±5.0 | 6.24±0.2 |
| 48 | 13.6±4.0 | 6.24±0.2 | 16.8±5.0 | 6.19±0.4 | 31.2±2.0 | 6.01±0.2 | 21.6±2.0 | 6.06±0.2 |
| 72 | 20.8±3.0 | 6.1±0.3 | 25.6±4.0 | 6.03±0.3 | 21.6±2.0 | 6.06±0.4 | 25.6±4.0 | 6.03±0.1 |
| 96 | 19.2±3.0 | 6.24±0.4 | 23.2±4.0 | 6.05±0.4 | 14.4±5.0 | 6.2±0.3 | 23.2±4.0 | 6.05±0.3 |
| 120 | 20.8±3.0 | 6.11±0.5 | 32.4±2.0 | 6.0±0.1 | 25.6±4.6 | 6.04±0.3 | 33.6±2.0 | 6.0±0.4 |
| 144 | 13.6±4.0 | 6.22±0.5 | 16.0±5.0 | 6.24±0.2 | 16.0±5.0 | 6.25±0.4 | 23.2±4.0 | 6.05±0.4 |
| 168 | 15.2±5.0 | 6.23±0.2 | 20.0±2.0 | 6.12±0.2 | 25.6±4.0 | 6.03±0.4 | 16.8±5.0 | 6.2±0.2 |
Values are mean±SD, n=3.
a, b, c, d indicates the value of solubilized P in µg/ml.
Effect of carbon sources on phosphate solubilization by bacterial isolates
The bacterial isolates were evaluated in the presence of five carbon sources by replacing glucose with sucrose, maltose, fructose, galactose and mannitol. The form available carbon greatly affected the growth as well as the phosphate solubilization and was more active in presence of hexoses and pentoses or disaccharides30. The role of carbon source is important in phosphate solubilization was affected by the carbon source31. All the six strains demonstrated diverse levels of phosphate solubilization activity in the presence of various carbon sources. Among the six phosphate solubilizing bacterial strains, K. pneumoniae S4C10 showed most efficient strain with the maximum P released with fructose of 85.6 µg/ml in NBRIP broth medium (Table 7). Mardad et al32.,reported that fructose was found to be best carbon sources of 112.46% and 104.25% of soluble phosphate liberated by Enterobacter sp. PSB6 and Bacterium DR172 PSB5 respectively in NBRIP broth medium as compared with the positive control Acitenobacter sp. and maximum drop in pH also reported due to the organic acids secreted to the medium. Fructose has been identified as the best carbon source for Rhodotorula minuta NCIM 3359 and Saccharomyc escerevisiae ATCC 9896 in cultures33. After K. pneumoniae S4C10, the strain K. quasipneumoniae S6C2 showed PS activity in all the carbon sources test, but the efficiency varied with the carbon source. This strain showed maximum PS activity of 83.2 µg/ml in glucose containing NBRIP medium, followed by maltose and sucrose containing medium. Hameeda et al34.,reported the bacterial isolates from the different composts, farm waste compost (FWC), rice straw compost (RSC), Gliricidia vermicompost (GVC) and macrofauna, showed rock phosphate (RP) solubilization in buffered medium in plate culture. The strains Enterobacter cloacae EB27, Serratia marcescens EB67, Serratia sp. EB75, Pseudomonas sp. BW75 showed solubilized RP in RP broth medium. In the presence of different carbon sources, both strains showed a drop in pH and solubilized RP, P released was maximum with glucose (1212 and 522 µmol) by Serratia marcescens EB67 and Pseudomonas sp. CDB35 respectively. Earlier, phosphate solubilizing bacteria NBRI0603, NBRI2601, NBRI3246 and NBRI4003 isolated from the rhizosphere of chickpea and alkaline soils shows diverse levels of phosphate solubilization activity under In vitro conditions in the presence of various carbon sources. Xylose, lactose, xylose and glucose reported to be the best carbon sources for phosphate solubilization by strains NBRI0603, NBRI2601, NBRI3246 and NBRI4003 respectively35. Sridevi et al36.,revealed that the isolates Rhizobium species from Crotalaria species (C. juncea, C. laburnifolia, C. retusa and C. verrucosa) are able to solubilize tricalcium phosphate (TCP) and glucose was found to be best carbon source for P solubilization among the other carbon sources. Rhizobium sp. from C. retusa and C. verrucosa showed maximum solubilization at 3% concentration of glucose. Maximum decrease in pH also revealed in glucose containing medium. Another B. subtilis and B. cereus also reported as efficient for phosphate solubilization in different carbon sources viz. Glucose, sucrose, lactose and mannitol. However, incorporation of glucose increased the rate of solubilization of phosphate37.
Table (7):
Effect of different carbon sources of P solubilization.
| Carbon sources | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Glucose | pH | Sucrose | pH | Maltose | pH | Fructose | pH | Galactose | pH | Mannitol | pH |
| S4C7 | 13.6±4.0 | 6.23±0.7 | 18.8±5.0 | 6.13±0.1 | 29.6±4.0 | 6.12±0.3 | 36.8±3.5 | 6.09±0.3 | 10.4±5.0 | 6.88±0.4 | 6.8±5.0 | 6.69±0.5 |
| S4C9 | 54.4±2.0 | 5.81±0.3 | 56.8±2.0 | 5.79±0.2 | 48.8±3.0 | 5.88±0.3 | 58.4±1.0 | 5.79±0.4 | 55.2±2.0 | 5.80±0.1 | 47.2±3.0 | 5.91±0.4 |
| S4C10 | 63.2±2.0 | 5.72±0.3 | 57.6±2.0 | 5.80±0.3 | 70.4±1.0 | 5.73±0.5 | 85.6±1.0 | 5.65±0.4 | 73.6±2.0 | 5.86±0.2 | 58.4±4.0 | 5.79±0.5 |
| S5C7 | 55.4±2.0 | 5.83±0.4 | 28.8±3.0 | 6.08±0.2 | 32.4±3.0 | 6.08±0.5 | 45.6±5.0 | 5.89±0.2 | 62.8±4.0 | 5.75±0.2 | 46.0±4.0 | 5.92±0.3 |
| S6C1 | 78.4±2.0 | 5.69±0.4 | 70.4±1.0 | 5.81±0.4 | 72.0±2.0 | 5.65±2.0 | 81.6±2.0 | 5.70±0.3 | 63.2±4.0 | 5.72±0.2 | 67.2±1.0 | 5.87±0.4 |
| S6C2 | 83.2±2.0 | 5.65±0.3 | 74.4±3.0 | 5.85±0.4 | 78.4±3.0 | 5.54±0.3 | 63.2±2.0 | 5.72±0.2 | 73.2±4.0 | 5.63±0.3 | 73.2±1.0 | 5.83±0.5 |
Values are mean±SD, n=3
Effect of nitrogen sources on phosphate solubilization by bacterial isolates
All the strains behave differently in the presence of different nitrogen sources. The efficiency of three different nitrogen sources on PS activity was studied. Phospho-solubilization is related to the excretion of protons (H+) that accompanies the breathing on the assimilation of NH4+ 38. Bacterial cultures increased their solubilzation when ammonium was added39,40.
The PSB strains utilized different nitrogen sources tested viz. Sodium nitrate, di-ammonium sulphate and urea. Di-ammonium sulphate supported maximum phosphorus solubilization (151.2 µg/ml and 144.0 µg/ml) by the strains K. pneumoniae S6C1 and E. asburiaeS5C7 respectively with concomitant decrease in pH (Table 8). While sodium nitrate is utilized by the strains K. pneumoniae S4C9 (66.4 µg/ml) and K. quasipneumoniae (50.4 µg/ml) for solubilization of P. B. subtilis showed the ability of phosphate solubilization activity in different nitrogen sources with insoluble form of phospahte sources like tricalcium phospate and rock phosphate. B. subtilis utilized ammonium sulphate at highest level among other nitrogen sources both in TCP and RP supplemented medium where the pH drifted from the neutral to acidic with all carbon source in presence of TCP41. Prabhavati and Mallaiah42 supported our result where the strain Rhizobium HGR19 isolated from the root nodules showed maximum PS activity containing ammonium sulphate in the medium. Kumar and Ram43reportedTherhizobial strains (Sinorhizobium sp. MRR101, Agrobacterium tumifaciens MRR102, Rhizobium sp. 103, Sinorrhizobium kostiense MRR104, Agrobacterium tumefaciens MRR105, Rhizobium sp. MRR106) isolated from the root nodules of Vigna tribolata plants utilized different nitrogen sources for the solubilization of phosphorus. Sinorrhizobium kostiense MRR104 and Rhizobium sp. MRR106 reported to be high phosphate solubilization in ammonium sulphate containing in PVK medium. Reduction in pH also reported in the strains when nitrogen sources are used.
Nahas44 investigated most of the phosphate solubilizing microorganisms are heterotrophs and depends on carbon and energy sources that can be found in the rhizosphere or by recycling crop residues. In addition, nitrogen sources may be considered as control factors as they influence microorganisms growth and consequently their solubilization capacity. Qualitative analysis of the phosphate solubilized by various groups correlated well with grouping based upon quantitative analysis of bacteria isolated from soil and effect of carbon and nitrogen sources45.
Table (8):
Effect of different nitrogen source of P solubilization.
Strains |
Sodium nitrate |
pH |
Di-ammonium sulphate |
pH |
Urea |
pH |
|---|---|---|---|---|---|---|
S4C7 |
46.4±3.0 |
5.79±0.2 |
129.6±3.0 |
5.31±0.1 |
6.4±4.0 |
6.8±0.5 |
S4C9 |
66.4±4.0 |
5.68±0.3 |
128.0±2.0 |
5.35±0.2 |
9.6±4.0 |
6.78±0.4 |
S4C10 |
36.0±2.0 |
6.08±0.5 |
140.8±2.0 |
5.25±0.3 |
7.2±5.0 |
6.82±0.3 |
S5C7 |
26.4±4.0 |
6.17±0.5 |
144.0±4.0 |
5.22±0.1 |
54.4±3.0 |
5.72±0.2 |
S6C1 |
42.4±4.0 |
5.81±0.2 |
151.2±1.0 |
5.07±0.2 |
10.4±4.0 |
6.83±0.5 |
S6C2 |
50.4±5.0 |
5.75±0.2 |
120.0±1.0 |
5.35±0.2 |
7.2±5.0 |
6.85±0.3 |
Values are mean±SD, n=3
This study has revealed that, most of the isolates released more soluble P in all different concentration of rock phosphate (RP) and phosphate solubilizing bacteria were efficient in P solubilization with the decrease of pH in the medium. K. pneumoniae S4C10 and K. quasipneumoniae S6C2 are the most potential strains among the other PSB strain. Also, addition of fructose or glucose in the NBRIP medium by K. pneumoniae S4C10 and K. quasipneumoniae S6C2 showed maximum P release. The contribution of these strains, individually or in combination, can increase the P nutrition, growth and yield of the plants.
- Khan, M.S., Zaidi, A., Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate solubilizing microorganisms In: Khan MS, Zaidi A, Musarrat J, editors. Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology. Springer International Publishing Switzerland, Switzerland. 2014; 31- 62, doi:10.1007/978-3-319-08216-5-2.
- Adesemoye, A.O., Kloepper, J. W. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Micro- biol. Biot., 2009; 85: 1–12.
- Oberson, A., Friesen, D.K., Rao, I.M., B¨uhler, S., Frossard, E. Phosphorus transformations in an Oxisol under contrasting land-use systems: The role of the soil microbial biomass. Plant Soil., 2001; 237: 197–210.
- Wild, A. Plant nutrients in soil: In soil conditions and plant growth, ed. A. Wild. Longman Scientific and Technical, Essex. 1988; 695- 742.
- Schröder, J.J., Cordell, D., Smit, A.L., Rosemarin, A. Sustainable use of phosphorus. Wageningen: Plant Resource International., 2010; 122.
- Reddy, M.S., Kumar, S., Khosla, B. Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger. Bioresource Technol., 2002; 84: 187–189.
- Koliari, A.A., Akbari, Gh.A., Armandpisheh, O., Labbafi, M.R., Zarghami, R. Effects of phosphate chemical fertilizers and biologic fertilizers in various moisture regimes on some morphological characteristics and seeds performance in maize S.C. 704. Asian J. Agr. Sci., 2011; 3: 223–234.
- Kang, S.C., Ha, C.G., Lee, T.G., Maheshwari, D.K. Solubilization of insoluble inorganic phosphates by a soil fungus Fomitopsis sp. PS 102. Curr. Sci., 2002; 82: 439-442.
- Duponnois, R., Colombet, A., Hien, V., Thioulouse, J. The mycorrhizal fungus Glomus intraradices and rock phosphate amendment influence plant growth and microbial activity in the rhizosphere of Acacia holosericea. Soil Biol Biochem., 2005; 37: 1460—1468.
- Chen, Y. P. et al. Phosphate solublizing bacteria from subtropical soils and their tricalcium solublizing abilities. Appl. Soil Ecol., 2006; 34: 33–41.
- Sugihara, S., Funakawa, S., Kilasara, M., Kosaki, T. Dynamics of microbial biomass nitrogen in relation to plant nitrogen uptake during the crop growth period in a dry tropical cropland in Tanzania. Soil Sci. Plant Nut., 2010; 56: 105–114.
- Sharma, S.B., Sayyed, R.Z., Trivedi, M.H., Gobi, T.A. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus., 2013; 2: 587.
- Singh, H.B., Singh, R.S., Sandhu, J.S. Herbal medicine of Manipur–A colour encyclopaedia. Daya Publishing House, Delhi; 2003.
- Kala, C.P. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. J Ethnobiol Ethnomed., 2005; 1: 1–8.
- Pandey, A., Pandey, R., Negi, K.S., Radhamani, J. Realizing value of genetic resources of Allium in India. Genet Resour Crop Evol., 2008; 55: 985–994.
- Nautiyal, C.S., Mehta, S. An Efficient Method for Qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol., 2001; 43: 51-56.
- Jackson, M.L. Estimation of phosphorus content. Soil chemical analysis, Printer Hall, New Delhi (India). 1973.
- Xiao, C., Chi, R., Pan, X., Liu, F., He, J. Rock phosphate solubilization by four yeast strains. Ann Microbiol., 2013; 63: 173—178.
- Vassilev, N., Vassileva, M., Nikolaeva, I. Simultaneous P solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol., 2006; 71: 137-144.
- Sharma, K., Dak, G., Agrawal, A., Bhatnagar, M., Sharma, R. Effect of phosphate solubilizing bacteria on the germination of Cicer arietinum seeds and seedling growth. J. Herb. Med. Toxicol., 2007; 1: 59-61.
- Kucey, R.M.N. Phosphate-solubilizing bacteria and fungi in various cultivated and Virgin Alberta soil. Can. J. Soil Sci., 1983; 63: 671- 678.
- Goenadi, D.H., Sugiarto, Y. Bioactivation of poorly soluble phosphate rocks with a phosphorus-solubilizing fungus. Soil Sci. Society America J., 2000; 64: 927- 932.
- Vazquez, P., Holguin, G., Puente, M., Cortes, A.E., Bashan, Y. Phosphate solubilizing microorganisms associated with the rhizosphere of mangroves in a semi arid coastal lagoon. Biol. Fertil. Soils. 2000; 30: 460- 468.
- Kaur, G., Reddy, S. Phosphate solubilizing rhizobacteria from an organic farm and their influence on the grown and yield of maize (Zea mays L.). J. Gen. Appl. Microbiol., 2013; 59: 295- 303.
- Himani, S., Reddy, M.S. Improvement of wheat and maize crops by inoculating Aspergillus spp. in alkaline soil fertilized with rock phosphate. Arch. Agron. Soil Sci., 2012; 58: 535- 546.
- Manzoor, M., Abbasi, M.K., Sultan, T. () Isolation of phosphate solubilizing bacteria from Maize rhizosphere and their potential of rock phosphate solubilization-mineralization and plant growth promotion. Geomicrobiology J., 2016; 81- 95.
- Alia, Afzal A, Khokhar SN, Jabeen B, Asad SA. Phosphate solubilizing bacteria associated with vegetables roots in different ecologies. Pak J Bot, 2013; 45: 535—544.
- Reddy, M.S., Kumar, S., Khosla, B. Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger. Bioresource Technol., 2002; 84: 187–189.
- Gomes, E.A., Silva, U.D.C., Marriel, I.E., Oliveira, C.A.D., Lana, U.G.D.P. Rock phosphate solubilizing microorganisms isolated from maize rhizosphere soil. Revista Brasileira de Milho e Sorgo., 2014; 13: 69- 81.
- Patil, M.G., Sayyed, R.S., Chaudhari, A.B., Chincholkar, S.B. Phosphate solubilizing microbes: A potential bio-inoculants for efficient use of phosphate fertilizers. 107-117. In: S. M. Reddy, S. Ram Reddy, M. A. Singarachary & S. Girisham (eds.) Proceeding of National Symposium Bioino culants for Sustainable Agriculture and Forestry. 2001; Scientific Publishers (India), Jodhpur.
- Di Simine, C.D., Sayer, J.A., Gadd, G.M. Solubilization of zinc phosphate by a strain of Pseudomonas fluorescensisolated from a forest soil. Biol. Fert. Soils., 1998; 28(1): 87–94.
- Mardad, I., Serrano, A., Soukri, A. Effect of carbon, nitrogen sources and abiotic stress on phosphate solubilization by bacterial strains isolated from a Moroccan rock phosphate deposit. J. Adv. Chem. Eng., 2014; 4: 1- 10.
- Narsian, V., Patel, H.H. Inorganic phosphate solubilization by some yeasts. Indian J Microbiol., 1995; 35: 127-132.
- Hameeda, B., Reddy, Y.H.K., Rupela, O.P., Kumar, G.N., Reddy, G. Effect of carbon substrates on rock phosphate solubilization by bacteria from composts and macrofauna. Curr. Microbiol., 2006; 53: 298-302
- Nautiyal, C.S., Bhadauria, S., Kumar, P., Lal, H., Mondal, R., Verma, D. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiology Letters., 2000; 182: 291- 296.
- Sridevi, M., Mallaiah, K.V., Yadav, N.C.S. Phosphate solubilization by Rhizobium isolates from Crotalaria species. J. Plant. Sci., 2007; 2: 635- 639.
- Maheswar, N.U., Sathiyavani, G. Solubilization of phosphate by Bacillus sps, from groundnut rhizosphere (Arachishypogaea L). J. Chem. Pharm. Res., 2012; 4: 4007- 4011.
- Illmer, P., Schinner, F. Solubilization of inorganic calcium phosphates solubilization mechanisms. Soil Biol Biochem., 1995; 27: 257-263.
- Asea, P.E.A., Kucey, R.M.N., Stewart, J.W.B. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol Biochem., 1988; 20: 459-464.
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv Agron., 2000; 69: 99-151.
- Selvi, B.K., Ravindran, A.D. Influence of different carbon and nitrogen sources on insoluble inorganic phosphate solubilization by Bacillus subtilis. I. J. A. B. R., 2012; 2: 441- 445.
- Prabhavati, E., Mallaiah, K.V. Effect of different carbon, nitrogen and cell wall affecting agents on phosphate solubilization by Rhizobium sp. nodulating Macrotyloma uniflorum (Lam.) Verdc. Internat. J. Agric. Sci., 2009; 5: 126- 128.
- Kumar, G.K., Ram, M.R. Phosphate solubilizing rhizobia isolated from Vigna trilobata. American J. Microbiol. Res., 2014; 2: 105- 109.
- Nahas, E. Phosphate solubilizing microorganisms: Effect of carbon, nitrogen and phosphorus sources. E. Velázquez and C. Rodríguez-Barrueco (eds.). First International Meeting on Microbial Phospate Solubilization. 2003; 111- 115.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


