This paper has reviewed investigates that obtained from peer-reviewed literatures on Shigellosis. Foodborne diseases related to unhygienic food handling practices remain a major public health problem across the globe. The problem is rigorous in developing countries due to restrictions in securing most possible hygienic food handling practices. Data demonstrates that an estimated 75% of cases of diarrheal diseases are linked with the using up of foods contaminated with pathogenic microorganisms. Bacillary dysentery (Shigellosis) is a severe human disease caused by Shigellae. It is one of the major sources of diarrhoea in developing countries. The precise estimates of morbidity and mortality due to shigellosis are deficient, however it is widespread and has been reported to cause many outbreaks. The limited information available specifies Shigella to be a vital food borne pathogen in developing countries. In this Review, it is renowned that pathogenic Shigella is still public health problem. Therefore, a large amount of information has been generated concerning the host, pathogen and environmental factors that impact the pathogenesis of shigellosis at the cellular and molecular level and summarizes what is currently known about Shigella, elementing those features that contribute to pathogenesis and investigating the existing progress in the development of safe and low-cost multivalent vaccine. Thus this review is focused upon the epidemiology, disease burden and the therapeutic challenges of Shigellosis in developing countries perception.
Shigellosis, Epidemiology, MDR, Pathogenesis, Shigella, Food-borne pathogens, Antibiotic Resistance.
In medicine, “diarrhoea” means “a flow through”, also defined as “the passage of three or more loose or liquid stools per day, more frequently than is normal for the personality” 1, 2. If left untreated, diarrhoea can lead to severe dehydration, which can result in hospitalisation or even death. Diarrhea disease is widespread all over the world, not only threatens human health but also greatly influence society and global economy. The fatality rate by diarrhea disease exceedingly ranks fourth among all the diseases, only lower than tumour, Cardiovascular or Cerebral vessels diseases and diabetes mellitus, effects are worse in developing countries and low revenue countries and it has became one of problems of the worldwide major public health. WHO treats the organize of diarrhea disease as global strategy and also according to the scheme of control of diarrhea disease was ratified in May, 1978 (Figure 1).
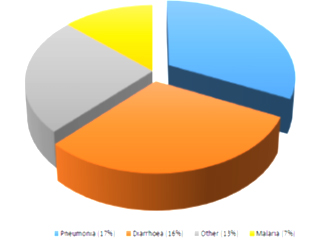
Fig. 1. Proportional distribution of cause of disease among children below five years of age in India (Source: Based on WHO, Global Burden of Diseases estimates to the most recent estimates for the total number of less than-five deaths)
It is usually the symptom of gastrointestinal infection, most cases of diarrhoea in children result from infection caused by a variety of viruses, bacteria or parasites, which disturb the normal fluid and nutrient assimilation of the intestines. As per UNICEF data: Monitoring the situation of Children and Women report June 2016, diarrhoeal diseases accounting for 9 percent of all deaths among the children under- five years of age making them the second largely common cause of child deaths worldwide. This translates into over 1,400 young children dying each day or nearly 1.34 million deaths a year 3, most deaths from diarrhoea occur along with children less than 2 years of age living in South Asia and sub-Saharan Africa. Over half of the fatality occurs in just five countries: India, Nigeria, Afghanistan, Pakistan and Ethiopia.
As it core, in developing countries like India, diarrhoeal infection remains a major health problem, due to infection constitute a major burden disease 4. Fifteen countries contribute three divisions of childhood causality due to diarrhoea in children less than five years of age worldwide out of which India ranks first 5. In India acute diarrhoeal diseases lead to 13% deaths under five years of age group, for the period of 2009, concerning 11.2 million cases with 1,762 deaths were accounted 6. According to a statistical survey, by National Institute of Cholera and Enteric Disease, Kolkata, (India), basic death rate due to diarrhoea in rural India is 9.3 for each 1,000 populations and the diarrhoeal deaths account for 22% of total rural deaths among 0 to six years age group children (NICED, Kolkata). Rotavirus is the single most common pathogen which causes gastroenteritis in together developed and developing countries but the inclination of bacterial enteropathogens vary largely between developed and developing countries 7.
The causes of relentless diarrhoea in populations are poorly understood. Five types of Escherichia coli are responsible for significantly 25% of all diarrhoeal diseases in developing countries. Shigella species are accountable for 10 to 15% of severe diarrhoeas in children less than 5 years of age. Other bacterial enteropathogens are Vibrio cholera, Campylobacter jejuni, Aeromonas species, Bacteroides fragilis and Providencia alkalifaciens appeared to be the most common etiological causes but certain situations are associated through and particularly high incidence of acute diarrhoeal diseases 8. The trends of bacterial enteropathogens causing gastroenteritis keep on altering with change in pattern of living and environmental hygiene 7. So the periodic renewal of the knowledge concerning the trends of the bacterial enteropathogens is very crucial. An epidemiologic revision of an infectious disease in a community is constantly measured to be a primary action towards the introduction of the proper involvements for overprotective of disease since the characteristics and the model of isolation of etiologic agents of the disease fluctuate from place to place depending on the confined meteorology, geography and socio-economic elements 9.
A preface to Shigella bacteria
Shigella is a species of enteric bacteria that originates diseases in humans and other primates 10, 11. The disease originated by the ingestion of Shigella bacteria is referred as Shigellosis. Shigellosis is a bacterial infection of the intestinal tract caused through bacteria of the genus Shigella and manifested by diarrhoea. This is a well known agent of bacillary dysentery, infantile diarrhoea all over the world and more serious than the common “stomach flu”. Shigella species was the second most widespread bacterial agents causing diarrhoea after Escherichia coli (E. coli) 12. Shigellosis is endemic to many developing countries and also occurs in outbreak causing substantial morbidity and mortality. The foremost symptom of this infection is bloody diarrhoea and the least infective dose is as low as 10-100 bacterial cells due to virtual resistance to stomach acid. Shigellosis is a major public health concern worldwide, mostly in developing countries 13, 14. Shigella infection is characterized with high fever (>38.5°C [101.3°F]), abdominal cramps, diarrhea, tenesmus, and polymorph nuclear leukocytes on a methylene blue stain of the faecal matter; extraintestinal appearances and complications also occur.
Evolution and Identification of Shigella
The numerous types of Shigella bacteria have been named after the lead workers who discovered each one 10, 11, 15. The first bacterium to be discovered, Shigella dysentriae, was named after Kiyoshi Shiga, a Japanese scientist who discovered it in 1896 as investigating a large epidemic of dysentery in Japan 16, 17. The bacterium was also referred to a genus of generally as the dysentery bacillus (the word “bacillus referring to a class of Gram-positive, rod shaped bacteria of which Shigella is a member) 17.
In summary published annually, the CDC provides an overview of the classification of various types (species) of Shigella bacteria as; the genus Shigella consists of four major serological groups belonging A to D according to their O-polysaccharide antigens subsequenting to the four species, correspondingly 18. In current taxonomy, these four serological subgroups are accepted as four species within the genus Shigella: Shigella dysentriae (S. dysentriae), Shigella flexneri (S. flexneri), Shigella boydii (S. boydii) and Shigella sonnei (S. sonnei). The four distinct species can be distinguished on the basis of serogrouping and biochemical analysis 19. Around 70% of the children with Shigellosis are infected with Shigella flexneri, 20% with Shigella sonnei and the remaining 10% with Shigella dysentriae or Shigella boydii.
General Characteristics and Epidemiology of Genus Shigella
The cells of the Shigella species are Gram-negative bacilli, nonmotile, non-spore-forming, facultative anaerobic rods 20. Shigella is differentiated from the closely related Escherichia coli on the basis of pathogenicity, physiology (failure to ferment lactose or decarboxylation lysine) and serology. They are generally catalase positive and oxidase and lactose negative. They ferment sugars, usually without forming gas. The strains are able to at temperatures ranging from 200C and 460C, with an optimum at 370C and at a pH range of 5.0 to 7.5. The common selective or differential agar media used for the culturing of Shigella are MacConkey (MAC), Xylose Lysine Deoxycholate (XLD), Hektoen (HEK), Salmonella-Shigella (SS), Deoxycholate Citrate Agar (DCA). The genus is divided into four serogroups with over 2,500 identified multiple serotypes 21, 22.
The genus Shigella was divided into four species viz., Shigella dysentriae (serogroup A), Shigella flexneri (serogroup B), Shigella boydii (serogroup C) and Shigella sonnei (serogroup D). Based on the variations in their O- polysaccharide portion of their LPS, the species were further classified into several serotypes, as S. dysentriae known to have 15 serotypes, S. flexneri have 14 serotypes and sub serotypes, S. boydii 20 serotypes and S. sonnei with a single serotype 23, 24 (Table 1).
Table (1):
Species and Serogroups of Shigella (Thomas and Gerald, 2000).
Species |
Serogroup |
Serotypes |
|
|---|---|---|---|
S.dysentriae |
A |
1-12 |
Most common, with outbreaks |
S.flexneri |
B |
1-6(with 15 subtypes) |
Developing Countries |
S.boydii |
C |
1-18 |
|
S.sonnei |
D |
1 |
Developed countries * |
(* Kotloff, 1999; Prado, 1999)
Shigellosis is a global human health problem. Annually, there are 165 million cases of shigellosis resulting in 1.1 million deaths in the developing world with high morbidity and mortality (99%) 25, 26. Of these 1.1 million deaths due to Shigella, 69% are in children aged less than five years 23, 25. It is endemic in most developing countries where substandard hygiene and unsafe water supplies, in densely populated areas and institutions 27, 28. Humans are the only natural hosts for Shigella. Both endemic and epidemic shigellosis is present in developing countries. Among 69% infections, 61% of all deaths were reported in the children less than 5 years of age. S. flexneri and S. sonnei were responsible for increased percentage of infections in the developing as well as in the industrialized countries. It has been reported that annually around 58 million travelers from industrialized countries were affected. Bacillary dysentery was also reported among the military troops 23. During a sporadic outbreak of dysentery in Kolkata, S. dysentriae type I and S. flexneri were the commonest serotypes found in the tested stool samples of the patients 29-32. A surveillance report of Shigella infections in Indonesia states that Shigella was isolated from 9.3% of diarrhea patients in the health centres. S. flexneri was found in 5.9% of patients, and was the most frequent species isolated, comprising 63.2% (36/57) of all Shigella species isolated. Shigella species were found significantly more often among children over 2 years old, and the rate of isolation increased with age. In a multicentre study of Shigella infection in six Asian countries, S. flexneri was found to be predominant in Bangladesh, China, Pakistan, Indonesia and Vietnam site whereas S.sonnei was common in Thailand; S. boydii was responsible for the series of infection in Bangladesh 33. Kimura et al., reported about S. sonnei outbreak in United States through the commercially prepared food 34. S. boydii was generally found only in the Indian subcontinent 25. Stool with mucus and/or blood were the main characteristics of Shigella infection in these patients 35. Meta-analysis from PubMed and Chinese biomedical literature database showed the prevalence of S. flexneri and S. sonnei in mainland China from 2001 until 2012 36.
The most frequently reported factor associated with the involvement of the infected worker was bare hand contact with the food followed by failure to properly wash hands, inadequate cleaning of processing or preparation equipment or utensils, cross-contamination of ready-to-eat foods by contaminated raw ingredients. In United States and Europe, children in day-care centres, migrant workers, travelers to developing countries and individuals in custodial institutions are infected most often 37.
Biology and Biochemistry of Shigella species
Upon ingestion, the bacteria continue to exist in the gastric environment of the stomach or abdomen and make its way to the large intestine. Where it attaches and enters the epithelial cells of the intestinal mucosa. Post invasion, Shigella multiplies intracellular and spreads to Neighbouring epithelial cells resulting in tissue destruction and representative pathology of shigellosis. Generally, Shigella adheres to the membrane of the cell and is internalized by an endosome which it consequently lyses to gain access to the cytoplasm where multiplication takes place 38. Shigella infects the host during the M-cells in the gut epithelia of the small intestine, as they cannot enter directly through the epithelial cells. Using a Type III secretion system acting as a biological syringe, the bacterium injects Invasion plasmid antigen D (IpaD) proteins into cells, triggering bacterial infect and the subsequent lyses of vacuolar membranes using IpaB and IpaC proteins 23. Extracellular Shigella is not motile but intracellularly it is able to move occupying the entire cytoplasm of the infected cell and between cells. It uses a mechanism for its motility by which its IcsA and IcsB proteins trigger action polymerization in the host cell (via N-WASP recruitment of Arp2/3 complexes) in a rocket propulsion fashion for cell-to cell spread 39. Specifically, movement between adjacent cells is facilitated by the IcsA protein.
After successful epithelial cell invasion and penetration of the colonic mucosa by the bacteria, there is deterioration of the epithelium and inflammation of the lamina propria culminating in desquamation and ulceration of the mucosa and subsequent leakage of blood, inflammatory elements and mucus into the intestinal lumen 23. Thus the characteristic passage of recurrent and measly dysenteric stool mixed with blood and mucus. Absorption of water by the colon is inhibited under these conditions 20. Some strains of Shigella produce enterotoxins and shiga-toxins similar to the verotoxins of E. coli O157:H7. The toxin has a molecular weight of 68kDa and is a multi-subunit protein consisting of one molecule of an A subunit (32,000 MW) and five molecules of the B subunit (7,700 MW). Both shiga-toxins and verotoxins are associated with haemolytic uremic syndrome (HUS), Haemolytic colitis and dysentery 39. The names of these conditions are dependent on the causative organism and symptoms range from severe diarrhoea, abdominal pain, vomiting and bloody urine. Each of the Shigella genomes includes a virulence plasmid that encodes conserved primary virulence determinants. The Shigella chromosomes share most of their genes with those of E. coli K12 strain MG1655 20. No antidote exists for these toxins. Thus supportive care requires maintenance of fluid and electrolyte levels and monitoring and support for kidney function. Inactivation of the toxin is achieved by steam treatment, oxidizing agents such as bleach and chemical sterilizing agents such as glutaraldehyde. The toxin acts on the lining of the blood vessels, the vascular endothelium. The B subunits of the toxin bind to a cell membrane component, Gb3, and the complex enters the cell. Once inside, the A subunit interacts with the ribosomes to inactivate them. The A subunit of the Shiga-toxin is a Glycosidase that modifies the RNA component of the ribosome to inactivate it and thereby bring a halt to protein synthesis of the cell leading to cell death 40.
The vascular endothelium has to continually renew itself. Hence, cell death leads to breakdown of the lining leading to haemorrhage. The primary response is characteristically bloody diarrhoea. For unexplained reasons, the toxin is seemingly effective against small blood vessels such as found in the digestive tract, kidneys and lungs but not against vessels such as the arteries or major veins. A specific target for the toxin appears to be the vascular endothelium of the glomerulus destroying the structures and concluding in kidney failure and the development of the often deadly and frequent debilitating haemolytic uremic syndrome. Food poisoning with Shiga-toxin often has an effect on the lungs and the nervous system 41.
Clinical Manifestation
Shigella spp. is the causative agent of the disease shigellosis. This severe intestinal infection is also called as bacillary dysentery. Globally, this disease is known as a major burden in public health care 23. It is first and foremost a disease of humans. The disease is characterized by injure of the colonic epithelium followed by intracellular and intercellular spread, infection in the nearby or near to cells and the host’s acute inflammatory reactions leads to colitis of the mucosa. It results in the leakage of blood and mucous in the intestinal lumen 41. Since the disease is acquired via the faecal-oral route, the rigorousness and the series of symptoms may be dependent upon the number of organisms ingested. The symptoms vary from mild watery diarrhea to severe inflammatory dysentery with the passage of frequent bloody and mucoid stools. The other symptoms include fever, malaise, abdominal cramping and convulsions. The beginning of symptoms usually occurs within 24 to 48 hours of ingestion of the etiologic agent. Like other complications of shigellosis include bacteraemia, septicaemia, hypoglycaemia, dehydration, haemolytic-uremic syndrome, reactive arthritis, toxic megacolon and other neurological problems 42. The persons infected with S. flexneri subsequently develop pain in their joints, irritation of the eyes and painful urination. This condition is called Reiter’s syndrome. It is the late complication of S. flexneri infection and lasts for months which lead to chronic arthritis. Shiga toxin of S. dysentriae type I is responsible for the Haemolytic-uremic syndrome. Usually, shigellosis is a self-limiting disease. Life-threatening are often seen in malnourished infants and young children less than 5 years of age, also in elderly people who have weak immune system 23, 43, 44.
Pathogenesis of Shigella
Infection is initiated by ingestion of Shigellae (usually via faecal-oral contamination). The organism enters the host and travels through the digestive system until it reaches the large intestine. In general the infection is limited to the intestinal mucosa. Pathogenesis is initiated by the invasion through the basal face of the intestinal epithelium by Shigella. Thus, upon reaching the large intestine, Shigella is taken up in vacuoles by microfold cell (M cells) which are specialized structure of the follicle associated epithelium which covers the mucosal lymphoid follicles, the stimulating site of the mucosal immune system 45, 46. The organism escapes from the vacuole and finally travels to underlying macrophages that are associated with M cell-associated lymphoid follicles 47. Shigella is phagocytised by macrophages in the dome area of these follicles, which then induces apoptosis which results in the escape of the pathogen to the basal side of the colonic epithelium. Studies also report that virulent S. flexneri causes destruction to the host cell mitochondria and triggers necrosis in the infected human monocyte derived macrophage 48. Another study indicated that Shigella induced mitochondrial dysfunction in non myeloid cells; result in caspase-independent necrotic cell death through a new pathway during oxidative cell stress in epithelial cells 49. Death of the macrophage results in the release of proinflammatory cytokine IL-12 which eventually results in the recruitment of polymorphonuclear (PMN) cells to the site of infection and the onset of inflammation 47, 50, 51.
Meanwhile, the bacteria induce their own uptake into colonic epithelial cells and spread laterally through the cells of the epithelium by a process known as action based motility (ABM) through the comet tail formation 52. This actin based filamentous tail propels the Shigellae into the protrusions on the contiguous enterocytes. During cell-cell spread the plasma membrane envelops the bacteria are lysed which results in the intracellular replication and intercellular bacterial spread 47, 53. As the inflammation persists and expands, the infiltration of the PMNs facilitates the entry of additional bacteria onto the epithelium. Ultimately, it is the cells of the host’s immune system that cause inflammation and ulceration of the mucosa to the colon and develops the symptoms associated with shigellosis 45, 54-57. It has been reported that the destruction of epithelial cells in the experimental models of shigellosis is due to the host inflammatory response and probably not by the intracellular multiplication of the pathogen 58.
Transmission of and Infection with Shigella
Shigella infections are very transmissible; transmission of the disease is mainly by person-to-person contact through contaminated hands 59. Outbreaks in children consistently occur under situation associating close physical contact, such as those come across in day-care centres, nursing homes, custodial institutions, cruise ships, aboriginal reservations and packed refugee camps, with poor hygiene practices and contaminated food or water serving as the vehicle for infection 61-63. It is approximated that up 80% of all infection is the result of shigellosis transmission; the infectious dose is as low as 10-100 organism. Numerous studies have exposed an increased frequency of shigellosis cases in young adult men residing in urban settings who have little, if any; disclosure to these traditionally recognised risk groups. Although some of these studies designated that sex between men able to be a risk-factor, most of these studies transpired before the HIV epidemic 64.
Shigella infections also may be acquired from consumption of contaminated food. In the developed and developing countries, incidence of foodborne illness is cited through foodNet, a reporting system used by public health agencies that occupies foodborne illness in over 13% of the population of the ten pathogens; Salmonella, Campylobacter and Shigella are responsible for most cases of foodborne illness. An estimated 20% of the total number of cases of shigellosis involved food as the vehicle of transmission 64.
Symptoms of Shigella infection
Shigellosis has mild infection cause low-grade fever (about 100.4 to 1020F [38 to 38.90C]) and watery diarrhea, fever, stomach cramps and abdominal pain one to four days after people exposed to the bacteria, symptoms may start 6-72 hours after injection. Some adults not have a fever. In adults, the first indications may be painful abdominal cramps and a continual longing to secrete. Passing stool may temporarily mitigate the pain. These symptoms may develop into more severe and appear more usually as the infection progresses. Serious infections may cause low-grade or moderate fever and watery diarrhea that progress to dysentery. Shigella bacteria produce toxins that can invasion the lining of the large intestine, lead to swelling, ulcers on the intestinal wall and bloody diarrhea. The severity of the diarrhea sets Shigellosis apart from routine diarrhea. In kids with Shigellosis, the primary bowel movement is often outsized and watery. Later bowel movements may be smaller, frequent, but stool may have blood, pus and mucus in it.
In very severe cases of Shigellosis, an infected person may have seizures, a rigid neck, a headache, acute tiredness and uncertainty. Shigellosis can also lead to dehydration and in odd cases, other complications, like arthritis, skin rashes and kidney failure. Some cases in children with serious cases of Shigellosis may need to be hospitalised. Shigellosis usually resolves in 5 to 7 days 65. A severe infection with high fever may be having with seizures in children less than two years old 28. Some persons who are infected may have no signs at all, but may still overtake the Shigella bacteria to others 10, 15.
Prevalence of Shigella infection
Most Shigella infections result sporadically, but huge Shigella epidemic have been traced to contaminated food and water. The CDC estimates that 4, 50,000 total of Shigellosis appear in the development countries every year 15, 66. In local Gulbarga district and surrounding region of Gulbarga (Karnataka state), diarrhoea has been estimated to be responsible for approximately 11-13% of all childhood illness, with a population of about 5, 32,031. Among the four species of Shigella, Shigella dysentriae and Shigella flexnerii were more predominant one 67. Shigellosis also characterized by seasonality with the largest percentage of accounted cases occurring between July and October and the smallest percentage occurring in January, February and March 28. Non-outbreak infections account for the majority of cases and in general, the precise means through which persons are infected (risk factors) are not yet well renowned or understood. Shigella is a particularly common reason of disease among young children, in large part since it is difficult to control the spread of the bacteria in day-care settings 10. The symptoms of shigellosis vary so widely that children shedding Shigella in their stool may show no indications of infection. A person infected with Shigella can be asymptomatic (show no symptoms of illness), suffer from moderate to rigorous diarrhea, or suffer complications up to and including death 15.
Antimicrobial Resistance in Shigella bacteria
Antimicrobial resistance in pathogenic bacteria is developing and increasing risk to human health 68, 69. Physicians are progressively attentive that antimicrobial resistance is increasing in bacterial pathogens and that, as a result, patients who are recommended antibiotics are at increased risk for promoting antimicrobial-resistant various infections 70. Certainly, “increased frequency of treatment failures for acute illness and increased relentless of infection may be signified by continued duration of illness, increased frequency of bloodstream infections, increased hospitalization or expanded fatality”. The need of antimicrobial agents in the feed of food animals is estimated by the FDA to be over 100 million pounds per year. It is estimated that 36% to 70% of all antibiotics produced in the United States are used in food animal feed or in preventative treatment to counter animal disease 68. In 2002, the Minnesota Medical Association printed an article by David Wallinga, M.D., M.P.H. who wrote: According to the [Union of Concerned Scientists], 70 percent of entire antimicrobials used in the United States and developing countries for all intents or about 24.6 million pounds annually are fed to poultry, swine and beef cattle for nontherapeutic point, in the non appearance of disease. Over half are “medically important” antimicrobials; identical or so closely related to human medications that resistance to the animal medicine can confer resistance to the similar human drug. Penicillin, tetracycline, macrolides, streptogramins and sulphonamides are prominent examples 71 (Figure 2).
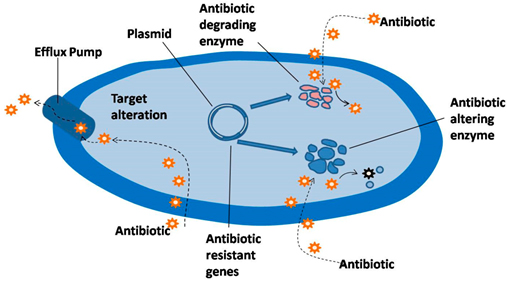
Fig. 2. Chief Bacterial targets and various ways of resisting the action of antibiotics (Source by; Harish Chandra et al, 2017)
Important enteric pathogens like Vibrio cholera, E.coli, Shigella spp, Salmonella spp and Campylobacter jejuni are becoming gradually more resistant to the major antibiotics that are needed for optimal treatment of patients and these bacterial pathogens are very diverse from one another. They cause quite different clinical syndromes; their ecology, epidemiology and method of transmission are distinct; and they are usually separated genetically. The fact that such different organisms are developing increasingly antibiotic-resistant underlines the pervasiveness of the pressures that lead to the emergence and spread of resistance. Over the past decades, Shigella species show a pattern of steadily increasing and have become even more resistant to most widely-used antimicrobials 72, 73. The increasing levels of antimicrobial resistance of Shigella isolates have complicated the treatment of shigellosis. The antimicrobial resistance mould of Shigella species differ according to geographic area and in the same place over time, leading to therapeutic problems 74-77. Periodic regional monitoring of disease with serotype breakdown and regular periodic antibiotic-susceptibility testing mode of isolates to conduct local empirical therapy are important factors for the adequate control of shigellosis.
The clinical consequences of antibiotic resistance differ among the pathogenic bacterial diarrhoea agents. For shigellosis, antibiotics are the primary treatment. Patients treated with an ineffective antibiotic may have more complications than condition that, they had not been treated, since the antibiotic is likely to affect the normal intestinal flora, thus actually supporting the growth of the resistant Shigella.
Infantile diarrhoea is a major disease and has been posing a vast communal health problem in developing countries like India over the years. The traditional antishigellosis drugs chlormaphenicol, ampicillin and sulphamethoxazole have developed into out of date. In recent years, fluoroquinolones, especially ciprofloxacin have been very successful in combating Shigellosis but unfortunately, resistant strains have emerged. The emergence of high-level ciprofloxacin resistance in Shigella sps has also been reported in India79 among Shigella poses a major therapeutic challenge to manage this disease. One of the reasons for emergence of multi-drug resistant Shigella sps is the unique capability of the pathogen to acquire resistance factors (transmissible genes) from the environment or from other bacteria. Antimicrobial resistance is usually conferred by certain genes. A large number of resistance related genes have reported for each group of antimicrobials. It is impossible to study all the reported genes, so most commonly isolated predominant isolates and reported genes were selected for this study. Fluoroquinolone, especially ciprofloxacin are the most regularly used drugs for Shigellosis treatment. Reduced susceptibility to the fluoroquinolone group of antibiotics is usually linked with point mutations in the bacterial target genes gyrA, gyrB encoding DNA gyrase and parC, parE encoding DNA topoisomerase IV.
At present, multi-drug resistance has complicated the assortments of empirical agents used for treatment of Shigellosis, particularly in children. The emergence of fluoroquinolone resistance in Shigella sps and their dissemination across the countries 80 due to;
- Point mutations, intrinsic resistance and extra chromosomal resistance that result in amino acid substitution in chromosomal genes intended for DNA gyrase and topoisomerase IV, the targets of fluoroquinolone action.
- Changes in expression of efflux pumps and outer membrane permeability to facilitate control the accumulation of these agents inside the bacterial cells 82.
- A wide range of molecular mechanism, such as the presence of β-lactamases dihydrofolate reductase, Chl acetyltransferase (CAT) enzymes and many others 83-85.
These acquired mechanisms of survival by Shigella sps have contributed to the persistence of this pathogen, thus making the antibiotic treatment therapy failure. Preparing the prevention and treatment protocols with natural patterns in this regard seems to be necessary.
Prevention and Control
The most useful methods for controlling shigellosis are prerequisite of safe and plentiful water and efficient faeces disposal. Prevention of dysentery caused by Shigella relies mainly on measures that prevent spread of the organism within the community and from person to person 26. These include:
- Hand-washing with soap
- Ensuring the availability of safe drinking water
- Safely disposing of human waste
- Breastfeeding of infants and young children
- Safe handling and processing of food
- Control of flies
These measures will not only reduce the occurrence of shigellosis, but of other diarrhoeal diseases as well. In all cases, health education and the cooperation of the community in executing control measures are important. In general, several studies and Edwards (1999) 86 reported that the most effective intercession approach to minimize morbidity and mortality would involve comprehensive media and personal outreach programs consisting of the following components:
- Education of all residents to actively avoid faecal contamination of food and water and to encourage
- Hand washing after defecation;
- Encourage mother to breast feed infants;
- Promote the use of oral rehydration therapy to offset the effects of acute diarrhea;
- Encourage mothers to provide convalescent nutritional care in the form of extra food for children
- Recovering from diarrhea or dysentery.
The input to avoid infection by Shigella is prevention of fecal contamination in drinking water and food supplies. Since the only source of this agent is infected humans, it is possible to control transmission by proper hygiene, waste management, water purification and treatment of the sick. Health education is vital to elevate public awareness and provoke behaviour change.
Vaccination: There is a strong need for an effective, safe and low-cost vaccine against shigellosis 44. The high disease burden of shigellosis in developing countries, children <5 yr of age as the core sufferers, difficulty in attaining adequate sanitation and personal hygiene in these regions and scarce therapeutic alternatives for emerging multiple drug resistnace Shigella point towards vaccination as a hope for effective and sustainable approach against shigellosis. Shigellosis is targeted by WHO as one of those enteric infections for which novel vaccines are most needed, the target populations being travellers from developed countries and military service personnel, as well as children existing in endemic areas 23, 44.
Even though the need for a Shigella vaccine is urgent, not much development has been done due to the antigenic complexity, lack of inter-species cross-protective epitopes, and gaps in understanding of the protective immune response. Numerous diverse types of vaccines against Shigella have been experimentally tested in animal models and in volunteer trials 87. Various live attenuated vaccines such as the old parenteral killed whole-cell vaccine was effective but produced strong side-effects because of LPS. Yang et al., (2001) 89 showed that two safe and useful vaccines are now licensed and available. One is based on defined subunit antigens (Vi polysaccharide, is given in a single dose Subcutaneous), the other on whole-cell live attenuated bacteria (the live oral vaccine Ty2la, available in enteric-coated capsule or liquid formulation 89. Vaccination against shigellosis before or during an outbreak condition should consequently be critically considered as an effective tool.
Shigellosis remains an important public health problem and produces a substantial global disease burden, particularly for young children in developing countries being of epidemiological importance. Outbreaks of infection with Shigella species are difficult to control because of the low infectious inoculum and ease of transmission. Antimicrobial therapy is advocated for shigellosis to shorten the duration of illness. However, in developing countries like India, antimicrobial resistance is an emerging problem in Shigella species, treatment options are becoming limited globally and resulting in reduced efficacies of antimicrobial therapy. Rapid emergence of resistance to antimicrobials over time warrants the need for continuous monitoring of sensitivity patterns and this has become a challenge in the management of shigellosis. So the practicing the prevention, management issue and develop into protocols with natural pattern consider to be essential.
ACKNOWLEDGMENTS
The authors are (Kelmani Chandrakanth Revanasiddappa and Prabhurajeshwar C) profusely thankful to the Department of Biotechnology (grant from DBT, Govt. of India, BT/PR1812/SPD/24/577/2011) for funding the project and Department of Biotechnology, Gulbarga University, Gulbarga for providing facilities for pursuing the research work at the Department.
ETHICAL APPROVAL
This research was conducted in compliance with the Institutional Animal Ethics Committee and other federal statutes and regulations relating to animals (Lcp/PQ.col/IAEC/P/86/2016, 346/CPCSEA).
- WHO, 2013. Diarrhoeal disease. Available from: http://www.who.int/ mediacentre/factsheets/fs330/en/ index.html.
- UNICEF/WHO, 2009. Diarrhoea: Why children are still dying and what can be done.
- Black, R. E., Cousens, S., Johnson, H. L., Lawn, J. E., Rudan, I., Bassani, D. G., Eisele, T. Global, regional, and national causes of child mortality in 2008: a systematic analysis. The lancet, 2010; 375(9730), 1969-1987.
- Ahs, J. W., Tao, W., Lofgren, J., & Forsberg, B. C. Diarrheal diseases in low-and middle-income countries: incidence, prevention and management. The Open Infectious Diseases Journal, 2010; 4(4), 113-124.
- WHO, U. Diarrhoea: why children are still dying and what can be done. Geneva: 2009; UNICEF/WHO.
- Estimation of the Burden of Diarrhoeal diseases in India, NICED Kolkata available from http://www.whoindia.org/linkfiles/commision on macroeconomic and health Bg P2 Estimation of the burden of diarrhoeal diseases in India.
- Lee, W. S., & Puthucheary, S. D. Bacterial enteropathogens isolated in childhood diarrhoea in Kuala Lumpur—the changing trend. The Medical Journal of Malaysia, 2002; 57(1), 24-30.
- Wolf, M. K., Taylor, D. N., Boedeker, E. C., Hyams, K. C., Maneval, D. R., Levine, M. M., Echeverria, P. Characterization of enterotoxigenic Escherichia coli isolated from US troops deployed to the Middle East. Journal of clinical microbiology, 1993; 31(4), 851-856.
- Haque, R., Mondal, D., Kirkpatrick, B. D., Akther, S., Farr, B. M., Sack, R. B., & Petri, W. A. Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. The American journal of tropical medicine and hygiene, 2003; 69(4), 398-405.
- DuPont, H.L., “Shigella species (bacillary dysentery),” in Mandell, Douglas, and Bennett’s Principles And Practice Of Infectious Diseases, Fifth Edition, 2000; 2363-9
- Hale, T. L., Keusch, G. T. Shigella’: Structure, Classification and Antigenic Types. Baron, The University of Texas Medical Branch at Galveston: Ch22 1996.
- Esmaeili Dooki M.R., Rajabnia R., Barari Sawadkohi R., Mosaiebnia Gatabi Z., Poornasrollah M., Mirzapour M. Bacterial enteropathogens and antimicrobial susceptibility in children with acute diarrhoea in Babol, Iran. Caspian J Intern Med. 2014; 5: 30-4.
- Pichel, M., Fraga, S. G., Terragno, R., Mulki, J., Gentile, A., Kremer, C., Binsztein, N. Analysis of clonal relationship among Shigella sonnei isolates circulating in Argentina. Epidemiology and infection, 2007;135(04), 681-687.
- Ranjbar, R., Dallal, M. M. S., Pourshafie, M. R. Epidemiology of shigellosis with special reference to hospital distribution of Shigella strains in Tehran. Archives of Clinical Infectious Diseases, 2008; 3(1).
- CDC, National Centre for Zoonotic, Vector-Borne, and Enteric Diseases, Shigellosis-General Information and Frequently Asked Questions 2009.
- Keusch, G.T., Acheson, D.W. “ShigellaInfection,” in Enteric Infections and Immunity 1996.
- Trofa, A. F., Ueno-Olsen, H., Oiwa, R., Yoshikawa, M. Dr. Kiyoshi Shiga: discoverer of the dysentery bacillus. Clinical infectious diseases, 1999; 29(5), 1303-1306.
- Hornick, R.B. Shigellosis. In: Hoeprich P, Paul D, editors. Infectious diseases. Hagerstown: Harper aid Row Publishers. 1977; 594.
- Orrett, F. A. Prevalence of Shigella serogroups and their antimicrobial resistance patterns in southern Trinidad. Journal of Health, Population and Nutrition, 2008; 456-462.
- Yang, F., Yang, J., Zhang, X., Chen, L., Jiang, Y., Yan, Y., Xue, Y. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic acids research, 2005; 33(19), 6445-6458.
- Thomas, L. H., Gerald, T. K. Baron’s Medical Microbiology. Shigella, 2000; 389-400.
- Ranjbar, R., Dallal, M. M. S., Pourshafie, M. R. Epidemiology of shigellosis with special reference to hospital distribution of Shigella strains in Tehran. Archives of Clinical Infectious Diseases, 2008; 3(1).
- Kotloff, K. L., Winickoff, J. P., Ivanoff, B., Clemens, J. D., Swerdlow, D. L., Sansonetti, P. J., Levine, M. M. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization, 1999; 77(8): 651-666.
- Niyogi, S.K. Shigellosis. Journal of Microbiology, 2005; 43(2):133-143.
- WHO. Shigella: Disease burden 2006.
- Michael, E., Mohammad, A., Mohammad, Y. Risk areas and neighbourhood-level risk factors for Shigella dysenteriae 1 and Shigella flexneri, Healthplace, 2008; 14: 96-105.
- Shane, A. L., Tucker, N. A., Crump, J. A., Mintz, E. D., Painter, J. A. Sharing Shigella: risk factors for a multicommunity outbreak of shigellosis. Archives of pediatrics & adolescent medicine, 2003; 157(6), 601-603.
- Gupta, A., Polyak, C. S., Bishop, R. D., Sobel, J., Mintz, E. D. Laboratory-confirmed shigellosis in the United States, 1989–2002: epidemiologic trends and patterns. Clinical Infectious Diseases, 2004; 38(10), 1372-1377.
- Dutta, S., Dutta, D., Dutta, P., Matsushita, S., Bhattacharya, S. K., Yoshida, S. I. Shigella dysenteriae serotype 1, Kolkata, India. Emerging infectious diseases, 2003; 9(11), 1471.
- Pazhani, G. P., Sarkar, B., Ramamurthy, T., Bhattacharya, S. K., Takeda, Y., Niyogi, S. K. Clonal multidrug-resistant Shigella dysenteriae type 1 strains associated with epidemic and sporadic dysenteries in eastern India. Antimicrobial agents and chemotherapy, 2004; 48(2), 681-684.
- Taneja, N. Changing epidemiology of shigellosis and emergence of ciprofloxacin-resistant Shigellae in India. Journal of clinical microbiology, 2007; 45(2), 678-679.
- Pazhani, G. P., Niyogi, S. K., Singh, A. K., Sen, B., Taneja, N., Kundu, M., Ramamurthy, T. Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. Journal of medical microbiology, 2008; 57(7), 856-863.
- Von Seidlein, L., Kim, D. R., Ali, M., Lee, H., Wang, X., Thiem, V. D., Mason, C. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med, 2006; 3(9), e353.
- Kimura, A.C., Johnson, K., Palumbo, M.S., Hopkins, J., Boase, J.C., Reporter, R., Goldoft, M., Stefonek, K.R., Farrar, J.A., Van Gilder, T.J., Vugia, D.J. Multistate shigellosis outbreak and commercially prepared food, United States. Emerging Infectious Diseases, 2004; 10(6):1147–1149
- Herwana, E., Surjawidjaja, J. E., Salim, O. C., Indriani, N., Bukitwetan, P., Lesmana, M. Shigella-associated diarrhea in children in South Jakarta, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health, 2010; 41(2), 418.
- Chang, Z., Lu, S., Chen, L., Jin, Q., Yang, J. Causative species and serotypes of shigellosis in mainland China: systematic review and meta-analysis. PLoS One, 2012; 7(12), e52515.
- WHO. Review of Shigella spp. 2001; 8: 21-30.
- Presterl, E., Zwick, R. H., Reichmann, S., Aichelburg, A., Winkler, S., Kremsner, P. G., Graninger, W. Frequency and virulence properties of diarrheagenic Escherichia coli in children with diarrhea in Gabon. The American journal of tropical medicine and hygiene, 2003; 69(4), 406-410.
- Levinson, W.E. Basic bacteriology. In Review of Medical Microbiology and Immunology. New York, NY: McGraw-Hill Medical Publishing Division 2006.
- Ito, H., Kido, N., Arakawa, Y., Ohta, M. Sugiyama, T. Kato, N. Possible mechanisms underlying the slow lactose fermentation phenotype in Shigella spp. Applied and Environmental Microbiology, 1991; 57, 2912-2917
- Schroeder, G. N., Hilbi, H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clinical microbiology reviews, 2008; 21(1), 134-156.
- Phalipon, A, Sansonetti, P.J. Shigella ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol, 1994; 85: 119-129.
- Ashkenazi, S. Shigella infections in children: New insights. Semin Pediatr. Infect. Dis., 2004; 15: 246-252.
- Seidlein, L., Kim, D. R., Ali, M., Lee, H., Wang, X., Thiem, V. D., Canh, D. G., Chaicumpa, W., Agtini, M. D. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med, 2006; 3: e353.
- Wassef, J., Keren, D. F., Mailloux, J. L. Role of M cells in initial bacterial uptake and in ulcer formation in the rabbit intestinal loop model in shigellosis. Infect. Immun. 1989; 57: 858-863.
- Perdomo, O. J., Cavaillon, J. M., Huerre, M., Ohayon, H., Gounon, P., Sansonetti, P. J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. Journal of Experimental Medicine, 1994; 180(4): 1307-1319.
- Sansonetti, P. J., Phalipon, A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, conse- quencesforthe disease process. Semin.Immunol, 1999; 11: 193-203.
- Koterski, J. F., Nahvi, M., Venkatesan, M. M., Haimovich, B. Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect Immun, 2005; 73: 504–513.
- Carneiro, L. A., Travassos, L. H., Soares, F., Tattoli, I., Magalhaes, J. G., Bozza, M. T., Girardin, S. E. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell host & microbe, 2009; 5(2): 123-136.
- Hathaway, L. J., Griffin, G. E., Sansonetti, P. J., Edgeworth, J. D. Human monocytes kill Shigella flexneri but then die by apoptosis associated with suppression of proinflammatory cytokine production. Infection and immunity, 2002; 70(7): 3833-3842.
- Sansonetti, P. J., Phalipon, A., Arondel, J., Thirumalai, K., Banerjee, S., Akira, S., Zychlinsky, A. Caspase-1 activation of IL-1â and IL-18 are essential for Shigella flexneri–induced inflammation. Immunity, 2000; 12(5): 581-590.
- Ruetz, T., Cornick, S., Guttman, J. A. The spectrin cytoskeleton is crucial for adherent and invasive bacterial pathogenesis. PloS one, 2011; 6(5): e19940.
- Philpott, D.J., Yamaoka, S., Israel, A., Sansonetti, P.J. Invasive Shigella flexneri activates NF-kB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J Immunol, 2000; 165: 903-914.
- Islam, D., Veress, B., Bardhan, P. K., Lindberg, A. A., & Christensson, B. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infection and immunity, 1997; 65(2): 739-749.
- Sansonetti, P. J. Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote–eukaryote cross-talks. FEMS microbiology reviews, 2001; 25(1): 3-14.
- Jennison, A. V., Verma, N. K. Shigella flexneri infection: pathogenesis and vaccine development. FEMS microbiology reviews, 2004; 28(1): 43-58.
- Torres, A. G., Li, Y., Tutt, C. B., Xin, L., Eaves-Pyles, T., Soong, L. Outer membrane protein A of Escherichia coli O157: H7 stimulates dendritic cell activation. Infection and immunity, 2006; 74(5): 2676-2685.
- Mantis, N., Prévost, M. C., Sansonetti, P. Analysis of epithelial cell stress response during infection by Shigella flexneri. Infection and immunity, 1996; 64(7): 2474-2482.
- Butler, T. Shigellosis. In: Goldman L, Bennett JC, editors. Textbook of medicine. 21st ed. Philadelphia: W. B. Saunders, 2000; 1685-7.
- Khatun, F., Faruque, A. S. G., Koeck, J. L., Olliaro, P., Millet, P., Paris, N., Luby, S. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980–2008). Epidemiology and infection, 2011; 139(03): 446-452.
- Venkatesan, M. M., Ranallo, R. T. Live-attenuated Shigella vaccines. Expert review of vaccines, 2006; 5(5): 669-686.
- Samie, A., Guerrant, R. L., Barrett, L., Bessong, P. O., Igumbor, E. O., Obi, C. L. Prevalence of intestinal parasitic and bacterial pathogens in diarrhoeal and non-diarrhoeal human stools from Vhembe district, South Africa. Journal of Health, Population and Nutrition, 2009; 739-745.
- Kumar, Y., Sharma, A., Sehgal, R., Kumar, S. Distribution trends of Salmonella serovars in India (2001–2005). Transactions of the Royal Society of Tropical Medicine and Hygiene, 2009; 103(4): 390-394.
- CDC, “Preliminary FoodNet Data on the Incidence of Infection with Pathogens Transmitted Commonly through Food-10 States, 2006,” Morbidity and Mortality Weekly Report, 2007; 56 (14), 336-9.
- American Public Health Association (APHA), “Shigellosis,” in Control of Communicable Diseases Manual, 2008; 556-60.
- Haley, C. C., Ong, K. L., Hedberg, K., Cieslak, P. R., Scallan, E., Marcus, R., Shiferaw, B. Risk factors for sporadic shigellosis, FoodNet 2005. Foodborne pathogens and disease, 2010; 7(7): 741-747.
- Prabhurajeshwar, Ajaykumar, O, Ashajyothi, C, Kelmani, C.R. Prevalence and antibiotic susceptibility pattern of fluoroquinolone resistant Shigella species isolated from infants stool in Gulbarga district, Karnataka, India. Asian Pac J Trop Dis. 2015; 5(1):930-934.
- Angulo, F. J., Baker, N. L., Olsen, S. J., Anderson, A., Barrett, T. J. Antimicrobial use in agriculture: controlling the transfer of antimicrobial resistance to humans. In Seminars in pediatric infectious diseases, 2004; 15(2): 78-85.
- Angulo, F. J., Nargund, V. N., Chiller, T. C. Evidence of an association between use of anti microbial agents in food animals and anti microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. Journal of Veterinary Medicine, Series B, 2004; 51(8 9), 374-379.
- Philpott, D. J., Edgeworth, J. D., Sansonetti, P. J. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 2000; 355(1397), 575-586.
- Roberts, T. Human illness costs of foodborne bacteria. American Journal of Agricultural Economics, 1989; 71(2), 468-474.
- Paula, C. M. D. D., Geimba, M. P., Amaral, P. H. D., Tondo, E. C. Antimicrobial resistance and PCR-ribotyping of Shigella responsible for foodborne outbreaks occurred in southern Brazil. Brazilian Journal of Microbiology, 2010; 41(4), 966-977.
- Berhanu, A., Afeworke, K., Ermias, D., Feleke, M., Molla, G. The prevalence and antimicrobial responses of shigella isolates in HIV-1 infected and uninfected adult diarrhoea patients in North West Ethiopia. Ethiop J Health Dev. 2006; 20(2), 99-105.
- Njunda, A. L., Assob, J. C., Nsagha, D. S., Kamga, H. L., Awafong, M. P., Weledji, E. P. Epidemiological, clinical features and susceptibility pattern of shigellosis in the Buea Health District, Cameroon. BMC research notes, 2012; 5(1), 54.
- Srinivasa, H., Baijayanti, M., Raksha, Y. Magnitude of drug resistant Shigellosis: A report from Bangalore. Indian journal of medical microbiology, 2009; 27(4), 358.
- Taneja, N., Mewara, A., Kumar, A., Verma, G., Sharma, M. Cephalosporin-resistant Shigella flexneri over 9 years (2001–09) in India. Journal of antimicrobial chemotherapy, 2012; dks061.
- Bhattacharya, D., Sugunan, A. P., Bhattacharjee, H., Thamizhmani, R., Sayi, D. S., Thanasekaran, K., Roy, S. Antimicrobial resistance in Shigella-rapid increase & widening of spectrum in Andaman Islands, India. Indian Journal of Medical Research, 2012; 135(3), 365.
- Pazhani, G. P., Niyogi, S. K., Singh, A. K., Sen, B., Taneja, N., Kundu, M., Ramamurthy, T. Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. Journal of medical microbiology, 2008; 57(7), 856-863.
- Capoor, M. R., Nair, D. Quinolone and cephalosporin resistance in enteric fever. Journal of global infectious diseases, 2010; 2(3), 258.
- Talukder, K. A., Khajanchi, B. K., Islam, M. A., Dutta, D. K., Islam, Z., Safa, A., Niyogi, S. K. Genetic relatedness of ciprofloxacin-resistant Shigella dysenteriae type 1 strains isolated in south Asia. Journal of Antimicrobial Chemotherapy, 2004; 54(4), 730-734.
- Talukder, K. A., Khajanchi, B. K., Islam, M. A., Islam, Z., Dutta, D. K., Rahman, M., Sack, D. A. Fluoroquinolone Resistance Linked to Both gyrA and parC Mutations in the Quinolone Resistance-Determining Region of Shigella dysenteriae Type 1. Current microbiology, 2006; 52(2), 108-111.
- Poole, K. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrobial Agents and Chemotherapy, 2000; 44(9), 2233-2241.
- Cabrera, R., Ruiz, J., Marco, F., Oliveira, I., Arroyo, M., Aladuena, A., Vila, J. Mechanism of resistance to several antimicrobial agents in Salmonella clinical isolates causing traveler’s diarrhea. Antimicrobial agents and chemotherapy, 2004; 48(10), 3934-3939.
- Cotton, M. F., Wasserman, E., Smit, J., Whitelaw, A., Zar, H. J. High incidence of antimicrobial resistant organisms including extended spectrum beta-lactamase producing Enterobacteriaceae and methicillin-resistant Staphylococcus aureus in nasopharyngeal and blood isolates of HIV-infected children from Cape Town, South Africa. BMC infectious diseases, 2008; 8(1), 40.
- Navia, M. M., Gascon, J., Vila, J. Analysis of the mechanisms of resistance to several antimicrobial agents in Shigella spp. causing travellers’ diarrhoea. Clinical microbiology and infection, 2005; 11(12), 1044-1047.
- Edwards, B.H. Salmonella and Shigella species. Clin Lab Med, 1999; 19: 469-487
- Venkatesan, M. M., Ranallo, R. T. Live-attenuated Shigella vaccines. Expert review of vaccines, 2006; 5(5), 669-686.
- Yang, H. H., Wu, C. G., Xie, G. Z., Gu, Q. W., Wang, B. R., Wang, L. Y., Wang, W. Y. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bulletin of the World Health Organization, 2001; 79(7), 625-631.
- Lin, F. Y. C., Ho, V. A., Khiem, H. B., Trach, D. D., Bay, P. V., Thanh, T. C., Schneerson, R. The efficacy of a Salmonella typhi Vi conjugates vaccine in two-to-five-year-old children. New England Journal of Medicine, 2001; 344(17), 1263-1269.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


