ISSN: 0973-7510
E-ISSN: 2581-690X
With the unwelcome re-emergence of brucellosis in different regions of the world, an accurate and timely diagnosis of this zoonosis has become a daunting challenge. Due to the vague symptoms of the disease, laboratory confirmation is intensely needed to clinch a definite diagnosis. Consequently, reliable laboratory tests can play a pivotal role in proper diagnosis and disease management. Employing standard tube agglutination test (STAT) as the reference method, this study aimed to evaluate the performance of different serological tests as well as quantitative real time PCR (qPCR) in the diagnosis of human brucellosis. Out of 100 serum samples included in this study, 95 samples yielded positive result with STAT. The highest sensitivity (96%) was recovered with Rose Bengal (RB) test, while the sensitivities of ELISA and qPCR were 79% and 65% respectively. Meanwhile, RB test revealed a 100% specificity, while both ELISA and qPCR had specificities of 80% and 40% respectively. The RB test has proven to be a reliable and appropriate screening test for brucellosis. Likewise, ELISA is an attractive option. Meanwhile, STAT, which is accurate, cost-effective, and easy-to-use, remains the most appropriate test for the diagnosis of human brucellosis, particularly in endemic regions. While PCR may be costly and technically demanding for most laboratories, STAT can be very well adopted by laboratories established in low resource settings. It can provide a definite diagnosis of human brucellosis with minimal labor as well as an affordable cost.
Brucellosis; ELISA; qPCR; STAT; Zoonosis.
With nearly half a million cases reported yearly to the WHO from 100 countries, brucellosis remains one of the major zoonoses around the world.1 Despite the continuous progress of brucellosis control measures, the disease still poses an enormous public health problem of grave economic impact. This in turn has necessitated re-evaluating the current tools employed in the diagnosis of brucellosis.2
The gold standard for the laboratory diagnosis of brucellosis has been the isolation of Brucella spp. from blood, bone marrow or other tissues by culture. However, isolation of Brucella spp. is often hazardous, time-consuming, and of low sensitivity.3
In the meantime, serological tests such as the standard tube agglutination test (STAT), Rose Bengal (RB) slide agglutination test, and ELISA have been the most common tests in the laboratory diagnosis of brucellosis. The STAT, which has been a cornerstone in the serological diagnosis of brucellosis, is based on the reaction of a known standardized volume and concentration of whole Brucella abortus and Brucella melitensis cell suspension with a standardized volume of doubling serum dilutions, usually ranging from 1:20 to 1:1280.4 Being a non-labor intensive test with a short turn-around time, STAT can be used to screen a large number of susceptible populations in endemic areas at a minimal cost5.
Meanwhile, RB test is based on the agglutination of serum with a suspension of whole Brucella abortus cells stained with Rose Bengal dye and buffered at pH 3.65 to inhibit non-specific agglutinins.4 This test can be readily and rapidly performed, with a high sensitivity in acute cases.6
Likewise, ELISA offers a simple and rapid assay that can reveal total and individual specific immunoglobulins (IgG, IgM, IgA) within 1-2 hours. ELISA is the test of choice for focal lesions and chronic cases, especially when other tests are negative and the case is under high clinical suspicion4. This test can detect incomplete antibodies especially in chronic cases.6
On the other hand, PCR can provide an additional means to detect and identify Brucella spp. While PCR-based tests are undoubtedly promising, they are still hindered with substantial shortcomings, including a high risk of contamination and high expenses. Moreover, infrastructure, equipment, and expertise are lacking, and a better understanding of the clinical significance of the results is still needed.7
In the present study, we compared the sensitivity and specificity of RB, ELISA, and quantitative real time PCR (qPCR) in the diagnosis of brucellosis, while employing STAT as the reference method.
Ethical consideration
Before commencement of the study, approval of the protocol was obtained from the Ethics Committee in the Department of Microbiology and Immunology, Cairo University.
An informed consent was taken from all enrolled subjects and a questionnaire was filled out for each patient including various factors such as age, gender, residence (urban or rural), recent ingestion of unpasteurized dairy products, and animal contact.
Population of study
This cross sectional analytical study was done on 100 serum samples obtained from 100 inpatients clinically suspected of having brucellosis in Imbaba Fever Hospital. Samples were collected during the period from November 2015 through May 2016.
Inclusion criteria
Patients with night fever (of 39-40oC) which returns to normal (37oC) during the day for more than 10 days duration, accompanied by night sweating, arthralgia and low back pain, along with a history of animal contact or ingestion of unpasteurized dairy products, were enrolled in this study.
Exclusion criteria
Patients with fever of another identifiable cause.
Sample processing
Blood samples were collected from patients and centrifuged at 3000 g for 10 minutes. The serum obtained was divided into two aliquots: one for serological testing and one for qPCR. Both aliquots were collected in cryo-tubes and stored at -80oC until processed. All reagents for serological testing and qPCR were brought to room temperature before use.
Standard tube agglutination test (STAT)
One milliliter of serially diluted serum sample (from 1:20 to 1:1280) and one drop (50 µl) of the antigen (BioMed-Brucel- Abortus / Melitensis) were mixed and incubated at 37°C for 24 hours. A positive control (positive serum sample included in the kit) and a negative control (saline) were included in the test8.
In the present study, the diagnosis of brucellosis was confirmed in patients who fulfilled the clinical criteria mentioned above, combined with a STAT titre >1/160 9.
Rose Bengal (RB) plate agglutination test
According to the manufacturer’s instructions (BioMed-Rose Bengal), a coarse speckled agglutination after mixing 50 µl of the patient’s serum and 50 µl of the antigen (killed B. abortus) on a slide and rotating for 4 minutes was considered a positive test10.
Enzyme-linked immunosorbent assay (ELISA)
The ELISA IgG tests were performed and interpreted according to the manufacturer’s instructions (Vircell SL, Granada, Spain). A microtiter plate composed of 96 wells coated with LPS antigen of Brucella abortus (strain S-99) was used. Positive control, negative control, two cut off sera, and patients’ samples were diluted 1:100 with dilution buffer. Five microliters of the tested serum sample, 5 µl of positive control, 5 µl of cut off control (in duplicate) and 5 µl of negative control were added into the corresponding wells followed by incubation at 37ºC for 45 min.
All wells were washed five times with 0.3 ml of washing solution. Anti-human IgG peroxidase conjugate solution (100 µl) was immediately added into each well and the plate was incubated at 37ºC for 30 minutes. The substrate solution, tetramethyl benzidine (100 µl) was immediately added into each well followed by incubation at room temperature away from light for 20 minutes. Stopping solution (50 µl) was immediately added into all wells11.
The optical density was read at 450/620 nm using STAT FAX 2100 ELISA reader. Antibody index was calculated according to the following equation:
Antibody index = (sample optical density / cut off serum mean optical density) x 10.
As per the manufacturer’s instructions, an antibody index less than 9 was considered negative, while an antibody index more than 11 was considered positive. On the other hand, antibody indexes between 9 and 11 were re-tested and confirmed either positive or negative.
Quantitative Real time Polymerase chain reaction (qPCR)
Real time PCR was performed at the Molecular Biology Unit, Biochemistry Department, Faculty of Medicine, Cairo University.
DNA extraction from 200 µl of serum was done using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturers’ instructions. Brucella genus all species, PrimerDesignTM genesig kit and oasig TaqMan 2x qPCR Mastermix were used for DNA detection using Applied Biosystem step one device. Cycling conditions consisted of 50 cycles, starting with Taq polymerase activation at 95oC for 2 minutes, followed by denaturation at 95oC for 10 seconds, then annealing and extension at 60oC for 90 seconds.12
Negative control (RNAse/DNAse free water) and standard were included in all runs. Meanwhile, an internal positive control was used for each sample. The result was considered negative when no amplification occurred or when the cycle threshold (Ct) value exceeded 38 cycles. The bacterial DNA load per milliliter of serum was calculated from the standard curve which included five concentrations of Brucella DNA ranging from 2 to 2 X 10 5 copies.13
Statistical Analysis
In this prospective analytical study, the predictive analytics software (PASW) version 18 was used. Data were statistically described in terms of frequencies (number of cases) and percentages. Chi-Square test of distribution was used to declare the significant difference between group distributions at P<0.05. The sensitivity and specificity of each of RB test, ELISA, and qPCR were calculated.
This study was conducted on 100 serum samples collected from 100 inpatients in Imbaba Fever Hospital, during the period from November 2015 through May 2016.
Serological tests
Out of the 100 enrolled patients with provisional clinical diagnosis of brucellosis, 95 patients yielded positive result with STAT (titre
> 160). Of these 95 patients, 68% had titres of 1/1280 (Figure 1).
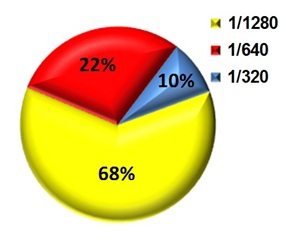
Fig. 1. Titres of positive serum samples as detected by STAT results
Meanwhile, 91 samples yielded positive results with RB test, while 76 samples were positive by ELISA.
Out of the 95 samples positive by STAT, RB was positive in 91 samples (96%) (Table 1), while ELISA was positive in 75 samples (79%) (Table 2).
Table (1):
Results of RB test compared to STAT
| STAT | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| RB | Positive | 91 | 0 | 91 |
| Negative | 4 | 5 | 9 | |
| Total | 95 | 5 | 100 | |
Table (2):
Results of ELISA compared to STAT
| STAT | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| ELISA | Positive | 75 | 1 | 76 |
| Negative | 20 | 4 | 24 | |
| Total | 95 | 5 | 100 | |
Real-time PCR
Real time PCR was positive for Brucella DNA in 65 out of 100 serum samples. The range of copies detected by PCR was 5-1000 copies/ml serum with a mean of 22 copies/ml serum. Meanwhile, out of the 95 positive samples by STAT, 62 (65%) were positive by qPCR as depicted in table 3.
Table (3):
Results of qPCR compared to STAT
| STAT | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| qPCR | Positive | 62 | 3 | 65 |
| Negative | 33 | 2 | 35 | |
| Total | 95 | 5 | 100 | |
Of note, qPCR positivity was highest (63%) at STAT titer of 1/1280 (Figure 2).
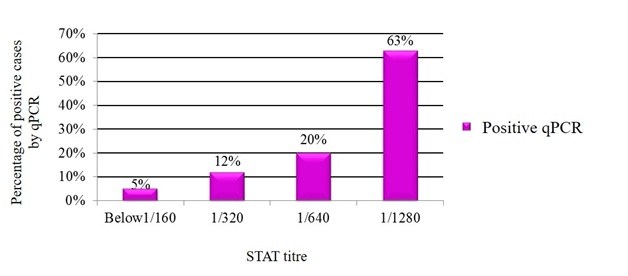
Fig. 2. Relation between STAT titre and qPCR
Table 4 summarizes the sensitivities and specificities of the tests employed in this study. The highest sensitivity (96%) and specificity (100%) were encountered with RB test.
Table (4):
Sensitivities and specificities of RB test, ELISA, and qPCR in the diagnosis of human brucellosis
Test |
Sensitivity |
Specificity |
|---|---|---|
RB |
96% |
100% |
ELISA |
79% |
80% |
qPCR |
65% |
40% |
Brucellosis is an endemic zoonosis in several parts of the world, particularly the Mediterranean basin, the Middle East, as well as central and South America. With the symptoms being nonspecific and often atypical, the clinical picture of brucellosis may masquerade as other clinical entities14. This asserts the need for reliable laboratory tests to reach a definite diagnosis.
In this study, out of 95 samples confirmed by clinical diagnosis and positive STAT, 91 (96%) samples were positive by RB. The sensitivity and specificity of RB test were 96% and 100%, respectively. This was in concordance with a previous study15 which demonstrated that each of the sensitivity and specificity of RB was 100%. Likewise, another study5 reported the sensitivity and specificity of RB as 100% and 96% respectively, compared to culture as a gold standard. On the other hand, Arabaci & Oldacay16 stated that the sensitivity and specificity of RB were 48% and 96% respectively.
In the present study, out of the 95 samples positive with STAT, 75 (79%) samples were positive by ELISA. The sensitivity and specificity of ELISA were 79% and 80% respectively. Earlier studies demonstrated results in line with this study. Kalem et al.17 stated that the sensitivity and specificity of ELISA were 93% and 80% respectively, compared to Brucella immune-capture agglutination as a gold standard. On the other hand, Shahrokhabadi et al.18 reported that considering culture as a gold standard, the sensitivity and specificity of ELISA were 78% and 28% respectively.
Meanwhile, Alshaalan et al.19 emphasized that while the sensitivity of ELISA ranges from 60 to 98%, it has the advantage of measuring different classes of reactive antibodies including IgG, IgA, and IgM. Hence, it can be used in the diagnosis of brucellosis especially in endemic areas.3
In the current study, the range of Brucella DNA copies detected by qPCR was 5-1000 copies/ml serum with a mean of 22 copies/ml serum. On the other hand, Sohrabi et al.20 reported that the range of copies detected by PCR was 64 – 580,000 copies/ml serum, while Tiwari et al.21 demonstrated that the range of copies of Brucella DNA detected by PCR was 101 -107 copies/ml serum.
In this study, out of the 95 positive samples, only 62 samples (65%) were positive by qPCR. Meanwhile, an earlier study revealed that PCR had a sensitivity and specificity of 64.4% and 77.5%, respectively.22 In concordance with this finding, Purwar et al.5 stated that there were brucellosis cases which were not detected by PCR, but had antibody levels above the cut-off value. This may be attributed to the fact that brucellosis is characterized by exacerbations and remissions. During remissions there may not be any bacteremia, and consequently, no DNA breakdown products can be found in serum to be picked up by PCR.
In addition, a study by Queipo-Ortuno et al.23 attributed the low detection rate of Brucella DNA by qPCR to the fact that it was done on serum specimens, while brucellae are intracellular pathogens. Moreover, the differences in PCR results among various studies can be attributed to the lack of uniformity and standardization of PCR protocols including optimal clinical samples, sample volume, extraction method, primers and target sequences, as well as storage conditions.24 In addition, the low sensitivity of qPCR in our study may be attributed to the use of proteinase K in the extraction kit.25 Furthermore, Scholz et al.26 pointed that a small amount of DNA in clinical samples is challenging even for assays with a very low detection limit as PCR.
On the other hand, Gemechu et al.27 demonstrated that the sensitivity of qPCR was only 12.6%, which was much less than the findings of other researchers, probably because at the time of specimen collection, the bacterial count in the peripheral circulation was below the detection limit of primers used. In the meantime, an extremely low bacterial load is needed to induce brucellosis,28 thus, the initial bacteremic course may run undetected due to the low number of circulating bacteria.
In this study, qPCR was positive in 5%, 12%, 20% and 63% of samples with STAT titres less than 1/160 (negative), 1/320, 1/640 and 1/1280 respectively. Variable results were obtained in earlier studies. El Kholy et al.29 demonstrated that PCR was positive in 12%, 24%, 32% and 32% in samples with STAT titres less than 1/160 (negative), 1/320, 1/640 and 1/1280 respectively. Another study by Refaat et al.30 stated that PCR was positive in 16%, 26%, 19% and 26% in samples with STAT titres less than 1/160 (negative), 1/320, 1/640 and 1/1280 respectively.
In the meantime, qPCR in this study was positive in 3, 5 and 16 samples which were negative by STAT, RB and ELISA respectively. This goes in line with a study by Singh31 who reported that among eight PCR-positive samples, three samples were negative by STAT.
Of note, serological tests and qPCR in this study were positive in a patient with history of brucellosis who had received two months of brucellosis therapy. Despite successful antibiotic therapy, Brucella DNA often remains detectable in the majority of brucellosis patients throughout treatment and follow-up, as well as years after clinical cure and in the absence of any symptoms.32
On the other hand, Mahmood et al.33 highlighted that although PCR is highly specific (up to 98%); yet, its sensitivity varies from 50 to 100%. In addition, Dias & Dias34 pointed that the use of PCR implies some limitations including; lack of standardization of extraction methods, as well as costly equipment and reagents. These limitations favor serology as the most useful tool for the laboratory diagnosis of brucellosis as it is reliable, easy to perform, does not need expensive equipment or training, and carries a relatively low risk of laboratory acquired infection.
- Hasanain, A., Mahdy, R., Mohamed, A., Ali, M. A randomized, comparative study of dual therapy (doxycycline–rifampin) versus triple therapy (doxycycline–rifampin–levofloxacin) for treating acute/subacute brucellosis. Braz. J. Infect. Dis. 2016; 30; 20(3):250-4.
- Pappas, G., Akritidis, N., Bosilkovski, M., Tsianos, E. Medical progress: Brucellosis. New Engl. J. Med. 2005; 352(22): 2325-63.
- Peeridogaheh, H., Golmohammadi, M.G., Pourfarzi, F. Evaluation of ELISA and Brucellacapt tests for diagnosis of human brucellosis. Iran. J. Microbiol. 2013; 5(1): 14-8.
- Araj, G.F. Update on laboratory diagnosis of human brucellosis. Int. J. Antimicrob. Ag., 2010; 36: 12-17.
- Purwar, S., Metgud, S.C., Mutnal, M.B., Nagamoti, M.B., Patil, C.S. Utility of serological tests in the era of molecular testing for diagnosis of human brucellosis in endemic area with limited resources. J. Clin. Diagn. Res., 2016; 10(2): 26-9.
- Nouri, H.R., Marashi, M.A., Rahimi, M.T., Baleghi Damavandi, S., Ebrahimpour, S. Diagnostic tests in human brucellosis. Int J Enteric Pathog., 2014; 2(3): 1-5.
- Bosilkovski M: Brucellosis: It is not only Malta! In: Zoonoses-Infections Affecting Humans and Animals. Springer Netherlands, 2015, pp. 287-315.
- Garshasbi, M., Ramazani, A., Sorouri, R., Javani, S., Moradi, S. Molecular detection of Brucella species in patients suspicious of brucellosis from Zanjan, Iran. Braz. J. Microbiology., 2014; 45(2): 533-8.
- Rahman, A.A., Dirk, B., Fretin, D., Saegerman, C., Ahmed, M.U., Muhammad, N., Hossain, A., Abatih, E. Seroprevalence and risk factors for brucellosis in a high-risk group of individuals in Bangladesh. Foodborne Pathog. Dis. 2012; 9(3):190-7.
- Mert, A., Ozaras, R., Tabak, F., Bilir, M., Yilmaz, M., Kurt, C., et al. The sensitivity and specificity of Brucella agglutination tests. Diagn. Micr. Infec. Dis., 2003; 46(4): 241-3.
- Ahmad, B., Jamil, S., Bashir, S., Bilal, M., Hassan, S., Khan, J. Incidence of Brucella abortus and Brucella melitensis in Peshawar and identification of active and passive infection. Life Sci. J., 2014;11.
- Surucuoglu, S., El, S., Ural, S., Gazi, H., Kurutepe, S., Taskiran, P. et al. Evaluation of real-time PCR method for rapid diagnosis of brucellosis with different clinical manifestations. Pol. J. Microbiol., 2009; 58(1): 15-9.
- Sohrabi, M., Mobarez, A.M., Khoramabadi, N., Doust, R.H., Behmanesh, M. Efficient diagnosis and treatment follow-up of human brucellosis by a novel quantitative TaqMan real-time PCR assay: a human clinical survey. J. Clin. Microbiol., 2014; 52(12): 4239-43.
- Gupte, S., Kaur, T. Diagnosis of human brucellosis. J. Trop. Dis., 2015; 4(1): 1-6.
- Marei, A., Boghdadi, G., Abdel-Hamed, N., Hessin, R., Abdoel, T., Smits H et al. (2011): Laboratory diagnosis of human brucellosis in Egypt and persistence of the pathogen following treatment. J. Infect. Dev. Cries, 2011. 5(11): 786-91.
- Arabaci, F., Oldacay, M. Evaluation of serological diagnostic tests for human brucellosis in an endemic area. J. Microbiol. Infect. Dis., 2012; 2(2): 50-65.
- Kalem, F., Ergün, A.G., Durmaz, S., Doan, M., Erturul, Ö., Gündem, S. Comparison of a new and rapid method: Brucella coombs gel test with other diagnostic tests. J. Clin. Lab. Anal., 2016; 30(5): 756-9.
- Shahrokhabadi, R., Rahimi, E., Mommtaz, H., Poursahebi, R., Doostmohamadi, S. The efficacy of multiplex PCR in comparison with agglutination and ELISA in diagnosis of human brucellosis. Zahedan. J. Res. Med. Sci., 2014; 16(4): 24-8.
- Alshaalan, M.A., Alalola, S.A., Almuneef, M.A., Albanyan, E.A., Balkhy, H.H., AlShahrani, D.A. et al. Brucellosis in children: Prevention, diagnosis and management guidelines for general pediatricians endorsed by the Saudi Pediatric Infectious Diseases Society (SPIDS). Int. J. Pediatr. Adolesc. Med., 2014; 1(1): 40-6.
- Sohrabi, M., Mobarez, A.M., Behmanesh, M., Khoramabadi, N., Doust, R.H. Evaluation of a new set of Real-Time PCR for Brucella detection within human and animal samples. J. Pharm. Health. Care. Sci., 2012; 1(2): 68-71.
- Tiwari A, Pal V, Afley P, et al. Real-time PCR carried out on DNA extracted from serum or blood sample is not a good method for surveillance of bovine brucellosis. Trop. Anim. Health. Prod., 2014; 46(8):1519-22.
- Lari, A.R., Karimi, A., Fallah, F., Angoti, G., Sanaei, A., and Azimi, L: The Efficacy of multiplex PCR in comparison with agglutination and ELISA in diagnosis of human brucellosis. Iran J Clinic Infect Dis., 2011; 6: 3-6.
- Queipo-Ortuño, M.I., Colmenero, J.D., Baeza, G., Morata, P. Comparison between Light Cycler Real-Time Polymerase Chain Reaction (PCR) assay with serum and PCR–enzyme-linked immunosorbent assay with whole blood samples for the diagnosis of human brucellosis. Clin Infect Dis. 2005; 40(2): 260-4.
- Asaad, A.M., Alqahtani, J.M. Serological and molecular diagnosis of human brucellosis in Najran, Southwestern Saudi Arabia. J Infect Public Health, 2012; 5(2): 189-94.
- Morata, P., Queipo-Ortuño, M.I., de Dios Colmenero, J. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J Clin Microbiol., 1998; 36(9):2443-6.
- Scholz, H.C., Pfeffer, M., Neubauer, H., Tomaso, H. (2007): Evaluation of genus-specific and species-specific real-time PCR assays for the identification of Brucella spp. Clin Chem Lab Med., 2007; 45(11): 1464-70.
- Gemechu, M.Y., Gill, J.P.S., Arora, A.K., Ghatak, S., Singh, D.K. Polymerase chain reaction (PCR) assay for rapid diagnosis and its role in prevention of human brucellosis in Punjab, India. Int J Prev Med., 2011; 2(3): 170-7.
- Pappas, G., Papadimitriou, P.: Challenges in Brucella bacteraemia. Int J Antimicrob Ag., 2007; 30(1): 29-31.
- El Kholy, A.A., Gomaa, H.E., El Anany, M.G., Abd El Rasheed, E. Diagnosis of human brucellosis in Egypt by polymerase chain reaction. East Mediterr Health J. 2009; 15(5): 1068-74.
- Refaat, H.M., Ahmed, O.I., Elabd, S.H., Sanad, M.M. Polymerase chain reaction (PCR) technique compared to conventional bacteriological and serological techniques in diagnosis of human brucellosis in Egypt. Afr J Microbiol Res. 2016; 10(2): 54-61.
- Singh, S. Prevalence of Human brucellosis among high risk, symptomatic cases in Anand district of Gujarat-India. Natl J Integr Res Med. 2015; 6(5): 22-7.
- Al Dahouk, S., Sprague, L.D., Neubauer, H. New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev Sci Tech. 2013; 32(1): 177-88.
- Mahmood, R., Ali, T., Waheed, U., Asif, M., Khan, Q.M. Application of serum based PCR and Fluorescence Polarization Assay for diagnosis of brucellosis among people occupationally at risk to disease. Int J Agric Biol. 2016; 18(2): 1-8.
- Dias, M., Dias, E. Comparative evaluation of various serological tests in the laboratory diagnosis of Brucellosis. CHRISMED J Health Res. 2015; 2(2): 136-9.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


