ISSN: 0973-7510
E-ISSN: 2581-690X
Scrub typhus is an arthropod-borne zoonotic bacterial infection caused by Orientia tsutsugamushi. It presents clinically as a non-specific febrile illness that needs a high index of clinical suspicion for diagnosis. The mortality rate can be as high as 30% if not treated appropriately. Laboratory diagnosis is therefore important for confirming the cause of illness prior to initiating appropriate therapy. Hence we aimed to detect scrub typhus in serum samples of undifferentiated febrile illness patients and to correlate with the socioeconomic status of these individuals. We also aimed to study the seasonal variation associated with the disease. Serum samples from 143 febrile patients who were negative for other febrile illnesses were subjected to scrub typhus IgM ELISA. Scrub typhus IgM antibodies were found in 14 (9.8%) individuals of which 41-60 years being the most affected age group. Scrub typhus positivity was high during the months of October to December (P value 0.0056) with the individuals from the rural areas being the most affected (P value 0.027). To conclude, this study emphasises the importance of serological tests to detect scrub typhus and to include it as a differential diagnosis among undifferentiated febrile illnesses.
IgM ELISA, Scrub typhus, Seasonal variation
Zoonoses are diseases that are naturally transmitted from vertebrate animals to human beings1. They can be bacterial, viral or parasitic origin with various modes of transmission from animals. It can be by direct or indirect contact with animals, by consuming raw or under cooked animal meat or by a bite of an arthropod vector2.
Scrub typhus is an arthropod borne zoonotic bacterial infection. It is a Reemerging disease. The causative agent, Orientia tsutsugamushi is transmitted by the bite of larval mites (chiggers) of Trombiculidae family (Leptotrombidium deliense and L.akamushi)3. The name of the disease “scrub” is derived from the vector’s habitat, terrain vegetation between woods and clearings and “typhus” in greek means “fever with stupor or smoke”4. The ‘tsutsugamushi triangle’ or “scrub typhus triangle” is an endemic area bounded by Japan, Northern Australia and Arabian peninsula in the north, south and the west respectively5.
Approximately 5 to 14 days after the trombiculid mite bite, non-specific flu-like symptoms, fever, rash, headache, myalgia, cough, generalized lymphadenopathy, nausea, vomiting, and abdominal pain are exhibited by these individuals6,7. The bite site has a characteristic black eschar, which is painless, non-pruritic lesion with surrounding erythema8. Eschar is a pathognomonic lesion in scrub typhus which is present in only 50 – 60% of patients9. Hence an intense search for eschar helps in early clinical diagnosis of scrub typhus. The clinical spectrum ranges from a mild, self-limiting disease to disseminated infection with protean manifestations, with 30-50% mortality if untreated10.
Poor socioeconomic conditions such as unhygienic sanitation practices, overcrowding and closer proximity to scrub vegetation or wood piles are key risk factors for scrub typhus11,12. Scrub typhus has been reported more frequently during the cooler months of the year which is attributed to the vector activity and habitat13. Advanced globalization and associated travel contributes to the increase in exposure in the non-endemic areas14. The antigenic and genetic diversity of Orientia tsutsugamushi strains, and its mysterious attribution to pathogenicity confound the epidemiological study of scrub typhus15.
At present, scrub typhus is difficult to diagnose because of its nonspecific clinical presentation mimicking other febrile illnesses. There is a low index of clinical suspicion especially in the absence of typical eschars. Hence laboratory diagnosis plays a vital role in providing timely appropriate treatment to the affected individuals. Various modes of diagnosis include both direct and indirect methods. Direct detection include cell culture isolation and molecular detection of specific gene targets and indirect methods are antibody detection by Enzyme linked immunosorbent assay (ELISA) and indirect immunofluorescence (IFA). Antibody detection by serological tests remain the mainstay of diagnosis especially when seroconversion in a paired sample is demonstrated. As it is not always feasible to obtain paired sample, a single cut off titre for ELISA is acceptable13.
Thus we aimed to detect scrub typhus antibodies by ELISA in undifferentiated febrile illness patients and to correlate with the socioeconomic conditions of these individuals.
Study design
This is a hospital based descriptive cross sectional study.
Ethical approval and informed consent
This study was carried out after obtaining approval from Institutional ethical committee. A written informed consent was obtained from all the study participants.
Study area and population
All febrile patients attending outpatient and inpatient departments of our Institute. The study population included both genders and all age groups.
Sample size and sampling method
By purposive sampling method, the serum samples of febrile patients who fit the sampling criteria were included in the study. A total of 143 eligible serum samples were selected.
Inclusion criteria: All febrile patients who were negative for Malaria, Typhoid, Dengue, Leptospirosis, and Chikungunya were included.
Exclusion criteria
Immunosuppressive patients like organ transplant and those receiving cancer chemotherapy or any other immunosuppressive drugs and immunocompromised patients.
Study period
The study was carried out over a period of one year from February 2019 to January 2020.
Study tool and data collection method: A predetermined proforma was used to collect demographic and clinical details of the patients.
All serum samples collected were screened for detection of IgM antibodies against scrub typhus by ELISA (InBios international Inc, WA, USA) according to manufacturer’s instructions. This ELISA uses Karp, Kato, Gilliam and TA716 recombinant proteins of the 56-kD outer membrane protein. Sensitivity and specificity were 84% and 98% respectively for the IgM ELISA16. In the absence of consensus on what constitutes a ‘Positive’ ELISA test in an endemic population, IgM cut-off values for optical density (OD) were set conservatively at >1.0.
Statistical analysis
The data collected are analyzed using SPSS software to know the level of significance.
Whenever a person reports to the hospital with complaints of fever, our primary suspects would be viral fever followed by Malaria and typhoid. Only when the person tests negative for the common illnesses, with a high index of clinical suspicion other diagnostic tests are ordered. This study was done to find out the frequency of one of the not so common and overlooked cause of fever especially in areas where scrub typhus is endemic.
Among all the serum samples tested for common febrile illnesses, 143 samples were found to be negative for Malaria, Typhoid, Dengue, Leptospirosis and Chikungunya.
Demographic details of serum samples of febrile patients
Among 143 serum samples, 84 (59%) were males and 59 (41%) were females. The patients’ age ranged from 0-80 years with majority of them in the age group of 21-40 years (39.16%) followed by 41-60 years (24.47%). Majority of the study participants (67%, n=96) were from rural areas as shown in Table 1.
Table (1):
Demographic details of serum samples of febrile patients.
| Variables | Frequency n = 143 |
Percentage (%) | |
|---|---|---|---|
| 1 | Age Group | ||
| 0-20 | 22 | 15.38 | |
| 21-40 | 56 | 39.16 | |
| 41-60 | 35 | 24.47 | |
| 61-80 | 30 | 20.97 | |
| 2 | Gender | ||
| Male | 84 | 58.74 | |
| Female | 59 | 41.25 | |
| 3 | Place of residence | ||
| Urban | 47 | 32.86 | |
| Rural | 96 | 67.13 | |
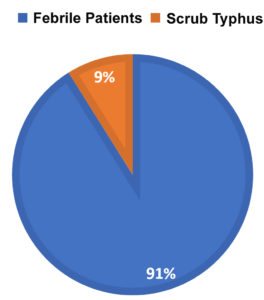
Among the 143 serum samples tested, 14 samples (9%) tested positive for IgM antibodies of scrub typhus. (Fig. 1).
It was surprising to find that, none of the samples in the age group of 0-20 years, tested positive for the scrub typhus. Most of the positive samples were distributed in the age group of 21-60 years. It was found that, comparatively females were more affected compared to males in the 21-40 years age group. (Table 2).
Table (2):
Gender wise distribution of positive scrub typhus samples by different age groups.
| Age group | Gender | No. of positives (%) | |
|---|---|---|---|
| Male | Female | ||
| 0-20 | 0 | 0 | 0 |
| 21-40 | 2 | 4 | 6 (42.8%) |
| 41-60 | 4 | 3 | 7 (50%) |
| 61-80 | 1 | 0 | 1 (7.2%) |
| 7(50%) | 7(50%) | 14(100%) | |
In this study, highest incidence of scrub typhus was seen during October to December followed by January to March. Majority of the patients affected by scrub typhus were from rural areas. (Table 3).
Table (3):
Seasonal and area distribution of positive scrub typhus samples.
| S.No | Variable | Frequency n = 14 |
Percentage (%) |
|---|---|---|---|
| 1 | Season | ||
| January – March | 3 | 21.42 | |
| April – June | 1 | 7.2 | |
| July – September | 1 | 7.2 | |
| October – December | 9 | 64.28 | |
| 2 | Area of residence | ||
| Rural | 11 | 78.57 | |
| Urban | 3 | 21.42 | |
This study shows that, there is a statistically significant association between seasonal variation and incidence of scrub typhus (P<0.05). It was found that, demographic differences between rural and urban areas were also found to have a statistically significant association with incidence of scrub typhus. (Table 4).
Table (4):
Association between incidence of scrub typhus and related variables.
| S.No | Variable | Scrub Typhus – Positive | Chi Square | P Value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| 1 | Season | ||||
| January – March | 3 | 140 | 12.594 | 0.0056* | |
| April – June | 1 | 142 | |||
| July – September | 1 | 142 | |||
| October – December | 9 | 134 | |||
| 2 | Area of residence | ||||
| Rural | 11 | 132 | 4.86 | 0.027* | |
| Urban | 3 | 141 | |||
*P – value < 0.05. Statistically significant at 95% Confidence Interval.
Scrub typhus is a vector borne re-emerging acute febrile illness associated with significant mortality if untreated. Scrub typhus occurs in geographic regions that are endemic for diseases that may present with similar clinical syndromes, such as malaria, dengue, typhoid, and leptospirosis. Clinically, scrub typhus can present with life-threatening complications such as respiratory dysfunction, shock, encepahalopathy and acute renal failure. The results of the study undertook in a tertiary care hospital to find out the frequency of occurrence of scrub typhus are discussed below.
In our study, scrub typhus was found in 9.8% of our study individuals. In a study done by Tay et al12, from Malaysia, community seroprevalence among different aboriginal groups was found to be 17.9%12, which is higher than our study. In another Malaysian study, the seroprevalence among healthy blood donors was 5.4% while that among febrile patients was higher (43.5%)17. Several hospital based studies in India have shown a wide prevalence of scrub typhus ranging from 8.8 per cent in the south to 59.6 per cent in the north18,19. This variation may be attributed to the lifestyle changes, extensive use of insecticides as well as empiric use chloramphenicol and tetracycline for acute febrile illnesses20,21.
Both male and female were equally affected in our study. This shows that scrub typhus is equally distributed without any gender disparity in our population. Equal male female ratio was also found by other studies done in China and Japan22,23. This may be explained in our population by the fact that women equally work in outdoors and fields in rural areas making them exposed to vectors and rodents. The most common age group affected with scrub typhus in our study is 41-60 years which is same as other studies22,24.
In our study most of the patients affected by scrub typhus were from rural areas 11 (78.57%) compared to urban areas (21.42%)25,26. Scrub typhus affects almost a million people every year mainly affecting population involved in occupation that encounter scrub vegetation or in their day to day life4. This may be due to delayed treatment seeking behaviour of the people as well as to increased exposure to mites/ mammals in the field and to the rodents during daily household activities27. Agricultural workers has a higher relative risk for scrub typhus26.
In our study highest incidence is seen during October to December followed by January to March as observed by Lee et al28. Few studies have reported higher rates of scrub typhus in rainy season which is attributed to the mite activity as observed by chigger index24,29,30. The discrepancy of the peak period of scrub typhus outbreaks might be due to the incubation period of the disease which ranges from 5 to 22 days31.
An eschar at the site of Trombiculid chigger bite is pathognomonic of scrub typhus. It begins as a papule which later ulcerates to form a black crust with surrounding erythema. When present, this might be the first symptom even before the onset of fever and other manifestations but is usually unnoticed6,32. The frequency of occurrence of eschar is 1% – 97% depending on the geographical area9.
Eschar was not present in any of our patients. Occurrence of eschar is rare in South-East Asian population who usually present with less severe illness without any eschar or rash33. As eschars are painless and the mites are too small to the naked eye, mite bite is rarely noticed unless it is intensely searched for. This is even more difficult in dark skinned individuals and when present in clothing covered areas. However, Varghese et al25 reported 55% incidence of eschar which is quite high while other authors Mahajan et al6 and Sonthayanon et al33 reported very low incidence of eschar as 9.5% and 7.2% respectively6,34.
The re-emergence of scrub typhus in the Asia-Pacific area attributed to massive deforestation and urbanisation along with the emerging antibiotic resistance elicits the urgency of developing and taking in effective control and preventive measures.
The best primordial preventive measure for high risk individuals is to avoid areas such as farms, bushy areas with abundance of rodents and mites. As this is not feasible, primary prevention plays a vital role in preventing the disease. This prioritises public education on case recognition, personal protection and avoidance of risk areas. WHO recommends awareness and educational ventures targeting schoolchildren, teachers and women in endemic areas. General population are at equal risk attributed to increased deforestation and overcrowding.
For farmers, field workers and those occupational individuals who cannot avoid the risk factors, taking the following precautions will help prevent the disease35-38.
- Protective full sleeved clothing/ gum boots
- Bathing and changing clothes after work
- Treating clothes and boots with permithrin
- Avoidance of sitting/lying down on bare grass
- Usage of Environmental Protection Agency (EPA)-registered insect repellents containing DEET (Diethyltoluamide) or 5% emulsion of diethyl phthalate or dibutylphthalate
- Chemoprophylaxis with doxycycline 100mg every 5 days for a total of 7 weeks
Scrub typhus is a zoonotic disease associated with significant mortality if untreated, due to serious life threatening complications. Scrub typhus is present in geographic areas that are endemic for other illnesses with similar clinical manifestations. Hence by our study we have emphasized the importance of considering Scrub typhus as an important differential diagnosis of any acute febrile episodes even in the absence of typical clinical presentation. To conclude, Scrub typhus antibodies were detected in 9% of the population with higher frequency in rural population and during cooler months of the year. Hence, high index of clinical suspicion, appropriate diagnostic tests and early onset of pertinent therapy can greatly reduce the case fatality rate.
ACKNOWLEDGMENTS
We would like to acknowledge our late professor Dr. K. Muthulakshmi for her constant support and guidance throughout the study. We would also like to acknowledge our phlebotomists and our lab technicians for their technical assistance during the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was a seed money project, partially funded by Meenakshi medical college hospital and research institute, Kanchipuram.
ETHICS STATEMENT
The study was approved by Institutional ethical committee, Meenakshi Medical College & Research Institute, Kanchipuram, Tamil Nadu, India.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- WHO | Zoonoses. WHO. Accessed August 20, 2020. http://www.who.int/topics/zoonoses/en/
- Zoonotic Diseases | One Health | CDC. Published February 19, 2020. Accessed August 20, 2020. https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html
- Chakraborty S, Sarma N. Scrub Typhus: An Emerging Threat. Indian J Dermatol. 2017;62(5):478-485.
Crossref - Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16(5):429-436.
Crossref - Koh GCKW, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of Scrub Typhus. Am J Trop Med Hyg. 2010;82(3):368-370.
Crossref - Mahajan SK, Rolain J-M, Kashyap R, et al. Scrub typhus in Himalayas. Emerging Infect Dis. 2006;12(10):1590-1592.
Crossref - Jeong YJ, Kim S, Wook YD, Lee JW, Kim K-I, Lee SH. Scrub typhus: clinical, pathologic and imaging findings. Radiographics. 2007;27(1):161-172.
Crossref - Bafna P, Kadhiravan T. Classical eschar in scrub typhus. Indian J Med Res. 2014;140(6):792.
- Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31(4):240-244.
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11(11).
Crossref - Lyu Y, Tian L, Zhang L, et al. A Case-Control Study of Risk Factors Associated with Scrub Typhus Infection in Beijing, China. Plos One. 2013;8(5):e63668.
Crossref - Tay ST, Zan HAM, Lim YAL, Ngui R. Antibody Prevalence and Factors Associated with Exposure to Orientia tsutsugamushi in Different Aboriginal Subgroups in West Malaysia. Plos Neglected Tropical Diseases. 2013;7(8):e2341.
Crossref - Prakash JAJ. Scrub typhus: risks, diagnostic issues, and management challenges. Research and Reports in Tropical Medicine.2017;8:73-83.
Crossref - Kuo C-C, Huang J-L, Shu P-Y, Lee P-L, Kelt DA, Wang H-C. Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl. 2012;22(6):1803-1816.
Crossref - Walker DH, Fishbein DB. Epidemiology of rickettsial diseases. Eur J Epidemiol. 1991;7(3):237-245.
Crossref - Blacksell SD, Tanganuchitcharnchai A, Nawtaisong P, et al. Diagnostic Accuracy of the InBios Scrub Typhus Detect Enzyme-Linked Immunoassay for the Detection of IgM Antibodies in Northern Thailand. Clin Vaccine Immunol. 2016;23(2):148-154.
Crossref - Tay ST, Kamalanathan M, Rohani MY. Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among Malaysian blood donors and febrile patients in the urban areas. Southeast Asian J Trop Med Public Health. 2003;34(1):165-170.
- Rajagopal V, Bhaskar M, Devi RR, Rajkumar P. Serological diagnosis of scrub typhus in patients attending a government hospital at Vellore, Tamil Nadu. Indian Journal of Medical Research. 2014;140(5):686.
- Khan F, Mittal G, Agarwal RK, Ahmad S, Gupta S, Shadab M. Prevalence of scrub typhus-A cause of concern in Uttarakhand Region, India. Int J Curr Microbiol App Sci. 2015;1:101-109.
- Chunchanur SK. Scrub typhus in India–An impending threat. Ann Clin Immunol Microbiol. 2018;1(1):1003.
- Zhang W-Y, Wang L-Y, Ding F, et al. Scrub Typhus in Mainland China, 2006–2012: The Need for Targeted Public Health Interventions. Plos Neglected Tropical Diseases. 2013;7(12):e2493.
Crossref - Ogawa M, Hagiwara T, Kishimoto T, et al. Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg. 2002;67(2):162-165.
Crossref - Bithu R, Kanodia V, Maheshwari RK. Possibility of scrub typhus in fever of unknown origin (FUO) cases: An experience from Rajasthan. Indian Journal of Medical Microbiology. 2014;32(4):387.
Crossref - Varghese GM, Abraham OC, Mathai D, et al. Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. J Infect. 2006;52(1):56-60.
Crossref - Devamani C, Schmidt W-P, Ariyoshi K, Anitha A, Kalaimani S, Prakash JAJ. Risk Factors for Scrub Typhus, Murine Typhus, and Spotted Fever Seropositivity in Urban Areas, Rural Plains, and Peri-Forest Hill Villages in South India: A Cross-Sectional Study. The American Journal of Tropical Medicine and Hygiene. 2020;103.
Crossref - Borkakoty B, Jakharia A, Biswas D, Mahanta J. Co-infection of scrub typhus and leptospirosis in patients with pyrexia of unknown origin in Longding district of Arunachal Pradesh in 2013. Indian Journal of Medical Microbiology. 2016;34(1):88.
Crossref - Lee SH, Lee Y-S, Lee IY, et al. Monthly Occurrence of Vectors and Reservoir Rodents of Scrub Typhus in an Endemic Area of Jeollanam-do, Korea. Korean J Parasitol. 2012;50(4):327-331.
Crossref - Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the himalayan region of India. Jpn J Infect Dis. 2005;58(4):208-210.
- Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24-28.
- Seong S-Y, Choi M-S, Kim I-S. Orientia tsutsugamushi infection:overview and immune responses. Microbes and Infection. 2001;3(1):11-21.
Crossref - Gupta V, Gautam V. Scrub Typhus – A Short Review. J Commun Dis. 2004;36(4):284-289.
- Kundavaram A, Jonathan A, Nathaniel S, Varghese G. Eschar in scrub typhus: A valuable clue to the diagnosis. Journal of Postgraduate Medicine. 2013;59(3):177.
Crossref - Sonthayanon P, Chierakul W, Wuthiekanun V, et al. Association of High Orientia tsutsugamushi DNA Loads with Disease of Greater Severity in Adults with Scrub Typhus. J Clin Microbiol. 2009;47(2):430-434.
Crossref - Sharma PK, Ramakrishnan R, Hutin YJF, et al. Scrub typhus in Darjeeling, India: opportunities for simple, practical prevention measures. 2009:103(11):1153-1158.
Crossref - Ritesh S. Scrub Typhus: Prevention and Control. JK Science : Journal of Medical Education & Research. 2010;12.
- Olson JG, Bourgeois AL, Fang RC, Coolbaugh JC, Dennis DT. Prevention of scrub typhus. Prophylactic administration of doxycycline in a randomized double blind trial. Am J Trop Med Hyg. 1980;29(5):989-997.
- Bailey CA, Ley HL. The Treatment and Prophylaxis of Scrub Typhus with Antibiotics. Annals of the New York Academy of Sciences. 1952;55(6):983-991.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.