ISSN: 0973-7510
E-ISSN: 2581-690X
Dengue is one among the acute viral infections with the probability of fatal complications. In 2017; NVBDCP reported 157220 positive dengue cases with 250 deaths in India; 17018 cases and 5 deaths in Karnataka. Most primary infections are uneventful. The critical illness like Dengue Haemorrhagic Fever and Dengue Shock Syndrome are generally attributed to serotype cross-reactivity. Identification of secondary dengue infection in the early onset of illness is beneficial. Therefore, methods to discrepate primary and secondary dengue infection are of significant prognostic value. The current study is a hospital based prospective analytical evaluation and was aimed to discriminate secondary from primary dengue virus infection in clinically suspected dengue cases presenting with fever and thrombocytopenia. Patients of all age groups attending Krishna Rajendra Hospital on outpatient and inpatient basis with clinically suspected dengue fever of less than 5 days associated with thrombocytopenia were included in the study. The samples were tested in the VRDL of the Microbiology Department for dengue NS1 antigen and IgM antibodies, positive for both were further subjected to IgG antibodies. IgM /IgG ratio was used to differentiate primary and secondary dengue infections. Dengue infection was categorized based on WHO guidelines. A total of 17,841 samples were tested from May 2017 to December 2023; out of 17841 samples tested 2111 (60.74%) were positive for dengue NS1 and IgM. Of the 2111 dengue NS1 and IgM positive cases, 1700 (80.5%) were having secondary dengue infection, whereas 411 (19.46%) were having primary infection. Early detection of secondary infection helps the clinician in anticipating dengue related complications with appropriate therapeutic intervention, thereby reducing further complications and mortality.
NS1 Antigen, IgM Antibody, IgG Antibody, Thrombocytopenia
Dengue fever is one of the acute viral infections that spreads with the aid of mosquitoes and could be lethal. The first ever epidemic of clinical dengue-like disease was documented in Madras in 1780, and in the year 1963-1964 the first diagnosed and virologically pinpointed dengue fever was recorded in Calcutta, India.1 The most common arboviral infection in the world is dengue infection; according to recent studies, around 390 million people are being infected annually.2 In tropical and subtropical region prime reservoir of the virus is an endophilic mosquito Aedes aegypti, thrives in and around houses and acts a vector in human to human transmission. Aedes albopictus also acts as a vector to a lesser extent.3 Majority of the states in India are endemic to dengue, frequent epidemics are also reported from several parts of the country.4 In 2017; NVBDCP reported 1,57,220 positive dengue cases with 250 deaths in India and 17,018 cases with 5 deaths in Karnataka. However, India witnessed 233251 and positive cases and 303 deaths and of which 9889 and 9 death cases were from were from Karnataka in the year 2022.5
The dengue virus belongs to the flaviviridae family and has four serologically related but antigenically definite serotypes (DENV-1, DENV-2, DENV-3, and DENV-4).
Symptoms of dengue infection varies from undistinguished fever to dengue fever (DF) and dengue hemorrhagic fever (DHF), prone to be mortal.6 Infection with one serotype provides immunity solely to the infecting serotype. Infection with one of the other three serotypes causes immune-enhanced illness, such as severe hemorrhagic fever or dengue shock syndrome.7 Patients with dengue who have a platelet count of less than 100,000 cells/mm3 are at higher risk of developing DHF and DSS and require close monitoring and appropriate treatment. In addition to its association with disease severity, thrombocytopenia can also impact the treatment of dengue patients. Platelet transfusions may be necessary in severe cases of thrombocytopenia to prevent bleeding complications, but their use must be carefully evaluated due to the risk of transfusion-related complications. Early recognition and monitoring of thrombocytopenia in dengue patients can help to improve outcomes and reduce the risk of complications.8
Across the world, the rate of primary and secondary dengue infection is 65.36% and 48.99% respectively,9 however the secondary dengue infection in central India is 77%.10 On a global scale, the severity of dengue due to secondary infection varies from 35 to 52%.11 Differentiation of dengue infection into primary dengue and secondary dengue is difficult in the acute phase of infection. Methods to distinguish primary and secondary DENV infection are essential in the anticipation and handling of complications. The gold standard test recommended by WHO to differentiate primary and secondary dengue infection is Hemagglutination inhibition (HI).12 The main disadvantages of the Hemagglutination inhibition test are that it is time-consuming and has numerous technical glitches. Pre-analytical concerns are 1) quality of the sample, 2) interference from non-specific inhibitors, 3) timing of the test, 4) due to non-specific inhibitors, higher rate of cross reactivity is observed and hence chemical pretreatment is mandatory. Post-analytical concerns include the need for paired serum samples and the difficulty of making an early diagnosis. The HI test has limited sensitivity. That is, it may not detect low levels of antibody or antibody that is not capable of completely inhibiting hemagglutination.13,14 Serological tests based on detection of specific antibodies (IgG and IgM) are simple and valuable for determination of primary or secondary infection.
The ratio of dengue IgM to IgG is an important parameter that can help to distinguish between primary and secondary dengue infections. In primary infections, the IgM antibody response is dominant, and the IgG response is relatively low. In contrast, in secondary infections, the IgG response is dominant, and the IgM response is relatively low.Therefore, a high ratio of dengue IgM to IgG may indicate a primary infection, while a low ratio may indicate a secondary infection. This information is critical for patient management, as secondary infections are more likely to develop severe dengue and require careful monitoring and prompt treatment. In addition, monitoring changes in the IgM/IgG ratio over time can provide valuable information on the progression of dengue infection and the effectiveness of treatment. A decreasing IgM/IgG ratio may indicate a shift from primary to a secondary infection, while an increasing ratio may indicate a successful immune response and recovery. As a result, when compare to HI test, the IgM/IgG ratio can be determined in a resource-constrained setting.15 With this background, the current study was aimed to discriminate secondary from primary DENV infection in clinically suspected dengue cases presenting with fever and thrombocytopenia.
The present study was implemented at Viral Research and Diagnostic Laboratory in the Department of Microbiology, MMC & RI, Mysuru. Institutional ethical clearance was obtained for the VRDL project, testing for dengue is one among the routine diagnostics, hence a separate ethical clearance was not obtained for the study. We followed consecutive method of sampling, a total of 17,841 suspected dengue fever cases from June 2017 to December 2023 were studied. Demographic, clinical, laboratory, and serological data were collected and documented for each case. Subjects of all age group presenting with fever and thrombocytopenia; ≤5 days were included in the study and those who presented after 5 days of fever were excluded from the study. Duration of illness was determined by considering the of onset of clinical manifestations such as fever, body ache, vomiting, retro-orbital pain etc. Blood was drawn and transferred to clot activating vacutainer. Upon collection, the blood samples were transported to VRDL (located within the premises of hospital) at room temperature within 2 hours of collection. Serum was separated the data pertaining to serology was obtained by evaluating dengue NS1 antigen and IgM antibodies within 24 hours of sample collection. Those samples which were positive for dengue NS1 and IgM were further subjected to Dengue IgG ELISA and the ratio of IgM/IgG was used to differentiate primary and secondary dengue.
Dengue NS1 testing: Blood samples collected within 5 days of onset of symptoms were tested for dengue NS1 antigen using Dengue NS1 Ag Microlisa from J. Mitra & Co. Pvt Ltd. Assay was carried out using 50 µl of 1:1 diluted sample as per the manufacturer’s instruction.
Dengue IgM antibody testing: Dengue IgM antibody detected by dengue IgM Antibody Capture ELISA (MAC-ELISA) kit developed by National Institute of Virology (NIV), Pune. Serum sample were diluted in the ratio of 1:100 and 50 µl of diluted sample was used for testing. The assay was performed as per the manufacturer’s instruction.
Dengue IgG antibody testing: Dengue IgG antibody was tested for all the NS1 and IgM positive cases. DENGUE IgG MICROLISA based GAC capture ELISA method was used to test Dengue IgG antibody from J. Mitra & Co. Pvt. Ltd. 100 µl of 1 in 100 diluted serum was used and further test was executed as per the manufacturer’s instruction.
Case definitions:
Thrombocytopenia
Platelet count less than 100,000 cells/mm3
Primary and Secondary dengue infection
Criteria for differentiating between primary and secondary dengue virus infections is based on the IgM and IgG antibody ratio of blood samples collected during the acute phase of the disease, usually between 3 and 5 days after symptom onset as described by WHO.
The criteria are as follows:
- A positive NS1 antigen test, a positive IgM antibody test, and a negative IgG antibody test, indicate a primary dengue virus infection.
- A positive IgG antibody test, but a negative IgM antibody test and a negative NS1 antigen test, indicates a previous dengue virus infection.
- A positive NS1 antigen, a positive IgM antibody test, and a positive IgG antibody test, with an IgM /IgG more than 1.2, indicate a primary dengue virus infection.
- A positive NS1 antigen, a positive IgM antibody test, and a positive IgG antibody test, with an IgM/IgG ratio of less than 1.2, indicate a secondary dengue virus infection as mentioned in Table 1.
Table (1):
WHO criteria for differentiating primary and secondary infection
Result of NS1 Antigen |
Result of IgM Antibody |
Result of IgG Antibody |
Ratio of 1gM/IgG |
Classification of dengue |
|---|---|---|---|---|
Positive |
Positive |
Negative |
NA |
Primary infection |
Negative |
Negative |
Positive |
NA |
Previous infection |
Positive |
Positive |
Positive |
Greater than 1.2 |
Primary infection |
Positive |
Positive |
Positive |
Less than 1.2 |
Secondary infection |
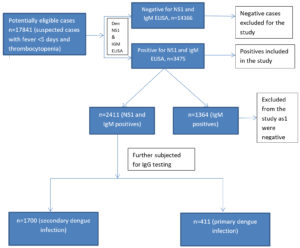
A total of 17,841 samples were tested; 3475 cases were positive for dengue either by dengue NS1 and or dengue IgM by ELISA method. Out of 3475 dengue positive cases, 2111 samples were positive for NS1 & IgM and remaining 1364 samples were positive only by dengue IgM ELISA, these samples were not considered for secondary testing as shown in the Figure. Samples which were positive for dengue NS1 and IgM were further tested for dengue IgG antibodies by ELISA. IgM/IgG ratio was determined. Of the 2111 cases studied, 1700 (80.53%) of them were having secondary infection and 411 (19.47%) were having primary infection as mentioned in Table 2. In our study, relatively all the age groups were infected and most frequently infected age group in both primary and secondary dengue was 21-30 years (Table 3). Both males and females were almost equally infected in both primary and secondary dengue infections as given in Table 4. Thrombocytopenia was more common in secondary dengue when compared to primary dengue and majority of secondary dengue cases had platelet count of 50,000 to 1,00,000 per mm3 (Table 5).
Table (2):
Year-wise distribution of positive cases
Year |
Samples tested |
Total positives |
Only IgM positives |
NS1 and IgM positives |
Dengue secondary infection |
Dengue primary infection |
|---|---|---|---|---|---|---|
2017 |
2909 |
1176 |
423 |
753 |
607 |
146 |
2018 |
2435 |
247 |
53 |
194 |
156 |
38 |
2019 |
3634 |
719 |
351 |
368 |
296 |
72 |
2020 |
1684 |
192 |
88 |
104 |
84 |
20 |
2021 |
2035 |
299 |
81 |
218 |
176 |
42 |
2022 |
2942 |
637 |
310 |
327 |
263 |
64 |
2023 |
2190 |
205 |
58 |
147 |
118 |
29 |
Total |
17841 |
3475 |
1364 |
2111 |
1700 |
411 |
Table (3):
Age-wise distribution of cases in primary and secondary infection
Age-wise Distribution (years) |
Total |
Secondary infection |
Primary infection |
|---|---|---|---|
0-10 |
355 (16.8%) |
300 (14.1%) |
55 (2.60%) |
11-20 |
560 (26.52%) |
410 (19.42%) |
150 (7.05%) |
21-30 |
616 (29.18%) |
466 (22.07%) |
150 (7.05%) |
31-40 |
299 (14.16%) |
243 (11.51%) |
56 (2.65%) |
41-50 |
131 (6.20%) |
131 (6.20%) |
0 |
51-60 |
75 (3.55%) |
75 (3.55%) |
0 |
61-70 |
75 (3.55%) |
75 (3.55%) |
0 |
Total |
2111 (100%) |
1700 (80.53%) |
411 (19.47%) |
Table (4):
Demographic and Laboratory data with respect to primary and secondary infection
Demographic, and laboratory data |
Total |
Secondary infection |
Primary infection |
|---|---|---|---|
Male |
1065 (50.45%) |
878 (82.44%) |
10 (17.5%) |
Female |
1046 (49.54) |
44 (78.5%) |
12 (21.4%) |
Mean Age |
25.4 |
25.5 |
23.8 |
Mean IgM OD |
0.483 |
0.533 |
0.275 |
Mean IgG OD |
1.714 |
2.071 |
0.238 |
Mean Platelet count |
89646.7 |
84385.5 |
116454.5 |
Table (5):
Platelet count in primary and secondary infection
Thrombocytopenia |
Total |
Primary infection |
Secondary infection |
|---|---|---|---|
>50000 |
149 (7.05%) |
19 (0.90%) |
130 (6.15%) |
51000 – 100000 |
1438 (68.11%) |
37 (1.75%) |
1401 (66.33%) |
101000 – 150000 |
524 (24.82%) |
355 (16.81%) |
169 (8.00%) |
Total |
2111 (100%) |
411 (19.46%) |
1700 (80.53%) |
Two thousand one hundred and eleven cases with thrombocytopenia were studied. One thousand seven hundred (80.5%) of them were having secondary dengue infection; both male and female were equally infected and the ratio was 1.06:1. This could be attributed to the dwelling of Aedes aegypti mosquito in both indoor as well as out door. Male:female ratio in our study was consistent with the study conducted by Kumar et al.16 from Telangana, south India where they found the ratio of 1.4:1. Majority of the positive cases were in the age group of 21-30 years with the mean age of 25.5 years and was discordant to the study by Lytton et al.17
The number of secondary dengue cases in Agarwal A’s study10 was consistent with our study and inconsistent with the study Kumar et al.16 Studies indicate that secondary dengue infections exist in dengue endemic regions18 suggesting that Mysore also belongs to endemic zone. In Karnataka the rate of dengue infection is markedly increasing since 20145; this could be the probable reason for the higher number of secondary cases in our study.
Like other viral infections, the host immune response is triggered by infection with dengue virus. Generally primary infections are mild compared to secondary infections; a successive infection with homotypic serotype ought to be neutralized by neutralizing antibodies. Although, secondary infection with heterotypic serotype perhaps pave a way to intensify the severity by antibody dependent enhancement. As the antibodies generated cause of primary infection does not have the potential to neutralize though they bind the virus. Thus formed virus-antibody complex produces cross reacting antibodies assisting virus antibody complex to attach and invade the cells carrying FCg receptors like dendritic cells, monocytes and macrophages resulting in amplified viral count. This eventually leads to the mobilization of elevated complexes such as pro-inflammatory type I cytokines (TNFa and TNFg) causing plasma leakage by influencing the vascular endothelial cells.19
Thrombocytopenia is a common feature of dengue fever, studies suggest several hypothetical and immunological hypotheses for thrombocytopenia in dengue infection: 1) DENV inhibit bone marrow progenitor cells by affecting directly or indirectly and thereby reducing the proliferating magnitude of hematopoietic cells,20 2) Activation of macrophages and subsequent phagocytosis of platelets in spleen21 these are the typical cases of hypothetical, whereas immunological hypotheses are: 3) Significant deregulation of plasma kinin system and immune-pathogenesis of dengue results in functional disruption of hematopoietic cells.22 Antiplatelet antibodies lyses the complement system, induces increased apoptosis causes destruction of platelets.23
Secondary dengue fever is associated with more severe symptoms, including more pronounced thrombocytopenia. In case of secondary dengue infection specific isotypes of IgG antibodies plays a crucial role in enhancing the disease severity. Thrombocytopenia in secondary dengue is attributed to the increasesed ratio of IgG1:IgG2 and existence of afucosylated IgG1 which in turn binds to the activating FcR Fcg IIIA+ immune cells and dengue virus immune complex leading to diminishing of platelet.24
Thrombocytopenia was more frequent in secondary dengue compared to primary dengue in our study. Similar to our observation, thrombocytopenia was consistent with secondary dengue in a study conducted by Dinkar et al from Uttar Pradesh.25 Sixty six percent of the secondary dengue cases had a Platelet count of 51,000-1,00,000.
Several studies have investigated the use of IgM and IgG ratios for differentiating primary and secondary dengue infections. In a study from Dehradun reported that an IgM to IgG ratio of 1.59 had a sensitivity of 85.11%, specificity of 92.3%, for secondary dengue infection.26 Another study in Central India the IgG/IgM ratio of ≥1.59 had a specificity of 90.8%, sensitivity of 100%, and in differentiating primary and secondary infection.10
We do have some of the limitations like, our study mainly concerned on tertiary care hospital setting, hence chances of getting more severe cases of dengue infection was higher. It’s worth considering analyzing our data with other parameters like age, bleeding and another clinical parameter. We have considered the standard cut off ratio IgM to IgG but we did not evaluate the same for our region. The IgM to IgG ratio can be helpful in differentiating primary and secondary dengue infections, it is not a foolproof diagnostic tool as it may get affected by cross reacting antibodies. In addition, we missed to categorize whether the antibodies are neutralizing or non-neutralizing antibodies. Further, we have not ruled out other etiological agent responsible for thrombocytopenia.
Other factors such as the timing and duration of antibody responses, the circulating DENV serotype(s), and individual variation in immune responses can influence the results. Therefore, clinical judgment and other laboratory tests, such as viral NS1, IgM and IgG antigen testing, should also be considered in making a definitive diagnosis of dengue fever.
In the present study, we have tested good number of samples for a period of six years, hence this study may be considered as an external validation for other population in endemic areas.
Our study highlights the importance of serodiagnosis in detecting secondary dengue infections, particularly in tertiary care hospitals where accurate and timely diagnosis is crucial for effective patient management. In our study, 80% of the cases had secondary dengue infection, thus evaluating IgM and IgG antibody ratios can help to differentiate between primary and secondary dengue virus infections. This information can help clinicians to provide appropriate medical care to patients and to better understand the progression of the disease. Hence dengue testing protocol should include wide parameters like NS1, IgM and IgG testing as part of routine diagnostics; also, the evaluation of IgM/IgG ratio can be determined even in in a finite resource setting.
ACKNOWLEDGMENTS
The authors are grateful to DHR/ICMR for their support and including our Institute in the Viral Research and Diagnostic Network. Authors sincerely thank all the staff of NIV, Pune for their constant assistance and express their heartfelt gratitude to Director and Dean, MMC&RI, Mysuru for their encouragement.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was financially supported by the Department of Health Research/Indian Council of Medical Research, New Delhi, India.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethical Committee, Mysore Medical College & Research Institute and Associated Hospitals, Mysore, India, with reference number DHR/ICMR VRDL/14/2018-19.
- Dinkar A, Singh J. Dengue infection in North India: An experience of a tertiary care center from 2012 to 2017. Ci Ji Yi Xue Za Zhi. 2020;32(1):36-40.
Crossref - Dengue and Severe Dengue – Key facts. WHO. 2018. http://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Kok BH, Lim HT, Lim CP, Lai NS, Leow CY, Leow CH. Dengue virus infection – a review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023;324:199018.
Crossref - Singh N, Singh AK, Kumar A. Dengue outbreak update in India: 2022. Indian J Public Health. 2023;67(1):181-183.
- Dengue Cases and Deaths in the Country since 2010, National Vector Borne Disease Control Programme. Dengue/DHF Situation in India: National Center for Vector Borne Diseases Control (NCVBDC) (mohfw.gov.in)
- WHO, TDR, Eds. Dengue Guidelines For Diagnosis, Treatment, Prevention And Control. WHO Press.2009. Accessed on 28th December 2023. www.who.int/publications/i/item/97892415478716
- Ma E, Cheng G. Host immunity and vaccine development against Dengue virus. Infect Med. 2022;1(1):50-58.
Crossref - Das S, Abreu C, Harris M, Shrader J, Sarvepalli S. Severe Thrombocytopenia Associated with Dengue Fever: An Evidence-Based Approach to Management of Thrombocytopenia. Case Rep Hematol. 2022;2022:3358325.
Crossref - Asish PR, Dasgupta S, Rachel G, Bagepally BS, Girish Kumar CP. Global prevalence of asymptomatic dengue infections – a systematic review and meta-analysis. Int J Infect Dis. 2023;134:292-298.
Crossref - Agarwal A, Jain RK, Chaurasia D, Biswas D. Determining the optimum cut-off IgM/IgG ratio for predicting secondary dengue infections: An observational hospital based study from Central India. Indian J Med Microbiol. 2022;40(4):492-495.
Crossref - Soo KM, Khalid B, Ching SM, Chee HY. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. PLoS One. 2016;11(5):e0154760.
Crossref - Palabodeewat S, Masrinoul P, Yoksan S, Auewarakul P, Komaikul J. A modified IgG avidity assay for reliability improvement of an in-house capture ELISA to discriminate primary from secondary dengue virus infections. J Virol Methods. 2021;289:114043.
Crossref - Chong ZL, Soe HJ, Ismail AA, Mahboob T, Chandramathi S, Sekaran SD. Evaluation of the Diagnostic Accuracy of a New Biosensors-Based Rapid Diagnostic Test for the Point-Of-Care Diagnosis of Previous and Recent Dengue Infections in Malaysia. Biosensors. 2021;11(5):129.
Crossref - Raafat N, Blacksell SD, Maude RJ. A review of dengue diagnostics and implications for surveillance and control. Trans R Soc Trop Med Hyg. 2019;113:653-660.
Crossref - Kabir MA, Zilouchian H, Younas MA, Asghar W. Dengue Detection: Advances in Diagnostic Tools from Conventional Technology to Point of Care. Biosensors. 2021;11(7):206.
Crossref - Kumar VP, Archana ARK, Swetha G, Sowjanya G. Differentiation of primary and secondary dengue by simultaneous detection of NS1, IGM and IgG by enzyme-linked immunosorbent assay: a clinico-serological study from South India. Journal of Cardiovascular Research. 2023;14(4):2344-2350.
- Lytton SD, Nematollahi G, Van Tong H, et al. Predominant secondary dengue infection among Vietnamese adults mostly without warning signs and severe disease. Int J Infect Dis. 2020;100:316-323.
Crossref - Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26(7):taz062.
Crossref - Wang WH, Urbina AN, Chang MR, et al. Dengue hemorrhagic fever – A systemic literature review of current perspectives on pathogenesis, prevention and control. J Microbiol Immunol Infect. 2020;53(6):963-978.
Crossref - Hsu AY, Ho TC, Lai ML, et al. Identification and characterization of permissive cells to dengue virus infection in human hematopoietic stem and progenitor cells. Transfusion. 2019 Sep;59(9):2938-2951.
Crossref - Masri MF, Mantri CK, Rathore APS, John AL. Peripheral serotonin causes dengue virus-induced thrombocytopenia through 5HT2 receptors. Blood. 2019;133(21):2325-2337.
Crossref - Sagar V, Kumar M, Rawat R, Kumar P, Kumar G, Yadav SK. To determine the correlation of severity of thrombocytopenia, with vitamin B12 deficiency in patients of dengue fever. Int J Health Sci. 2022;6(S2):5579-5590.
Crossref - Chao CH, Wu WC, Lai YC, et al. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathogens. 2019;15(4):e1007625.
Crossref - St John AL, Rathore APS. Adaptive immune responses to primary and secondary dengue virus infections. Nat Rev Immunol. 2019;19(4):218-230.
Crossref - Dinkar A, Singh J, Kumar N, Kumar K, Singh SK, Singh AK. Impact of secondary infections on dengue presentation: A cross-sectional study in a tertiary care hospital in Uttar Pradesh, India. J Infect Public Health. 2023;16(12):1925-1932.
Crossref - Kalra C, Mittal G, Agarwal RK, Ahmad S. Role of IgM: IgG ratio in Differentiating Primary And Secondary Dengue Virus Infection In A Tertiary Care Hospital, A Cross Sectional Study. Int J Food Nutr Sci. 2022;11(11):17844-17857.
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.