ISSN: 0973-7510
E-ISSN: 2581-690X
Coronavirus disease (COVID-19) is an infectious disease caused by a newly discovered coronavirus. Following infection, antibodies are formed against the spike (S) and nucleocapsid (N) proteins, which are the primary viral antigens of SARS-CoV-2. This study aims to determine the antibody response three weeks post-infection and its persistence. To study antibody responses in COVID-19-positive individuals and to compare the degree of antibody response in symptomatic and asymptomatic individuals. The persistence of the antibody response was also assessed. Adult patients (> 15 years of age) who were diagnosed as COVID-19-positive by RT-PCR, three weeks after swab positivity were tested for total antibody levels against COVID-19 antigens by electrochemiluminescence assay. Out of 226 individuals, 129 were symptomatic and 97 were asymptomatic. Among the 129 symptomatic individuals, 74 exhibited an antibody response, whereas in the asymptomatic individuals, only 10 exhibited an antibody response. The antibody response was found to be significant in symptomatic individuals compared to that in asymptomatic individuals (p < 0.05). All follow-up individuals were seropositive at the end of both 6 and 8 months. Antibodies against SARS-CoV-2 persist for 8 months following infection. Despite the waning of antibodies against the nucleocapsid antigen, there was no complete disappearance of antibodies.
COVID-19, RT-PCR, Electrochemiluminescence assay
Coronavirus disease (COVID-19) is an infectious disease caused by a newly discovered coronavirus.1 Most people exposed to the COVID-19 virus will experience mild to moderate respiratory illness and recover with symptomatic management without requiring special treatment. The geriatric population and those with comorbidities, such as cardiac disease, diabetes, chronic respiratory illness, and malignancies, are more likely to develop serious illness with complications.2 Following infection, antibodies are formed against the spike (S) and nucleocapsid (N) proteins, which are the primary viral antigens of SARS-CoV-2.3
After infection by SARS-CoV-2, different categories of antibodies circulate in the serum, such as immunoglobulin G (IgG), immunoglobulin M (IgM), and immunoglobulin A (IgA), mainly targeting two viral proteins, the S protein and nucleoprotein (NP).4,5 The N protein plays an important role in viral pathogenesis, replication, and RNA packaging. Antibodies to the N protein are frequently detected in COVID-19 patients.6
Seroconversion occurs between days 10 and 21 in most individuals infected with SARS-CoV-2.7,8 In cases involving a mild infection, it takes longer (four weeks or more) for seroconversion, and in a few cases, antibodies to SARS-CoV-2 are not detected during the follow-up period.9 Stronger antibody responses are related to a more severe disease status in patients with SARS-CoV-2 infection.10
While serological tests are now widely available, the correlates of immunity are not well established. Therefore, there remains a great need to measure antibody responses and determine seroconversion rates. While such serological assays are not well suited to detect acute infections, they help to identify antibody responses that correlate with protection from SARS-CoV-2.11 There are several types of serological tests, including neutralization test, chemiluminescent immunoassay (CLIA), enzyme-linked immunosorbent assay (ELISA), and lateral flow assay (LFA). Serological assays could be employed to identify individuals who have been infected with SARS-CoV-2 and were asymptomatic or had mild symptoms, which include nasal congestion, rhinitis, and sore throat, thereby providing a better understanding of how the virus is widely spread within a population.12 This study sought to study the antibody response three weeks post-infection and its persistence.
Place of study
This prospective cross-sectional study was conducted at the Institute of Microbiology, Madras Medical College, Chennai, Tamil Nadu, India.
Period of study
6 months (July 2020–December 2020).
Study design
Prospective cross-sectional study.
Ethics
Ethics Committee approval was obtained from the Institutional Ethics Committee of Madras Medical College, Chennai-3 (EC Reg. No. ECR/270/Inst./TN/2013/RR-16).
Statistical analysis
Statistical analysis was performed using SPSS 16. software The significance of the obtained results was determined using the chi-square test.
Adult patients (> 15 years of age) who were diagnosed as COVID-19-positive by RT-PCR three weeks after swab positivity were enrolled in the study. To evaluate the persistence of the antibody response, follow-up testing was performed at intervals of 3, 6, and 8 months.
Written consent was obtained after explaining the details of the study to all enrolled cases individually. Patients unwilling to provide consent and adult patients (> 15 years of age) who had a history of contact or symptoms suggestive of COVID-19 infection but were negative by PCR were excluded.
Under strict aseptic precautions, 4 mL of venous blood samples were collected and the serum was separated and stored at -20°C.
All collected samples were tested by electrochemiluminescence assay (Elecsys® kit) for in vitro qualitative determination of the total antibody titer to SARS-CoV-2 (nucleocapsid antigen), which is a test based on the principle of double-antigen sandwich assay.
Twenty microliters of serum samples were incubated with a mix of biotinylated and ruthenylated nucleocapsid (N) antigens. In the presence of the corresponding antibodies, double-antigen sandwich immune complexes were formed (9 minutes).
Double-antigen sandwich immune complexes bind to the solid phase via the interaction of biotin and streptavidin after the addition of streptavidin-coated microparticles. After an incubation period of 9 min, the reagent mixture was transferred to the measuring cell, where the microparticles magnetically captured onto the substances were subsequently removed. By applying voltage, electrochemiluminescence was induced, and the measurement was performed using a photomultiplier. The signal yield increased with the antibody titer. Based on the manufacturer’s instructions, the results were interpreted as reactive if the cut-off index was ≥ 1.0, and non-reactive if the cut-off index was ≥ 1.0.
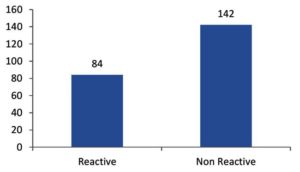
During the study period, 226 adult patients (> 15 years of age), 131 of whom were male and 95 female, diagnosed as COVID-19 positive by a positive RT-PCR test, were included in the study. Blood samples were collected three weeks after swab positivity and were tested for total antibodies against COVID-19. Out of the 226 samples, 84 were reactive and 142 were non-reactive.
Approximately 74 (57%) of the 129 symptomatic individuals and 10 (10%) of the asymptomatic individuals exhibited positive antibody responses. Applying the chi-square test, the antibody response was found to be significant in symptomatic individuals compared to that in asymptomatic individuals (p < 0.05).
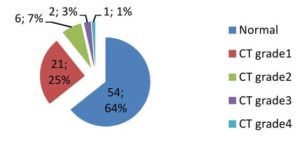
Among the reactive individuals, only 30 (36%) individuals came for the follow-up test after 3 months. The majority of them were under the category of grade 1 by CT chest examination (6%). Subsequently, at 6 and 8 months, 3 and 7 individuals, respectively, were lost to follow-up.
Out of 84 reactive individuals, 23 (27% ) underwent the complete follow-up. Although some were lost to follow-up, individuals who reported for the entire period were seropositive at the end of both 6 and 8 months. The seroconversion rate among various grades of CT changes was not statistically significant.
The first human case of COVID-19 was first reported by officials in Wuhan City, China, in December 2019, and it was found to be caused by a novel coronavirus causing COVID-19, subsequently named SARS-CoV-2.11 On 7 March 2020, a 45year old man who had returned from Oman with a history of fever, cough, and breathing difficulty was the first to test positive in the Indian state of Tamil Nadu. Following this case, there has been continuous reporting of SARS-CoV-2 positivity. To understand protective immunity, a serological assay based on the electrochemiluminescence assay principle was used in this study. The study included 226 adult patients (> 15 years of age) who were diagnosed as COVID-19-positive by RT-PCR and completed 3 weeks after swab positivity. Of these, 131 were males and 95 were females. RT-PCR is sensitive and specific for the diagnosis of COVID-19 early in the illness.13 An electrochemiluminescence assay method was used to assess the antibody response to nucleocapsid protein following infection in both symptomatic and asymptomatic individuals. Antibodies to the nucleocapsid protein are the most sensitive targets for the antibody response.14 There were 142 samples that were non-reactive and 84 that were reactive (Figure 1). In the study population, 129 (57%) individuals were symptomatic and 97 (43%) were asymptomatic. Approximately 74 of the 129 symptomatic individuals exhibited an antibody response compared to only 10 out of the 97 asymptomatic individuals. The antibody response was higher in the symptomatic individuals (57% vs. 10%; p < 0.05) than in those who were asymptomatic (Table 1). As the study involved only RT-PCR COVID-19 positives, recovery from infection in asymptomatic patients can be attributed to T-cell responses in the absence of humoral immunity. Asymptomatic positive subjects may benefit from some protective factors capable of limiting the spread of the virus within the body, or a more effective innate immune T cell-based response, as reported by Grifoni et al., 15 These factors could reduce the need for an adaptive response by the body, and consequently limit the production and persistence of antibodies.16 Tahmina et al.’s study involving 171 patients, of whom 108 were mildly symptomatic and 63 asymptomatic, also found a significant increase in the geometric mean titers of IgM, IgA, and IgG in mildly symptomatic patients compared to asymptomatic patients.17,18 In their study involving 214 patients, Jiang et al. observed seroconversion of immunoglobulin G in both asymptomatic and symptomatic patients, whereas immunoglobulin M seroconversion was less common in asymptomatic than in symptomatic patients (p-value < 0.001).19 With regard to the correlation of the antibody response with CT chest findings (Figure 2), of the 84 seropositive individuals, 54 had normal CTs, and the majority of cases (21 patients, or 25%) had CT grade 1 changes. Six (7%) exhibited grade 2, two (3%) had CT grade 3, and one (1%) had grade 4 changes. Disease severity has been associated with the magnitude and duration of the antibody response.20,21 The persistence of antibody response was studied by performing follow-up testing at 3, 6, and 8 months. Out of 74 symptomatic, antibody-reactive individuals, 30 were followed up for persistence of the antibody response at 3, 6, and 8 months (Table 2). At 6 months, 3 individuals were lost to follow-up, and at 8 months, 7 were lost to follow-up. The reason for the loss to follow-up could be related to travel restrictions and the financial burden on individuals during the pandemic. All of the followed up individuals were seropositive at the end of 3, 6, and 8 months. The seroconversion rate and antibody duration for SARS-CoV-2 vary significantly across studies, depending on the serological tests used and the disease stage.22,23 Dan et al. analyzed cross-sectional data describing the dynamics of SARS-CoV-2 memory B cells, CD8+ T cells, and CD4+ T cells for more than 6 months after infection. The authors found a high degree of heterogeneity in the magnitude of adaptive immune responses, which persisted into the immune memory phase to the virus.24,25 Gudbjartsson et al. showed that the magnitudes and the durations of the antibody responses against SARS-CoV-2 are heterogeneous and vary widely between individuals.26,27 In our study follow-up at 6 months, there was a decline in the antibody titer compared to the early months following post-infection. Although there was a decline in the total antibody response, complete disappearance of antibodies was not observed throughout the entire follow-up period. Studies showed that the antibody titers of patients with mild SARS-CoV-2 infection declined more quickly than those reported for SARS-CoV patients, and waning immunity was confirmed 5 months after infection.28 Choudhry et al. also reported waning of antibodies against nucleocapsid antigen, but not a complete disappearance after 16 weeks.29 The antibody response predicts both mid-term and long-term clinical outcomes, and consequently the use of monoclonal antibodies or boosting the antibody response with vaccination may be potential strategies to prevent COVID infection.30 Further follow-up of the persistence of antibodies was not carried out as COVID-19 vaccination was started and there currently are no methods to differentiate vaccine-induced antibodies from antibodies resulting from natural infection.
Table (1):
Antibody response in symptomatic vs asymptomatic.
| Category | Symptomatic(n=129) | Asymptomatic(n=97) |
|---|---|---|
| Reactive | 74 | 10 |
| Non Reactive | 55 | 87 |
| P value | 0.045 (P<.05)significant | |
Table (2):
Duration of Antibody response.
| Follow up month | CT grade1 | CT grade2 | CT grade3 | CT grade4 |
|---|---|---|---|---|
| 3mts(n=30) | 21 | 6 | 2 | 1 |
| 6mts(n=27) | 18 | 6 | 2 | 1 |
| 8mts(n=23) | 15 | 5 | 2 | 1 |
| P value | 0.999(not significant) | |||
Antibodies against SARS-CoV-2 persist for at least 8 months following infection. Despite the waning of antibody levels against the nucleocapsid antigen, there was no complete disappearance of antibodies. In addition to the antibody response, T-cell responses may play a role in limiting infection. As efforts are underway to vaccinate the majority of the adult population with two doses of vaccine, the findings of this study may be useful in making decisions regarding the timing of booster doses.
Limitations
Quantitative estimation of antibody responses and individual isotypes could not be carried out because of the non-availability of commercial kits and cost factors during the study period.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Ethical clearance was obtained from the Institutional Ethics Committee (IEC), Madras Medical College, Chennai, India (EC Reg .No.ECR/270/Inst./TN/2013/RR-16).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470-473.
Crossref - Coronavirus disease (COVID-19) pandemic,World Health Organisation. https://www.who.int › Diseases.Accessed July 1, 2020.
- Lu X, Chen Y, Bai B, et al. Immune responses against severe acute respiratory syndrome coronavirus induced by virus-like particles in mice. Immunology. 2007;122(4):496-502.
Crossref - Marchi S, Viviani S, Remarque EJ, et al. Characterization of antibody response in asymptomatic and symptomatic SARS-CoV-2 infection. PLoS ONE. 2021;16(7):e0253977.
Crossref - Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227-1230.
Crossref - Bai Z, Cao Y, Liu W, Li J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses. 2021;13(6):1115.
Crossref - Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848.
Crossref - Havers FP, Reed C, Lim T, et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180(12):1576-1586.
Crossref - To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565-574.
Crossref - Zhang X, Lu S, Li H, et al. Viral and Antibody Kinetics of COVID-19 Patients with Different Disease Severities in Acute and Convalescent Phases: A 6-Month Follow-Up Study. Virol Sin. 2020;35(6):820-829.
Crossref - Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033-1036.
Crossref - Norman M, Gilboa T, Ogata AF, Maley AM, Cohen L, Cai Y. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat Biomed Eng. 2020:1180-1187 (2020).
Crossref - Coronavirus Disease (COVID-19) Situation Reports, World Health Organisation https://www.who.int › diseases › novel-coronavirus-2019. Accessed August 13, 2020.
- urbelo PD, Riedo FX, Morishima C, et al. Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients. MedRxiv. 2020.
Crossref - Grifoni A, Weiskoff D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489-1501.
Crossref - Milani GP, Dioni L, Favero C, et al, UNICORN Consortium. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Sci Rep. 2020;10(1):20048.
Crossref - Shirina T, Bhuiyanb TR, Charles RC, Aminb S, Bhuiyane I, Kawser Z. Antibody responses after COVID-19 infection in patients who are mildly symptomatic or asymptomatic in Bangladesh. Int J Infect Dis. 2020;101:220-225.
Crossref - Grossberg AN, Koza LA, Ledreux A, et al. A multiplex chemiluminescent immunoassay for serological profiling of COVID-19-positive symptomatic and asymptomatic patients. Nat Commun. 2021;12:740-741.
Crossref - Jiang C, Wang Y, Hu M, et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Transl Immunol. 2020;9(9):e1182.
Crossref - Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiol. 2020;5(12):1598-1607.
Crossref - Whitcombe AL, McGregor R, Craigie A, et al. Comprehensive analysis of SARS-CoV-2 antibody dynamics in New Zealand. Clin Transl Immunol. 2021;10(3):e1261.
Crossref - Peghin M, De Martino M, Fabris M, et al. The fall in antibody responseto SARS-CoV-2: a longitudinal study of asymptomatic to critically ill patients up to 10months after recovery. J Clin Microbiol.. 2021;59(11):e01138-21.
Crossref - Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533-540.
Crossref - Dan JM, Mateus J, Kato Y, et a l.Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063.
Crossref - Hall V, Foulkes S, Charlett A, et al. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv. 2021.
Crossref - Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in patients with COVID-19. Sci Immunol. 2020;5(52):eabe5511.
Crossref - Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724-1734.
Crossref - Choe P, Kim K, Kang C, et al. Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection. Emerging Infectious Diseases. 2021;27(3):928-931.
Crossref - Choudhary HR, Parai D, Dash GC,et al. IgG antibody response against nucleocapsid and spike protein post-SARS-CoV-2 infection. Infection. 2021;49(5):1045-1048.
Crossref - Garcia-Abellan J, Padilla S, Fernandez-Gonzalez M, et al. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: a Longitudinal Study. J Clin Immunol. 2021;41(7):1490-1501.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.