ISSN: 0973-7510
E-ISSN: 2581-690X
All surfaces of human body are colonized by many microbial communities but gut is colonized by greater densities known as the microbiota or commensally microflora which is mainly influenced by the plant extracts in the diet. In this study, 4 different plant materials, the leaves of Camellia sinensis, Mentha piperita and Petroselinum crispum in addition to the Pimpinella anisum seeds were collected and extracted with either hot water or methanol. The antimicrobial activity was determined using agar well diffusion method. All the extracts showed antibacterial activity against some bacterial pathogens including Escherichia coli, Salmonella typhimurium, Pseudomonas earuginosa, Enterococcus faecalis, E. faecium Staphylococcus aureus and Streptococcus agalactiae which was used as a control. The water and methanol extracts of Camellia sinensis and the water extract of Pimpinella anisum and Petroselinum crispum showed significant lower antibacterial activity against all the tested probiotic bacteria like Lactobacillus and Bifidobacteria. MICs values of the water extracts of the 4 tested plants were recorded for all the tested bacterial pathogens in addition to the tested probiotic bacteria. Concerning the pathogenic bacteria, MIC was ranged from 50-250 µg/ml, 100-150 µg/ml, 150 µg/ml and 75-125 µg/ml for Camellia sinensis, Pimpinella anisum, Petroselinum crispum, respectively. Concerning the probiotics, the MIC of the 4 tested plants was greater than 250 µg/ml except for L. plantarum, where the MIC of Camellia sinensis was 250µg/ml. The presence of plant extracts slightly decrease the rate of growth L. acidophilus and the decrease was clear in case of Camellia sinensis> Mentha piperita > Pimpinella anisum> Petroselinum crispum. In conclusion, the tested plant extracts affect significantly the growth of pathogenic bacteria but the effect was lower on the tested gut bacteria, thus they can be used safely to improve human health
Plant extract, Gut microbiota, Mentha piperita, Pimpinella anisum, Petroselinum, Camellia sinensis.
More than 1000 different bacterial species and 1014 bacterial cells colonised the adult human gastrointestinal (GI) tract and among individuals, the count vary greatly according to dietary components and host age and health (Hooper et al. 2002, Eckburg et al. 2005). There is interactive associations and symbiotic relationship between these bacteria and the host cells. Within the tract, they have critical roles in motility, nutrient absorption, protection from invading GI pathogens, fermentation the unused substrates, production vitamins and conservation of mucosal immune function (Flint et al. 2007, Ley et al. 2008, Zoetendal et al. 2008). The bacteria break down the ingested polysaccharides to monosaccharides, which are then fermented to form short-chain fatty acids as a final metabolic product. In this shared environment, the host gains carbon and energy, and the bacteria are provided with glycans and protection. Unbalanced bacterial community in the gut plays a role in some diseases and gut disorders. There is an interest to remove gut disorders by influencing the composition and activities of resident microflora. Probiotics, are a group of bacterial genera, found mainly in some foods, known as functional foods, that protect against gut disorder by targeting particular groups of bacteria in the gut. Probiotics already present in the GI tract and prebiotic supplements as dietary substances may enhance their growth and development (Probert 2004). Certain carbohydrates such as arabinoxylan or other plant materials or extracts, which are prebiotics, are selectively metabolised by gut bacteria, thereby changing the gut ecosystem towards a more beneficial structure (Swennen et al. 2006). Brune et al. (2000) described the gut ecosystem as a programmed bioreactor with bacteria that degrade indigestible polysaccharides and alteration of the microbiota composition of the intestinal allowed to some bacterial pathogens to grow, multiply, colonize and initiate intestinal disorders. Some plant extracts and antibiotic may affect the intestinal physiology and inhibit or enhance microbiota in human and animals (Manzanilla et al., 2004). The aim of the present study was to evaluate the inhibitory effect of some used tradition plants on microbiota and some bacterial pathogens.
Plant collection and extraction
In this study, four medicinal plants including the leaves of Camellia sinensis, Mentha piperita and Petroselinum crispum in addition to the Pimpinella anisum seeds were collected and identified at Biology Department, Faculty of Science, KAU, Saudi Arabia. The collected samples were washed, dried, grinded and extracted with either hot water or methanol (Aly and Bafeel, 2010). About 50 g of each sample were macerated in 200 ml hot water or 200 ml methanol for 4 hours at room temperature, 22°C, and then the mixtures were filtered through muslin cloth filter paper. Further extraction of the residue was carried out and all extracts were collected and dried using either rotary evaporator for organic extracts or lyophilizer for water extracts. All dried extracts were kept in the deep freezer at -80°C until used for the antimicrobial susceptibility studies.
The tested bacteria
Clinical isolates of the Gram negative Escherichia coli, Salmonella typhimurium and Pseudomonas earuginosa, and the Gram positive Enterococcus faecalis, Enterococcus faecium, Staphylococcus aureus and Streptococcus agalactiae were obtained from the culture collection from King Fahd General Hospital, Jeddah, Saudi Arabia. Gut normal flora strains Lactobacillus acidophilus, L. bulgaricus, L. plantarum, Bifidobacterium and Streptococcus thermophilus were obtained from Culture collection Unit, Faculty of Agriculture, Ain Shams, Egypt.
The identification of all the used bacteria was confirmed using microscopic examination, Gram and spore stains, growth on selective medium and API20. Selective Strep Agar (Cat. no. A70) was used for Streptocooccus growth in presence of CO 2 for 24 hr., MRS medium was used for Lactobacillus and Bifidobacterium strains growth under aerobic and anaeronic conditions, respectively at 37°C for 2 days for Lactobacillus and 4 days for Bifidobacterium.
The antibacterial activity
All extracts were dissolved in DMSO and bacterial growth inhibition was tested by using agar well diffusion method while the MIC was determined by the micro dilution method. For each of the extract, multiple plates (3 replications) were prepared and the plates were then maintained for 2 h at room temperature to allow extract diffusion, incubated at 37°C for 24 h and the zones of inhibition were subsequently measured in mm (Mukherjee et al., 1995a, b). Dilution micromethod for MIC determination was used (de Paiva et al, 2003). The water extracts of the four tested plants were selected for further tests and to calculate their MIC by dilution method. This test was performed in sterile 96-well microplates the g dilution procedure. The microdilution was performed in 96-well microtiter plates with U-shaped wells. Each culture was grown in Müeller-Hinton broth for 12 h and the absorbance was adjusted to 0.5 McFarland turbidity (5×105 CFU/ml). Controls with 0.5 ml of only culture medium without plant extract was used. The wells were filled with 100 µl of sterile H2O and 100 µl of the plant extracts were added to the wells by serial two fold dilution from the suspension of plant extract stock solution. Each well was inoculated with 100 µl of 0.5 McFarland standard bacterial suspensions and one drop of phenol red solution was added to each well. All plates were covered, placed in plastic bags and incubated at 37°C for 24 h. The MIC was the lowest concentration of plant extracts that exhibited no bacterial growth (yellow color and followed by red color) by visual observations.
The effect of different water extracts of the 4 tested plants (250 µg/ml) on the growth of Lactobacillus acidophilus, grown in MRS broth medium (de Man et al., 1960) was determined by measuring the growth (absorbance at 540 nm ) each 2 hours up to 24 h by spectrophotometer and calculating log cfu/ml.
In Arab area, different plants were used traditionally as hot drinks. In this study, 4 different plant materials, the leaves of Camellia sinensis, Mentha piperita and Petroselinum crispum in addition to the Pimpinella anisum seeds were collected and extracted with either hot water or methanol. Table 1 showed the studied plants, their common names, the used plant part and the type of extraction. The antimicrobial activities of the two extracts of the tested plants were determined for some Gram positive and negative pathogens (Figure 1). The antibacterial activity of the tested aqueous extracts were determined against the tested bacterial pathogens and compared with that of Streptococcus agalactiae which was used as a control (Table 2). The water extract of Camellia sinensis showed significant antibacterial activity against both Enterococcus faecalis and E. faecium, while Pimpinella anisum extract showed significant antibacterial activity against Salmonella typhimurium and Pseudomonas earuginosa. The water extract of Petroselinum crispum has antibacterial activity against Escherichia coli, S. typhimurium, P. earuginosa and E. faecalis. Moreover, Mentha piperita recorded significant antibacterial activity against E. coli, S. typhimurium, P.earuginosa. The bacterial index showed that the maximum activity was recorded for water extract of Petroselinum crispum, followed by Pimpinella anisum and finally Camellia sinensis and Mentha piperita. Similarly, the methanolic extract of Camellia sinensis showed significant antibacterial activity against both E. faecalis and E. faecium, while the methanol extract of Pimpinella anisum showed significant abtibacterial activity against E. coli and Staphylococcus aureus (Table 3). Furthermore, Petroselinum crispum extract showed antibacterial activity against E. coli, P. earuginosa and Staphylococcus aureus, while Mentha piperita recorded significant antibacterial activity against E. coli, S. typhimurium, P. earuginosa, E. faecium and S. aureus. Bacterial index showed that the maximum activity was recorded for the methanol extract of Mentha piperita and Petroselinum crispum, followed by Camellia sinensis and finally Pimpinella anisum.
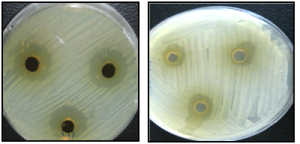 Fig. 1. Inhibitory effect of water extract Petroselinum crispum on Escherichia coli (A) and Streptococcus agalactiae (B).
Fig. 1. Inhibitory effect of water extract Petroselinum crispum on Escherichia coli (A) and Streptococcus agalactiae (B). Table (1):
The Used Medicinal Plants.
Scientific name |
Common name |
Family |
Used part |
Type of extraction |
|---|---|---|---|---|
Camellia sinensis |
Green tea |
Theaceae |
Leaves |
Hot water, methanol |
Mentha piperita |
Mint |
Labiatae |
Leaves |
Hot water, methanol |
Petroselinum crispum |
parsley |
Apiaceae |
Leaves |
Hot water, methanol |
Pimpinella anisum |
Anise |
Umbelliferae |
Seeds |
Hot water, methanol |
Table (2):
The Antibacterial activity of the aqueous extracts of 4 tested plant extracts against some bacterial pathogens.
| Tested extract Bacterial pathogen | Diameter of the inhibition zone (mm) | |||
|---|---|---|---|---|
| Camellia sinensis | Pimpinella anisum | Petroselinum crispum | Mentha piperita | |
| Escherichia coli | 11.4±0.0 | 14.0±0.7 | 21.6±0.1* | 11.0±0.1* |
| Salmonella typhimurium | 11.6±0.5 | 16.3±1.6* | 18.6±1.9* | 11.3±0.5* |
| Pseudomonas aeruginosa | 10.6±1.5 | 16.6±1.6* | 20.0±1.4* | 10.7±0.4* |
| Enterococcus faecalis | 15.0±1.0* | 12.6±0.9 | 19.3±1.0* | 12.7±0.1 |
| Enterococcus faecium | 15.3±1.1* | 12.3±1.0 | 14.3±1.8 | 13.6±1.6 |
| Staphylococcus aureus | 11.3±0.5 | 11.3±1.7 | 14.0±1.8 | 13.0±1.4 |
| Streptococcus agalactiae (control) | 11.6±0.5 | 11.6±0.7 | 14.3±1.0 | 13.3±1.5 |
| Bacterial index | 12.4 | 13.5 | 17.4 | 12.2 |
*: significant difference at p< 0.05
Table (3):
Antibacterial activity of the methanolic extracts of the 4 tested plant extracts against some tested bacterial pathogens.
| Tested extract Bacterial pathogen | Diameter of the inhibition zone (mm) | |||
|---|---|---|---|---|
| Camellia sinensis | Pimpinella anisum | Petroselinum crispum | Mentha piperita | |
| Escherichia coli | 17.6±0.0 | 16.0±0.0* | 19.6±0.5* | 17.0±1.0* |
| Salmonella typhimurium | 17.6±0.5 | 13.3±1.5 | 14.6±1.5 | 23.3±1.5* |
| Pseudomonas aeruginosa | 14.6±1.5 | 13.6±1.5 | 23.0±1.0* | 19.3±2.0* |
| Enterococcus faecalis | 13.0±1.0* | 13.6±0.5 | 14.3±1.5 | 14.6±1.1 |
| Enterococcus faecium | 12.3±1.1* | 15.3±2.0 | 14.3±1.5 | 17.0±1.0* |
| Staphylococcus aureus | 14.3±0.5 | 18.3±1.1* | 18.0±1.7* | 18.0±1.0* |
| Streptococcus agalactiae (control) | 17.0±0.5 | 15.6±0.5 | 13.3±1.5 | 12.3±0.5 |
| Bacterial index | 15.2 | 14.5 | 16.6 | 17.3 |
*: significant difference at p< 0.05
The effect of different water extracts of the 4 tested plants (250 µg/ml) on the growth of Lactobacillus acidophilus grown in MRS medium was determined each 2 hours up to 24 hours and compared to control medium, without plant extract (Figure 3). The used water extracts increase bacteria growth. In the control medium, the bacterial growth increased to 12 hours, then the rate of growth was decreased. The presence of plant extracts slightly decrease the rate of growth of L. acidophilus and the decrease was clear in case of Camellia sinensis>Mentha piperita >Pimpinella anisum>Petroselinum crispum(Figure 3).
 Fig. 2. MIC of the water extracts of Petroselinum crispum on Some Bacterial Pathogens using micromethod technique
Fig. 2. MIC of the water extracts of Petroselinum crispum on Some Bacterial Pathogens using micromethod techniqueLower activity of the water and methanolic extracts of the four tested plants was recorded against the probiotic bacteria (Lactobacillus acidophilus, L. bulgaricus, L. plantarum, Bifidobacterium and Streptococcus thermophilus) as shown in Table 4. Significant lower antibacterial activity was recorded for all tested water extracts against the tested probiotic bacteria compared to Streptococcus agalactiae as a control bacterium. Moreover, the methanol extract of Camellia sinensis, Pimpinella anisum and Petroselinum crispum showed significant lower antibacterial activity compared to control while Mentha piperita extract showed no significant antibacterial activity against all tested bacteria except Lactobacillus acidophilus. The bacterial index was higher for the methanol extracts compared to water extracts, thus water extracts was selected for determination of the MIC values for all the tested bacteria. The water and methanol extract of Camellia sinensis and the water extract of Pimpinella anisum and Petroselinum crispum showed significant lower antibacterial activity against all the tested probiotic bacteria. MICs values of the water extracts of the 4 tested plants were recorded for the test bacterial pathogens in addition to the tested probiotic bacteria (Table 5) using micromethod (Figure 2). Concerning the pathogenic bacteria, MIC was ranged from 50-250 µg/ml,100-150 µg/ml, 150 µg/ml and 75-125 µg/ml for Camellia sinensis, Pimpinella anisum, Petroselinum crispum, respectively. Concerning the probiotics, the MICs of the 4 tested plants were greater than 250 µg/ml except for L. plantarum, the MIC of Camellia sinensis was 250 µg/ml.
Table (4):
The Antibacterial activity of the aqueous and methanolic extracts of the 4 tested plant extracts against some gut bacteria.
| Tested extract Gut bacteria | Diameter of the inhibition zone (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Camellia sinensis | Pimpinella anisum | Petroselinum crispum | Mentha piperita | |||||
| Aqueous | methanol | Aqueous | methanol | Aqueous | methanol | Aqueous | methanol | |
| Lactobacillus acidophilus | 5.3±0.1* | 7.1±0.1* | 7.0±1.5* | 7.3±1.0* | 7.3±0.5* | 7.6±0.5* | 7.3±0.1* | 10.0±1.0* |
| Lactobacillus bulgaricus | 7.3±1.5* | 11.1±1.0* | 7.0±1.5* | 12.3±1.0 | 7.3±1.5* | 11.6±1.5 | 10.3±1.0* | 14.0±1.5 |
| Lactobacillus plantarum | 7.3±2.3* | 11.0±0.5* | 7.0±0.1* | 12.4±1.3 | 7.3±2.3* | 10.6±0.5* | 11.0±1.0 | 13.3±1.3 |
| Bifidobacterium | 9.9±1.1* | 11.6±1.5* | 7.0±1.5* | 12.6±1.0 | 9.9±1.1* | 11.0±1.0 | 10.3±0.3* | 13.9±1.5 |
| Streptococcus thermophilus | 9.0±0.5* | 10.6±1.0* | 9.0±1.2* | 10.6±0.9 | 7.0±0.5* | 11.3±1.5 | 11.0±0.3 | 14.0±0.2 |
| Streptococcus agalactiae (control) | 11.6±0.5 | 17.0±0.5 | 11.6±0.7 | 15.6±0.5 | 14.3±1.0 | 13.3±1.5 | 13.3±1.5 | 12.3±0.5 |
| Bacterial index | 8.6 | 11.0 | 9.0 | 11.8 | 7.8 | 11.2 | 10.6 | 13.5 |
*: significant difference at p< 0.05
Table (5):
MIC of the aqueous extracts of the 4 tested plant extracts against some tested bacterial pathogens and gut microbiota.
| Methanolic extracts | MIC (µg/ml) | ||||
|---|---|---|---|---|---|
| Camellia sinensis | Pimpinella anisum | Petroselinum crispum | Mentha piperita | Control antibiotic | |
| Escherichia coli | 150 | 100 | 150 | 100 | 3 |
| Salmonella typhimurium | 75 | 150 | 150 | 75 | 3 |
| Pseudomonas aeruginosa | 75 | 150 | 150 | 125 | 3 |
| Enterococcus faecalis | 125 | 150 | 150 | 75 | 1 |
| Enterococcus faecium | 250 | 100 | 150 | 75 | 1 |
| Staphylococcus aureus | 50 | 100 | 150 | 125 | 2 |
| Streptococcus agalactiae | 50 | 100 | 150 | 75 | 2 |
| Lactobacillus acidophilus | 250 | >250 | >250 | >250 | <1 |
| L. bulgaricus | >250 | >250 | >250 | >250 | 1 |
| L. plantarum | 250 | >250 | >250 | >250 | 1 |
| Bifidobacterium | >250 | >250 | >250 | >250 | <1 |
| Streptococcus thermophilus | >250 | >250 | >250 | >250 | 1 |
The effect of different water extracts of the 4 tested plants (250 µg/ml) on the growth of Lactobacillus acidophilus grown in MRS medium was determined each 2 hours up to 24 hours and compared to control medium, without plant extract (Figure 3). The used water extracts increase bacteria growth. In the control medium, the bacterial growth increased to 12 hours, then the rate of growth was decreased. The presence of plant extracts slightly decrease the rate of growth of L. acidophilus and the decrease was clear in case of Camellia sinensis> Mentha piperita > Pimpinella anisum> Petroselinum crispum (Figure 3).
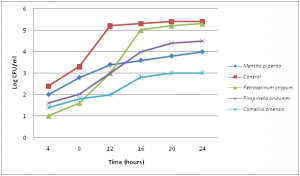 Fig. 3. Effect of different water extracts on the growth of Lactobacillus acidophilus, grown in MRS medium after 24 hours
Fig. 3. Effect of different water extracts on the growth of Lactobacillus acidophilus, grown in MRS medium after 24 hoursIntestinal normal flora are belonging to several major bacterial divisions, Firmicutes, Spirochaeates, Bacteroidetes, Proteobacteria, Actinobacteria and Fusobacteria (Rajilic-Stojanovic et al. 2007) and flora composition may be influenced by diet and stress (Mitsuoka, 1984). Previous investigations have shown that differences in intestinal bacteria cause many diseases (Hill, 1995). Plant extracts and/or probiotics are the most probable alternatives to antibiotics to the establishment of a beneficial intestinal population which antagonistic to harmful microbes (Gunal et al, 2006). Recently, much interest has focused on medicinal plants in relation to human health because they are largely free from harmful adverse effects and many plants are being investigated as natural sources of biologically important substances that may positively influence animal and human health (Aly and Bafeel, 2010, Aly et al., 2013). In this study, Camellia sinensis, Mentha piperita, Petroselinum crispum and Pimpinella anisum are traditionally used as drinks at least three times per day in Arabic region to improve human health. They are used to treat high cholesterol levels, cancer, rheumatoid and repair immune function due to the presence of powerful antioxidant e.g catechin polyphenols especially epigallocatechin gallate. Mentha piperita has many health and medicinal uses for thousands of years as a popular flavoring for food and drink. The infusions prepared with peppermint leaves was used in complementary and alternative medical therapy include: biliary disorders, dyspepsia, enteritis, flatulence, gastritis, intestinal colic, and spasms of the bile duct, gallbladder and gastrointestinal tract (McKay and Blumberg, 2006). Parsley leaves and root are high in iron content and rich in vitamins A, B, C, trace minerals, boron and fluoride might help against bone thinning and osteoporosis. Aniseeds are used as flavouring, digestive, carminative, and relief of gastrointestinal spasms. Consumption of aniseed in lactating women increases milk and also reliefs their infants from gastrointestinal problems (Zargari, 1996).
The aqueous extracts of the four studied plants showed antibacterial activity against the tested bacterial pathogens with MIC values ranged from 50-250 µg/ml. Higher activities were recorded for the methanolic extracts for the selected gut pathogens and probiotics, thus aqueous extracts were selected for more detail studies. Many others reported that extraction with organic solvents was more effective as compared to aqueous extraction and many previous studies reported that methanol was a better solvent for more consistent extraction of antimicrobial substances from medicinal plants as compared to other solvents such as water and ethanol (Ahmad et al., 1998, El Sayed and Aly, 2014).
The antimicrobial activity against bacterial pathogens was due to large number of active components in medical plants which may be effective against bacteria. Plants have several major components, including allicin, ajoene, thymol, and carvacrol, that have many biological activities e.g., antimicrobial, antioxidant, and antiseptic activities (Lee and Ahn, 1998) and the use of these material or the whole plant extract as food additives can decrease the number of intestinal pathogens (Pelicano et al., 2005). Camellia sinensis (Green tea) is a heterogeneous product, rich in various components such as caffeine, tannins, amino acids, vitamins and saponins, and increase Intestinal microorganisms that participate in normal physiological functions and decrease significantly that cause various diseases (Ahn et al., 1990 b). Extracts of leaves from the tea plant Camellia sinensis contain polyphenolic components with activity against a wide spectrum of microbes (Ahn et al., 1990a). Studies conducted over the last 20 years have shown that the green tea polyphenolic catechins can inhibit the growth of a wide range of Gram-positive and Gram-negative bacterial species. They are useful in control of common oral infections, such as dental caries and periodontal disease, the ²-lactam resistant Staphylococcus aureus, methicillin-resistant S. aureus (MRSA). Catechin gallates from green tea intercalate into phopsholipid bilayers and affect the bacterial cytoplasmic membrane functions.
In this study, no significant effects of the tested aqueous extracts were recorded against the microbial probiotics Lactobacillus and Bifidobacterium and the MIC values were more than 250 µg/ml. The two previous benefit bacterial isolates may have an antagonistic effect against human pathogens, thus their protection from the plant extract action is very important. They are important probiotics and used in the food industry. They have a range of beneficial health effects, including the inhibition of harmful bacteria and pathogens, the modulation of systemic and local immune responses, the vitamins production and improve the gut mucosal barrier. Many studies reported that the microbial probiotics have many beneficial effects (Gusils et al., 1999) including the competitive exclusion of pathogenic strains of Campylobacter jejuni ( Morishita et al., 1997) and E. coli (Watkins et al., 1982). In chickens, Lactobacillus acidophilus enhance the growth and viability of the other beneficial gut microflora (Hosoi et al., 2000), inhibit the pathogenic Escherichia coli and Salmonella enterica serovar Enteritidis (Pascual et al., 1999); and improved digestion and absorption of nutrients (Thomke and Elwinger, 1998). Previous studies have shown that Lactobacillus rhamnosus (Alander et al., 1999), Lactobacillus plantarum (West and Warner, 1988), Lactococcus lactis (Spelhaug and Harlander, 1989), and Pediococcus pentosaceus (Graham and McKay. 1985) inhibited the growth and development of Clostridium spp. Teo and Tan (2005) found five strains of lactic acid bacteria were antagonistic toward C. perfringens ATCC 13124 without production of a zone of inhibition while two strains of Bacillus subtilis, PB3 and PB6, exhibited antimicrobial activity against C. perfringens ATCC 13124. Moreover, Noodles from red bean stimulated the growth of microflora in the large intestine to 109-1010 cfu/ml but in the presence of plant extracts, garlic, onion and oregano, the count decreased to 108 cfu/ml (Gumienna et al., 2007).
Antimicrobial mechanisms of natural compounds found in herbs or spices have been discussed (Brul and Coot, 1999). Cinnamomum extract has excellent antibacterial activities and an inhibitory effects on the growth of enteric bacteria (E. coli O157:H7 and Salmonella typhimurium) due to prominent outer membrane disintegration activity and the increase in cytoplasmic membrane permeability to ATP (Helander et al., 1998). It has been reported that extracts from Panax ginseng not only enhanced the growth of bifidobacteria, but also inhibited selectively various clostridia (Ahn, 1990 b) Green tea extract has selective growth-inhibitory activity against various strains of clostridia including C. perfringens, C. dificile and C. paraputrijjicum (Ahn et al., 1990 a). They added that the daily intake of green tea might be expected to alter the growth and composition of the microbial community and to modulate the genesis of potentially harmful products such as carcinogenic N-nitroso compounds or aromatic steroids within the intestinal tract, thus protecting from a variety of diseases and helping to maintain optimal human health. In conclusion, the most common aqueous extracts of Camellia sinensis, Mentha piperita, Petroselinum crispum and Pimpinella anisum can be used to inhibit gut pathogens without any bad effects on gut benefit bacteria, Lactobacillus and Bifidobacterium.
ACKNOWLEDGMENTS
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (54/247/1433), the author, therefore, acknowledge with thanks DSR technical and financial support.
- Ahmad I., Mehmood Z. and Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. Journal of ethnopharmacology, 1998; 62: 183-193.
- Ahn YJ, Kim M, Yamamoto T, Fugisawa T, Mitsuoka T. Selective growth responses of human intestinal bacteria to Aruliuceue plant extracts. Microbial Ecology in Health and Disease, 1990b; 3: 169-175.
- Ahn YJ., Sakanakat S.,. Kim M J, Kawamurat T, Fujisawas T and Mltsuoka T. Effect of Green Tea Extract on Growth of Intestinal Bacteria. Microbial Ecology In Health and Disease, 1990a; 3: 335-338.
- Alander M., Satokari R., Korpela R., Saxelin M., Vilpponen-Salmela T., Mattila-Sandholm T., and von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosusGG, after oral consumption. Appl. Environ. Microbiol., 1999; 65:351-354.
- Aly MM and Bafeel S. Screening for antifungal activities of some medicinal plants used traditionally in Saudi Arabia. J. Appli. Anim. Res. 2010; 38: 39-44.
- Aly MM, Al-Ghamdi M, Bafeel SO and Khedr AM. Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Lavandula dentata and Lectranthus tenuiflorus, Collected From Al Baha Region, Saudi. Arabia. Life Sci J; 2013; 10(4): 3302-3309
- Bauer S, Tholen A, Overmann J, Brune A. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood- and soil-feeding termites by molecular and culture-dependent techniques. Arch Microbiol., 2000; 173(2):126-37.
- Brul B, Coot P. Reservative agents in foods mode of action and microbial resistance mechanisms. Inter J Food Microbiol, 1999; 50:1-17.
- de Paiva SR, Figueiredo MR, Aragao TV, and Kaplan MUC. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Memorias do Instituto Oswaldo Cruz, 2003; 98: 959–961.
- de Man, J.D.; Rogosa, M.; Sharpe, M.E. “A Medium for the Cultivation of Lactobacilli”. J Appl Bact., 1960; 23:130–135.
- Eckburg P., Bik E., Bernstein C., Purdom E., Dethlefsen L., Sargent M., et al. Diversity of the human intestinal microbial flora. Science, 2005; 308: 1635–1638.
- El Sayed H A and Aly M M. Antibacterial Activities of six medicinal plants used traditionally by Saudi people to treat common diseases. British Biotechnology Journal, 2014; 4(4): 499-510
- Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol.; 2007; 9:1101–1111.
- Graham D. C. and McKay L. L. Plasmid DNA in strains of Pediococcus cerevisiaeand Pediococcus pentosaceus. Appl. Environ. Microbiol., 1985; 50: 532
- Gumienna M., Lasik M., Czarnecki Z. Effect of plant extracts addition on phenolic compounds activity and intestinal microflora increase in the gastrointestinal tract model. Pol. J. Food Nutr. Sci., 2007; 57/4(A), 219–223
- Gunal, M, Yayli G, Kaya O, Karahan N, Sulak O. The effects of antibiotic growth promoter, probiotic or organic acid supplementation on performance, intestinal microflora and tissue of broilers. Int. J. Poult. Sci., 2006; 5:149–155
- Gusils C., S. N. Gonzalez, and Oliver G. Some probiotic properties of chicken lactobacilli. Can. J. Microbiol., 1999; 45: 981-987.
- Helander I M, Alakomi H, Kala K , Mattila-Sandholm T, Pol I, Smid E J, Gorris L G. and Wright A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590″3595
- Hill M.J. Role of gut bacteria in human toxicology and pharmacology. UK Taylor & Francis Ltd., 4 John St., London WC1N 2ET USA Taylor & Francis Inc., 1900 Frost Road, Suite 101, Bristol, PA 19007.
- Hooper L., Midwedt T., Gordon J. How host microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr., 2002; 22: 283–30.
- Hosoi, T., Ametani A., Kiuchi K. and Kaminogawa S. Improved growth and viability of lactobacilli in the presence of Bacillus subtilis(natto) or subtilin. Can. J. Microbiol, 2000; 46:892-897
- Lee H. S and Ahn Y. J. Growing-inhibiting effects of cinnamomun cassia bark-derived materials on human intestinal bacteria. J. Agric. Food Chem 1998: 46:8–12.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology—human gut microbes associated with obesity. Nature,2006; 444:1022–1023
- Manzanilla, E. G., Perez J. F., Martin M., Kamel C., Baucells F and Gasa J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 2004; 82:3210–3218.
- McKay D. L. and Blumberg J B. A Review of the Bioactivity and Potential Health Benefits of Peppermint Tea (Mentha piperita L.). Phytotherapy Research Phytother Res., 2006; 20: 619–63.
- Mitsuoka T. Taxonomy and ecology of bifidobacteria. Bifidobacteria and Microflora, 1984; 3(1): 11-28.
- Morishita, TY, Aye PP, Harr BS, Cobb CW, and Clifford JR. Evaluation of an avian-specific probiotic to reduce the colonization and shedding of Campylobacter jejuniin broilers. Avian Dis, 1997; 41:850-855.
- Mukherjee P.K., Balsubramanian R, Saha K., Pal M. and Saha B. P. Antibacterial efficiency of Nelumbo nucifera ( Nymphaeaceae) rhizome extract. Indian drugs, 1995a; 32: 274- 276.
- Mukherjee P.K., Giri S. N., Saha K., Pal M., and Saha B. P. Antifungal screening of Nelumbo nucifera (Nymphaeaceae) rhizome extract, Indian Journal of Microbiology, 1995b; 35:320-327.
- Pascual, M., Hugas M., Badiola J. I., Monfort J. M., and Garriga M. Lactobacillus salivariusCTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microbiol., 1999; 65: 4981-4986.
- Pelicano E. R. L., Souza P. A., Souza H. B. A., Figueiredo D. F., Boiago M. M., Carvalho S. R., and Bordon V. F. Intestinal mucosa development in broiler chickens fed natural growth promoters. Braz. J. Poult. Sci., 2005; 7: 221–229.
- Probert HM, Apajalahti JH, Rautonen N, Stowell J, Gibson GR. Polydextrose, lactitol, and fructo-oligosaccharide fermentation by colonic bacteria in a three-stage continuous culture system. Appl Environ Microbiol., 2004; 70(8):4505-11.
- Rajiliæ-Stojanoviæ M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S., de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology, 2011; 141: 1792–1801.
- Swennen K, Courtin CM, Delcour JA. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr.; 2006; 46(6)11-20.
- Teo A Y and Tan H, Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl Environ Microbiol., 2005; 71(8): 4185–4190.
- Thomke, A., and K. Elwinger. Growth promotants in feeding pigs and poultry. III. Alternatives to antibiotic growth promotants. Ann. Zootech., (Paris), 1998; 47:245-271.
- Watkins, B. A., Miller B. F., and Neil D. H. In vivoeffects of Lactobacillus acidophilus against pathogenic Escherichia coli in gnotobiotic chicks. Poult. Sci., 1982; 61: 1298-1308.
- West, C. A., and Warner PJ. Plantacin, B, a bacteriocin produced by Lactobacillus plantarumNCDO 1193. FEMS Microbiol. Lett., 1988; 49:163.
- Zargari A. (1996). Medicinal Plants. Tehran,: Tehran University Press; Iran.
- Zoetendal EG, Akkermans ADL and de Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human faecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol.; 1998; 64:3854–3859.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


