ISSN: 0973-7510
E-ISSN: 2581-690X
Monoterpenes, such as Geraniol (G), Geranyl acetate (GA), Citral (CT), Limonene (LN), and Linalool (LL), are the most widely used phytochemicals in the aroma, food, and pharmaceutical industries. Here, we screened several bacteria and fungi to assess their potential to biotransform the selected monoterpenes (G, GA, CT, LN, and LL) through the substrate toxicity test. Three bacteria Pseudomonas fluorescens MTCC2421, Streptococcus mutans MTCC497, and Escherichia coli were found to be resistant to G, GA, and LN while two P. aeruginosa, and S. epidermidis MTTC 435 to GA and LN. In general, all fungal strains did not show resistance to any of the monoterpenes used, except Candida albicans and Fusarium oxysporum, which were slightly resistant to lower concentrations (0.05-0.1%) of GA. Interestingly, none of the bacteria and fungi showed any resistance to CT. The maximum concentrations of monoterpenes to which bacteria exhibited resistance ranged from 0.05-0.2%. The growth and biomass profiles of bacteria revealed that P. fluorescens and S. mutans grew well in the presence of monoterpenes GA and LN. Based on this, Pseudomonas fluorescens was capable of biotransforming GA and LN, while S. mutans only LN. The biotransformation of GA by P. fluorescens produced G and LL on the day 5th and 7th of the incubation. Hence, the study revealed the three potential bacteria, which may be useful in producing new aromatic derivatives from selected monoterpenes through biotransformation.
Monoterpenes, Citral, Geraniol, Geranyl Acetate, Limonene, Biotransformation, Substrate Toxicity Test
Monoterpenes are C10-containing compounds that belong to the isoprenoids family of secondary metabolites. They are the main constituents of the essential oils of aromatic plants. They impart a unique aroma to the essential oils. Several monoterpenes such as geraniol (G), geranyl acetate (GA), citral (CT), limonene (LN), and linalool (LL) are highly popular and widely used in fragrances, cosmetics, hygiene, household products, food, and pharmaceuticals.1-4 They exhibited a wide range of biological activities such as antibacterial, analgesic, anti-inflammatory, anticancer, antidiabetic, antiobesity, and modulators of the gut microbiota.1,5,6 Citral and linalool are also involved in the synthesis of vitamins A, E, and ionones.7-9 At present, many reports are being published on geraniol and citral, demonstrating their potential anticancer effects and their scope in alternative cancer therapy.10
With the rapidly increasing importance of monoterpenes in the production of human fragrances and tastes, demand for new monoterpenes on the market will continue to rise. At present, most of the flavouring products available in the market are produced by chemical synthesis. Besides, many such products are also isolated from plants by solvent extraction and hydro-distillation. However, chemical synthesis has several demerits, such as the formation of undesirable chemical mixtures, inappropriate high operating temperatures, and the side or adverse effects of chemically synthesized products. In view of this, consumers now prefer products, which are produced by green synthesis to chemically synthesized products. However, in plants, these products are produced in a very limited quantity, so plants may not be reliable sources for large-scale extraction of such compounds. Therefore, microbial biotransformation relying on microorganisms and their biocatalysts has been proposed as an alternative approach for the production of novel monoterpenoids. This has several advantages over chemical processes. Recently, Mittal et al. studied the biotransformation of monoterpenes by microorganisms and plant cell and organ cultures.11 Considering the increasing and vastly varied significance of monoterpenes viz., geraniol (G), geranyl acetate (GA), citral (CT), limonene (LN), and linalool (LL) in the aroma industry and expanding the field of microbial biotransformation, the present study has been undertaken to screen monoterpene resistant microorganisms from soil samples, which may be used for the production of important monoterpenes through biotechnological approaches. The monoterpene-resistant microbes were screened using the substrate toxicity test.
Chemicals
Authentic geraniol, geranyl acetate, citral, limonene, and linalool were procured from Sigma-Aldrich, India.
Microorganisms
Ten bacteria namely Escherichia coli (MTCC901), Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas putida, Staphylococcus aureus (MTCC96), Streptococcus mutans MTCC497, Staphylococcus epidermidis MTTC 435, Shigella boydii MCC 2408, Acinetobacter baumannii, Bacillus mycoides and the three fungi Alternaria brassicicola, Fusarium oxysporum and Candida albicans were obtained from the CSIR-Institute of Microbial Technology, Chandigarh, India. Bacteria were inoculated on nutrient agar (NAM) and fungal cultures on potato dextrose agar (PDA). Pure colonies were sub-cultured and stored on slant agar at 4°C and 80% glycerol stocks at -20°C.
Substrate-toxicity test
Substrate toxicity was performed to screen monoterpene-resistant microorganisms in accordance with previous methods.12,13 The culture plates were prepared by displacing 30 ml sterilized NAM in pre-sterilized Petri dishes. Each 1 ml (1.0 x 105 CFU/ml) inoculum is evenly distributed to the agar medium with a sterile glass rod. Wells were bored in agar plates using a sterile cork borer (6 mm). To the wells, 25, 50, 75, and 100 µl of monoterpenes equal to concentrations 0.05-0.2% were added. Bacterial and fungal plates were incubated separately at 37°C, 24 h, and 27°C, 48 h, respectively. Simultaneously, positive and negative control plates were also incubated. The plates were observed and the mean diameter of the inhibition zone (mm) was measured. Each experiment was performed in triplicate.
Microbial growth rates
The cultures were incubated in a rotary shaker at 30°C and 150 rpm for seven days to measure microbial growth. Bacterial growth rates were measured in terms of absorbance at 660 nm. The biomass of fungal strains was filtered and assessed by wet weight. Finally, the microbiological growths were compared to a control without monoterpenes.
Biotransformation assay
Biotransformation of GA by P. fluorescens was performed in 150-mL Erlen Mayer flasks containing 50 ml nutrient broth medium (yeast extract 2 g L−1, beef extract gL−1, peptone 5gL−1, sodium chloride 5 g L−1, pH 7). An inoculum of P. fluorescens and GA (each 25μl) was added to the nutrient medium and the bacteria were allowed to grow on an orbital shaker at 150 rpm and 37°C. Samples (5 ml) were aseptically taken from the cultures at regular intervals (24 h) for 8 days. Two controls were used: a media-only (without the inoculums and substrate), and bacterial control without substrate.
Biotransformation products of GA
The biotransformation products from the samples were extracted after removing the bacterial cells by centrifugation. The supernatant was extracted thrice with 25 ml of diethyl ether. Thus, the pooled extract was washed three times with distilled water (10 ml), dried over anhydrous sodium sulfate, and filtered through Whatman paper No. 1. The sample was evaporated to dryness and subjected to thin-layer chromatographic separation (TLC).14 Samples and standards were loaded directly onto silica gel-G plates. The plates were developed in a solvent system consisting of toluene: ethyl acetate (96:4 v/v) at 4°C. The plates were then removed and dried at room temperature. Spots were visualized by exposing plates to iodine vapour. Identification of spots was done by comparing relative frontal (Rf) values of the standards used. Analysis of the biotransformation products was also performed by gas chromatography-mass spectrometry (GC-MS).
Statistical analysis
The mean and standard deviation of minimum inhibition zone (MIZ) diameter (mm) were calculated based on percent zone reduction in comparison to the control plate.
Biotransformation potential of microbes
The results of the substrate toxicity tests are presented in Tables 1 and 2. Five of the ten bacterial strains P. fluorescens MTCC2421, P. aeruginosa, S. mutans MTCC497, S. epidermidis MTTC435P, and E. coli were found to be resistant while the remaining five Shigella boydii MTCC2408, P. putida, Acinetobactor baumannii, Bacillus mycoides, and S. aureus highly sensitive to all monoterpenes used. Four bacteria P. fluorescens MTCC2421, P. aeruginosa, S. mutans MTCC497, and E. coli, showed resistance to GA and LN at all concentrations 0.05-0.2%. Three bacteria including P. fluorescens MTCC2421 and E. coli and S. epidermidis MTTC435P showed resistance to both G and LL, whereas the other three P. aeruginosa, S. mutans MTCC497, and E. coli were found sensitive to G. Among all, only S. epidermidis MTTC435P was susceptible to GA and LN. Three other P. fluorescens MTCC2421, P. aeruginosa, S. mutans MTCC497 were highly sensitive to linalool. Interestingly, all bacterial strains were susceptible to CT at all concentrations from 0.05-0.2% but three of them namely P. fluorescens MTCC2421, E. coli, and P. putida were highly susceptible with the zone of inhibition values of 30-90 mm (Table 1). The toxicity assay revealed that all the fungal strains were very sensitive to all monoterpenes used; however,
C. albicans and F. oxysporum showed little resistance to GA (0.05%) (Table 2).
Table (1):
Monoterpene-resistant behavior of the bacterial strains
| Bacterial Strains | Concentration (%) | Zone of inhibition (mm) | ||||
|---|---|---|---|---|---|---|
| Geraniol | Geranyl Acetate | Citral | Linalool | Limonene | ||
| Pseudomonas fluroscens MTCC 2421 | 0.05 | NO | NO | 35 | 15 | NO |
| 0.1 | NO | NO | 45 | 20 | NO | |
| 0.15 | NO | NO | 55 | 26 | NO | |
| 0.2 | NO | NO | 78 | 32 | NO | |
| P. aeruginosa | 0.05 | 15 | No | 30 | 13 | NO |
| 0.1 | 20 | No | 38 | 20 | NO | |
| 0.15 | 28 | No | 45 | 26 | NO | |
| 0.2 | 36 | No | 45 | 26 | NO | |
| Staphylococcus epidermidis MTTC 435 | 0.05 | No | 15 | 24 | No | 13 |
| 0.1 | No | 21 | 33 | No | 20 | |
| 0.15 | No | 29 | 42 | No | 25 | |
| 0.2 | No | 35 | 48 | No | 29 | |
| Streptococcus mutans MTCC497 | 0.05 | No | No | 35 | 12 | No |
| 0.1 | No | No | 40 | 16 | No | |
| 0.15 | No | No | 40 | 20 | No | |
| 0.2 | No | No | 50 | 20 | No | |
| E. coli | 0.05 | 11 | No | 40 | No | No |
| 0.1 | 20 | No | 60 | No | No | |
| 0.15 | 32 | No | 90 | No | No | |
| 0.2 | 32 | No | 90 | No | No | |
| Shigella boydii MTCC2408 | 0.05 | 11 | No | 18 | 18 | 25 |
| 0.1 | 15 | 10 | 25 | 28 | 25 | |
| 0.15 | 20 | 16 | 30 | 40 | 25 | |
| 0.2 | 25 | 25 | 30 | 40 | 25 | |
| Acinetobactor baumannii | 0.05 | 10 | No | 15 | 24 | No |
| 0.1 | 18 | 15 | 25 | 30 | 1 | |
| 0.15 | 20 | 20 | 25 | 30 | 15 | |
| 0.2 | 28 | 20 | 30 | 35 | 15 | |
| S. aureus | 0.05 | 23 | No | 20 | 20 | 20 |
| 0.1 | 15 | No | 20 | 20 | 20 | |
| 0.15 | 15 | 15 | 20 | 24 | 25 | |
| 0.2 | 20 | 15 | 30 | 24 | 25 | |
| P. putida | 0.05 | 14 | 13 | 30 | 20 | 18 |
| 0.1 | 20 | 13 | 40 | 20 | 24 | |
| 0.15 | 27 | 19 | 50 | 24 | 30 | |
| 0.2 | 35 | 26 | 60 | 24 | 30 | |
| Bacillus mycoides | 0.05 | 14 | 12 | 20 | 20 | 20 |
| 0.1 | 26 | 25 | 20 | 20 | 20 | |
| 0.15 | 35 | 25 | 20 | 24 | 25 | |
| 0.2 | 48 | 25 | 20 | 24 | 25 | |
Table (2):
Monoterpene-resistant behavior of the fungal strains
| Fungal Strains | Concentration (%) | Zone of inhibition (mm) | ||||
|---|---|---|---|---|---|---|
| Geraniol | Geranyl Acetate | Citral | Linalool | Limonene | ||
| Candida albicans | 0.05 | 14 | No | 40 | No | 18 |
| 0.1 | 20 | No | 50 | 15 | 25 | |
| 0.15 | 24 | 15 | 65 | 20 | 32 | |
| 0.2 | 30 | 15 | 75 | 20 | 38 | |
| Alternaria brassicicola | 0.05 | 25 | 45 | 65 | 65 | 65 |
| 0.1 | 33 | 54 | 70 | 76 | 78 | |
| 0.15 | 45 | 69 | 90 | 90 | 90 | |
| 0.2 | 60 | 80 | 90 | 90 | 90 | |
| Fusarium oxysporum | 0.05 | 15 | No | 35 | 30 | 25 |
| 0.1 | 20 | No | 45 | 42 | 25 | |
| 0.15 | 20 | 14 | 60 | 50 | 36 | |
| 0.2 | 20 | 14 | 80 | 80 | 42 | |
Analysis of biomass profiles
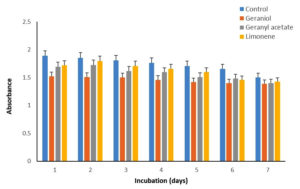
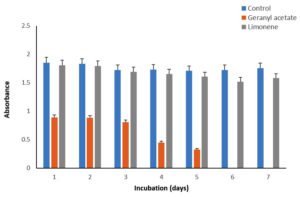
Biomass of P. fluorescens, P. aeruginosa, and S. mutans accumulated in the media in the presence of G, GA, and LN was measured recording the absorbance at 660 nm and compared with the control (Figure 1-3). For P. fluorescens MTCC242, the biomass accumulated was highest at ~100% during 1-4 days in the presence of GA and LN, which slightly decreased by 4% in GA but increased by 13% in LN on day 7 as compared to the control (Table 3). However, the biomass decreased significantly from 35 to 56% in the presence of G during the incubation period of 1-7 days. We did not observe any biomass accumulation for P. aeruginosa during the incubation period in the presence of G (Table 2). Overall, the biomass of bacteria declined significantly by 50-100% in the presence of GA and decreased by a comparatively very low margin of 10% in the presence of LN. In the case of S. mutans, the biomass was highest at ~118% in the presence of GA and LN on day 7 compared to the control. The biomass, however, decreased from 10 to 21% in the presence of G and LN from 1-4 days of incubation and again increased by 100% and 118% at day 7 (Table 3). Thus, these results revealed that P. fluorescens MTCC242 and S. mutans are suitable for the biotransformation of GA and LN and P. aeruginosa for LN.
Table (3):
Biomass (%) of monoterpene-resistant bacteria
| Treatment | Days | Biomass accumulation (%) | ||
|---|---|---|---|---|
| P. fluorescens MTCC 2421 | P. aeruginosa | S. mutans MTCC497 | ||
| Control | 1st | 100 | 100 | 100 |
| 4th | 88 | 94 | 95 | |
| 7th | 82 | 94 | 79 | |
| Geraniol | 1st | 64 | 00 | 79 |
| 4th | 53 | 00 | 83 | |
| 7th | 44 | 00 | 100 | |
| Geranyl acetate | 1st | 100 | 49 | 100 |
| 4th | 100 | 28 | 99 | |
| 7th | 96 | 00 | 118 | |
| Limonene | 1st | 100 | 89 | 89 |
| 4th | 87 | 89 | 87 | |
| 7th | 113 | 100% | 118 | |
Biotransformation of GA
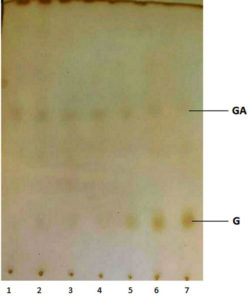
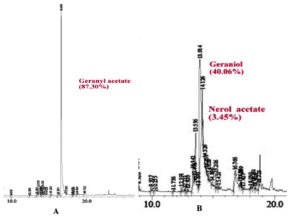
The main product of the biotransformation of GA by P. fluorescens was G (Figure 4). Geraniol was first detected on the day 5th of incubation, which was transformed into LL on the 7th day (Figure 4). In addition, some other products were produced, which could not be resolved on TLC, most likely they were hydrocarbons. The rate of biotransformation of GA varied with the incubation time. On the day 5th, the biotransformation of GA using P. fluorescens produced 50% geraniol. The presence of GA and G was further confirmed by GC-MS (Figure 5).
Figure 4. A thin-layer chromatogram of the biotransformation products of geranyl acetate. Lines 1-7 represent the incubation period from 1-7 days
Microbial biotransformation is a fast-growing alternative method of chemical synthesis for the production of many human products such as flavors, fragrances, food additives, and more. This method relies on microbes (bacteria, fungi, and yeast) and their enzymes, which are capable of transforming selected compounds into desirable products with multiple benefits. Since the availability of potential microbes is the first essential requirement for any biotransformation, in the present study, we screened a few bacteria that can transform the monoterpenes G, GA, LL, and LN through a substrate toxicity assay.
Results of the toxicity assay revealed that microbes had varying degrees of tolerance to the selected monoterpenes used at concentrations from 0.05-0.2% (Tables 1 and 2). It was found that P. fluorescens and S. epidermidis MTTC 43 effectively tolerated G (0.2%), while others were unable to tolerate >0.05% G. The toxicity test indicated that P. aeruginosa, P. fluorescens, S. mutans MTCC497, and E. coli MTCC901 tolerated 0.05-0.2% GA while S. boydii MCC2408, A. baumannii, and S. aureus could survive only in the presence of 0.05% GA. Two bacterial strains S. epidermidis MTTC 435 and E. coli MTCC901 grew well in the presence of 0.05-0.2% LL, whereas the other four P. aeruginosa, P. fluorescens, S. mutans MTCC497, and E. coli MTCC901 in 0.05-0.2% LN. Fungal strains C. albicans and F. oxysporum were more sensitive and could survive only in 0.05-0.1% GA (Table 2). These results revealed a correlation between microbial growth and monoterpene concentrations. In general, microbes exhibited resistance to varying concentrations (0.05 to 0.2%) of monoterpenes. Hence, any of these concentrations may be used to perform the microbial biotransformation of the monoterpenes. Several previous studies have also suggested a concentration range of monoterpenes from 0.1 to 0.2% most suitable for their biotransformation by Pseudomonas, Saccharomyces spp. and Penicillium, Aspergillus spp.13,15-17
Although simple microbial resistance to monoterpenes with added carbon sources does not guarantee high biotransformation activity, it is an essential property of a biotransformation agent. Therefore, we performed initial physiological studies to characterize microbial growth behaviour in the presence of monoterpenes. Two bacteria P. fluorescens and S. mutans showed the best growth profiles in the presence of GA. The biomass content of these bacteria was almost equal to the control throughout the incubation of 1-7 days. However, the biomass of P. aeruginosa significantly declined by 51-100%. These results suggest the rapid consumption of GA in P. fluorescens, S. mutans, and P. aeruginosa, which are most likely to have substrate-degrading metabolic pathways.15 The biomass is directly proportional to the growth rates, therefore, the higher the biomass the higher will be the growth. Here, the maximum microbial growth was recorded within the first two days of incubation compared to the control. Fungal growth was reduced only at the lower concentrations (0.05%) of GA on day 1 of incubation (Table 2).
Thus, the substrate toxicity test and biomass accumulation profiles suggest that P. fluorescens and S. mutans MTCC497, P. aeruginosa have the potential for biotransformation of GA and LN. Previous studies have reported that P. fluorescens and P. putida biotransformed limonene into limonene-1,2-oxide and perillyl alcohol.18,19 In contrast, in the present study, P. putida was found to be sensitive to limonene. Besides the biotransformation of GA and LN, the biotransformation of geraniol can be carried out by S. mutans MTCC497 and P. fluorescens. Earlier, we reported an enzyme, geranyl acetate esterase (GAE) from lemongrass leaves that catalyzes the biotransformation of GA into G.20 However, in the literature, no report is available on the biotransformation of GA by microbial enzymes. Here, we carried out the biotransformation of GA by P. fluorescens producing G, LL, and other products (Figures 4 and 5). This action of P. fluorescens can be attributed to homologous esterase and synthase enzymes. In accordance with a previous study, optimization of several parameters like the catalyst, reaction medium, stirring rate, molar ratio, and temperature is being carried out to improve the efficiency of the microbial biotransformation system.21 Certainly, the outcomes of this study may be used to carry out the biotransformation of geranyl acetate, geraniol, and other monoterpenes to produce newer commercial aromatic derivatives.
The present study revealed three potential bacteria P. fluorescens, S. mutans, and P. aeruginosa with an ability to biotransform GA, G, and LN. However, none of the fungi was found capable of biotransforming the selected monoterpenes. The biotransformation with various monoterpenes can be carried out utilizing particular bacteria in order to choose the finest strains beneficial for industrial applications. Thus, the current work underlines the importance of the screening of microorganisms as the first step in the biotransformation processes.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Ashok Kumar Chauhan, Founder President and Mr. Atul Chauhan, Chancellor of Amity University, Uttar Pradesh, Noida, India, for providing the necessary facilities and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Ganjewala D, Gupta AK. Lemongrass (Cymbopogon flexuosus Steud.) Wats essential oil: Overview and biological activities. Recent Prog Med Plants 2013;37:235-271.
- Rehman R, Asif Hanif M, Mushtaq Z, Mochona B, Qi X. Biosynthetic factories of essential oils: The Aromatic Plants. Nat Prod Che Res. 2016;04(04):17-160.
Crossref - Sommano SR, Chittasupho C, Ruksiriwanich W, Jantrawut P. The Cannabis terpenes. Molecules. 2020;25(24):5792-5794.
Crossref - Maczka W, Winska K, Grabarczyk M. One Hundred Faces of Geraniol. Molecules. 2020:25(4):3303.
Crossref - Zielinska-Blajet M, Feder-Kubis J. Monoterpenes and their derivatives-Recent development in biological and medical applications. Int J Mol Sci. 2020; 21(19):7078.
Crossref - Alvarenga DJFR, Genaro B, Costa BL, et al. Monoterpenes: current knowledge on food source, metabolism, and health effects. Crit Rev Food Sci Nutr. 2023;63(10):1352-1389.
- Sharma S, Habib S, Sahu D, Gupta J. Chemical properties and therapeutic potential of citral, a monoterpene isolated from lemongrass. Med Chem. 2021;17(1):2-12.
Crossref - Kamatou GPP, Viljoen AM. Linalool- A review of a biologically active compound of commercial importance. Nat Prod Comm. 2008;3:1183-1192.
Crossref - An Q, Ren JN, Li X, Fan G, et al. Recent updates on bioactive properties of linalool. Food Func. 2021;21:10370-10389.
Crossref - Mukarram M, Choudhary S, Khan MA, et al. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants. 2021.
Crossref - Mittal R, Srivastava G, Ganjewala D. An update on the progress of microbial biotransformation of commercial monoterpenes. Z Naturforsch C J Biosci. 2022;77(5-6):225-240.
Crossref - Bier MCJ, Poletto S, Soccol VT, Soccol CR, Medeiros ABP. Isolation and screening of microorganisms with potential for biotransformation of terpenic substrates. Braz Arch Biol Technol. 2011;54(5):1019-1026.
Crossref - Rottava I, Cortina PF, Grando CE, et al. Isolation and screening of microorganisms for R-(+)-Limonene and (−)-β-Pinene biotransformation. Appl Biochem Biotechnol. 2010;162(3):719-732.
Crossref - Lees TM, DeMuria PJ. A simple method for the preparation of thin layer chromatography plates. J Chromat. 1962;8:108-109.
Crossref - Deepthi Priya K, Petkar M, Chowdary GV. Isolation, screening and Identification of terpene resistant microorganisms from decayed yellow orange Citrus fruits. Res Rev: J Pharm Pharm Sci. 2014;3(1):12-21.
- Molina G, Bution ML, Bicas JL, Dolder MAH, et al. Comparative study of the bioconversion process using R-(+)- and S-(-)-limonene as substrates for Fusarium oxysporum 152B. Food Chem. 2015;174:606-613.
Crossref - Bicas JL, Fontanille P, Pastore GM, Larroche C. A bioprocess for the production of high concentrations of R-(+)-a-terpineol from R-(+)-limonene. Process Biochem. 2010;45(4):481-486.
Crossref - Bicas JL, Fontanille P, Pastore GM, Larroche C. Characterization of monoterpene biotransformation in two Pseudomonads. J Appl Microbiol. 2008;105(6):1991-2001.
Crossref - Chatterjee T, Bhattacharyya D. Biotransformation of limonene by Pseudomonas putida. Appl Microbiol Biotechnol. 2001;55(5):541-6.
Crossref - Ganjewala D, Luthra R. Geranyl acetate esterase controls and regulates the level of geraniol in lemongrass (Cymbopogon flexuosus nees ex steud.) mutant cv. GRL-1 leaves. Z Naturforsch C J Biosci. 2009;64(3-4): 251-259.
Crossref - Yadav GD, Kamble MP. A green process for synthesis of geraniol esters by immobilized lipase from candida antarctica B fraction in non-aqueous reaction media: Optimization and kinetic modeling. Int J Chem React Engg. 2018;16(7).
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.