ISSN: 0973-7510
E-ISSN: 2581-690X
Different Bacillus strains were isolated and selected to assess their capability to inhibition of rice soil borne pathogens and evaluated in terms of plant growth promotion activities. Out of seventeen strains, four strains B-1, B-2, B-3 and B-4 showed strongly antagonistic activities against pathogens and showed significant plant growth promoting (PGP) activity. Based on the morphological and several biochemical approaches like P-solubilization, IAA production, HCN production, different specific C-utilization, catalase, oxidase activities and mechanism of antagonism was studied through protease and chitinase activities, all these six isolates were identified as Bacillus sp. All the strains showed strongly inhibited (upto 90%) the growth of phytopathogens like Rhizoctonia solani, Sclerotium rolfsii and Sclerotium oryzae invitro. For Plant growth promoting assay, strains B-1, B-2 and B-3 were capable of enhancing the vegetative growth and yield parameters (shoot/root length, biomass, chlorophyll content and yield) and showed significant increase than non-inoculated control plants. The present findings suggest that these three indigenous isolates may be exploited as a potential bioinoculant agent for biocontrol as well as plant growth promoter.
Bacillus; phytopathogens; Rice; R. Solani; S. Rolfsii; S. oryzae.
Due to uncontrolled population growth the demand for rice in India is increasing (Joshi et al. 2009), so farmers heavily dependent on the use of high chemical fertilizers and pesticides to achieve the higher yields (Tilman et al. 2002). This dependence on chemical fertilizers and fungicides associated with problems such as environmental pollution, health hazards, interruption of natural ecological nutrient cycling and destruction ecological communities especially on soil microflora that directly supports the soil and crop health (Ramirez et al. 2010). Plant diseases which lead to the loss of crops are one of the major concerns in the agricultural scenario in India and there is need to control the diseases caused by the different pathogens. like soil borne and foliar, soil borne pathogens inflicts many diseases on rice like stem rot, seedling blight and sheath blight caused by Sclerotium rolfsii, Sclerotium oryzae and Rhizoctonia solani, respectively and causes loss of yield. A variety of alternative approaches have also been considered for control of pathogens (Ajilogba et al. 2013; Compant et al. 2005; Karlidag et al. 2007; Foldes et al. 2000; Leelasuphakul et al. 2008). Hence crop production and diseases management have to be achieved with fewer detrimental inputs or through biofertilizers and biocontrol agents (BCA) is important for improving and sustaining the crop productivity (Roberts et al. 1988; Leelasuphakul et al. 2008; Sethi and Adhikary, 2009a; Sethi and Adhikary, 2009b; Karlidag et al. 2007; Foldes et al. 2000). Several microorganisms are being used in controls of pest and diseases of various crops included Trichoderma, Pseudomonas and Bacillus sp. Rhizospheric Bacillus subtilis based biocontrol agents are quite important in the management of pest and plant diseases (Jacobsen et al. 2004; Leelasuphakul et al. 2008; Ajilogba et al. 2013) is also known as effective biocontrol agent against several soil borne pathogens indicating their effectiveness in inducing disease resistance in the crop plants in addition; the increased growth response induced by these species has also been reported for many kinds of crops (Compant et al. 2005; Foldes et al. 2000; Karlidag et al. 2007). It was found that the efficient strains from out side a particular agro-climatic zone often fail to achieve the desired result as plant growth promoter (Sethi and Adhikary, 2014; Sethi and Adhikary, 2015; Azad, 1994). Therefore it becomes necessary to isolate and screen the native Bacillus strain and testing the efficacy of biocontrol and plant growth promoting activity.
Therefore in this present study the efficacies of indigenous Bacillus strains isolated from rice rhizospheric and non-rhizospheric soil of different regions of Odisha and compare their plant growth promoting and biocontrol activities against R. Solani, S. rolfsi, S. oryzae of rice plant.
Isolation and maintenance of Bacillus sp.
Soil samples were collected from rhizosphere and non-rhizospheric soils in and around Odisha. Bacillus sp. was isolated by soil dilution method. Soil samples (10g) from rice rhizosphere transferred to 90 ml sterile distilled water and mixed thoroughly by shaking the flask on a rotatory shaker for 5 min. After serial dilution 0.1 ml suspension was spread over presterilized and cooled down nutrient agar plates in triplicates. The inoculated plates were incubated at 30 ± 2 æ%C for 2days. Rough and abundant colonies with waxy growth and irregular spreading edge were obtained and maintained on the Nutrient Agar Medium and kept at 40C for further use.
Physiological and biochemical characterization of isolates were performed by using HiBacillus Identification Kit KB013 and Hi Carbohydrate TM Kit Hi25TM (Himedia) followed by as per the manufacture’s instruction. The culture strains were identified following the Bergey’s Manual of Determinative Bacteriology15.
Bacillus sp. inoculum preparation
The inoculum preparation was carried out according to the method described (Sethi and Adhikary, 2009a; Sethi and Adhikary, 2009b). Loopfuls of each isolated bacillus sp. from 2-3 days old cultures were transferred separately to 50 ml nutrient broth (NB) medium and incubated overnight at 28±2°C for 2-3 days. Viability was confirmed by taking loopful of cultures and restreaked on nutrient agar medium. This inoculum was prepared to be used in vitro for antifungal activities of the Bacillus isolates and also their biocontrol activities in vivo. The inoculums also used to net house experiments were prepared from a 24 h old shaken culture of each of the Bacillus isolate incubated at 28±2°C. Bacillus culture suspension containing 107 cfu/ml was used as inoculant.
Isolation and preparation of phytopathogenic fungal sp.
Samples of diseased leaf stem, root parts and seeds were randomly collected and these fungal pathogens were isolated from infected plants and diseased seeds following water agar and blotter techniques. Besides some other standard fungal pathogens were also used in this study such as Rhizoctonia solani, Sclerotium rolfsii and Sclerotium oryzae obtained from different agricultural research institutes, India. Fungal strains and inoculum was prepared by culturing it on Potato dextrose agar (PDA) for 10 days in Petridishes. The microconidial suspension of these three pathogens was prepared by pouring 1 ml of sterile water in each petridish. The inoculum was then scrapped with the help of a sterile spatula from the surface of the Petridishes. The spores from the mycelia of the fungi to get a concentration of 106 spore/ml concentration and applied in sterile soils as method described (Ajilogba et al. 2013; Adebay and Ekpo 2005).
Phosphate Solubilisation
Phosphate solubilization is an important and complex phenomenon for selectively screening the bacteria having P-solubilizing activity which have the ability to release inorganic phosphate from tricalcium phosphate. Pikovskaya’s medium, is a selective medium for phosphate solubilizing microorganisms (PSM) was used to which tricalcium phosphate (TCP) has been added as it will enhance formation of halo zones. The medium was sterilized and poured into sterile petridishes (Himedia). Isolates were streaked on the Petridishes and incubated for 3 days at 28±2°C. Bacillus isolates that were able to solubilize developed clear zones around colonies (Pikovskaya 1948).
Determination of Indole Acetic Acid Production (IAA)
Luria Bertani Broth (Himedia) was prepared and freshly grown cultures were inoculated into 10 ml LB broth in each test tube and incubated at 28±2°C for 24 h at 120rpm. Grown cultures removed from each test tube and centrifuged at 10,000 rpm for 15 min. An aliquot of 1 ml of supernatant was transferred into a fresh tube to which two drops of orthophosphoric acid were added. The mixture was incubated at room temperature for 25 minutes. The development of a pink colour indicated the presence of indole acetic acid (Gordon and Weber 1951.).
Detection of Hydrogen Cyanide (HCN) Production
Production of HCN was detected according to the method described (Lorck 1948). 24h freshly grown cells were spread on agar medium which contained 4.5 g/l glycine had been added and a sterilized filter paper saturated with 1% solution of picric acid and 2% sodium carbonate was placed in the upper lid of the Petridish, which was then sealed with parafilm and incubated at 28±2°C for 4 days, a change in colour of the filter paper from yellow to reddish brown was an index of cyanogenic activity while no colour change represent no cyanogenic activity.
Determination for the production of different cell wall degrading enzymes of Bacillus isolates Chitinase activity
The different isolates obtained were screened for chitinase production based on the halo produced on plates with minimal salts medium amended with chitin. The qualitative assay for chitinase production was performed following the method (Dunne et al. 1997) and modified method given by (Roberts and Selitrennikoff , 1988). Isolates were inoculated by spotting on the plates having chitin minimal medium (CMM) as sole source of carbon and incubated at 30±2°C for 10 days. These plates were examined for development of clear zones around colonies.
Preparation of colloidal chitin
5.0 g of chitin was added to 60 ml of concentrated HCl (acid hydrolysis) by constant stirring using a magnetic stirrer at 4°C and kept in refrigerator overnight. The resulting slurry was then added to 2000 ml. of ice-cold 95% ethanol and kept at 26°C for overnight (ethanol neutralization). Then it was centrifuged at 3000 rpm for 20 min at 4°C. The pellet was washed with sterile distilled H2O. The final colloidal chitin was stored at 4°C until further use.
Final chitinase detection medium
The final chitinase detection medium per litre comprises of 4.5 g colloidal chitin, 0.3 g magnesium sulphate, 3.0 g ammonium sulphate, 2.0 g potassium dihydrogen phosphate, 1.0 g citric acid monohydrate, 15 g agar, 0.15 g bromocresol purple and 200 µl of tween-80. The pH of the media was maintained at 4.7 and autoclaved at 121°C for 15 min. The fresh culture of Bacillus isolates to be tested for chitinase activity were inoculated into the sterile plates containing chitinase detection medium and incubated at 28±2°C for seven days and observed for the colored zone formation. Chitinase activity was identified due to the formation of purple colored zone. Color intensity and diameter of the purple coloured zone were taken as the criteria to determine the chitinase activity after three days of incubation.
Protease activity
The production of protease, a fungal cell wall degrading enzymes which is an important mechanism of fungal growth inhibition was detected for all selected isolates. Protease activity was determined according to the modified method described (Berg et al. 2002). Skim milk agar medium (5.15/100ml) was used for detection of protease activity, 48h old freshly grown cultures were inoculated on skim milk agar medium and incubated at 28±2°C for two to three days. Bacillus sp. which shows the protease activity gave a clearance zone indicating the production of protease enzyme. The degree of protease activities were measured based on the diameter of clearance zone.
Carbon Utilization study
Different carbon utilization of Bacillus sp. was studied by the HiCarbo Utilization kit (Himedia). Hi carbokit was used to study the biochemical profile of organisms is a standardized colorimetric identification system utilizing 35 carbohydrate utilization test. The test was based on the principle of change of pH and substrate utilization by selected bacteria. On incubation or organism undergo metabolic changes which are indicated by a spontaneous colour change in the media. A combination of 35 tests for utilization of carbohydrate tests. Kit contains Part A, Part B each having 12 carbohydrates utilization tests and Part C containing 11 sugars and 1 control. The organism was pure cultured and culture inoculums turbidity was 0.5 OD at 620 nm, then inoculate each well with 50µl of the above inoculums by surface inoculation methods and incubate at 28±20C for 24 hr and data was interpreted as per result interpretation chart.
Antagonistic activity of Bacillus sp.
The test was carried out to see the effect of the Bacillus isolates on the three important rice phytopathogens in vitro. Pathogen inhibition test was performed by dual culture plate assay One 5-mm disc of a pure culture of the pathogen was placed at the corner of a Petri dish containing NA+PDA (Nutrient agar with Potato dextrose agar). A loopful of Bacillus culture was streaked over the surface of NA+PDA plate at opposing corners. Plates were incubated for 10 days at 28±20C, and examined for inhibition of fungus growth by the Bacillus isolates. The growth diameter of the pathogen was measured and compared to control growth, where the bacterial suspension was replaced by sterile distilled water. Each experiment was used a single pathogen isolate was run in triplicate. The Results were expressed as the mean value with standard error deviation in inhibition distance between the growths of the fungus isolate and the presence of bacillus isolate tested. Percent inhibition was calculated using the following formula: % inhibition = [1-(Fungal growth/Control growth)] x100
Control experiment with only growth of Bacillus isolates and each pathogen in each separate Petridishes of three replicates were observed.
Pot experiment for Efficacy of Bacillus on biomass and yield
Healthy seeds of rice were treated with different treatments of Bacillus isolates and directly sown into 24cm-diameter pots. A loopful of bacterial isolate (starter culture) was transferred to a 500 ml flask containing sterile liquid medium and grown aerobically in a rotary shaker at 120-160 rpm for 48h at 28±20C (Lamsal et al. 2013; Sethi and Adhikary 2009a,b; Sethi and Adhikary 2014; Sethi and Adhikary 2015). The bacterial culture suspension was diluted to become 109 CFU/ml as per method described23. Seeds were surface sterilized for 30 min in 20% sodium hypochloride, followed by rinse with 70% ethanol before sown in earthen pot (4 cm diameter with hole). Each pot contained sterile garden soil. The outlined of the experimental design using 10 replicates (each replicates comprised of 3 plants) for each of the Bacillus isolates, a negative control with no fungus and a positive control with inoculation of fungus. Seeds were treated with bacterial suspension (containing 108 CFU/ml) of each Bacillus sp. in the sterile trays 24h before showing in nursery bed. Seedlings were transplanted in to 30cm diameter pots each pots contain sterile soil. The different treatments with Bacillus isolates were applied as outlined in the experimental design using seven trials where each trial composed with ten replicates and a negative control with no pathogens. Plants watered daily in the net house at for 8 weeks. At the end of 8 weeks, 10 plants (one plant of each replicates) were harvested to assess the effect of the various Bacillus isolates on the different vegetative growth parameters. Significant difference was assessed from the mean of each of the different treatments. The experiment was repeated twice. The growth parameters assessed include: length of shoot and root length, biomass and randomly selected seedlings were used to determine each parameter per treatment. Cholorophyll content was also measured with a chlorophyll meter (SPAD meter, USA)
Statistical analysis
All experiments were performed twice. Analysis of Variance and Duncan’s multiple range test (DMRT) was employed to test for significant differences between treatments at p = 0.05 (Gomez and Gomez, 1984).
Isolation of Bacillus sp.
All the isolates (six isolates) showed as gram positive, rod shaped, endospore former with white dry and fold, opaque and irregular edge colonies on nutrient agar medium. All of them are fast grower and found positive for catalase, oxidase, beta galactosidase (ONPG) activity glucose, manitol, sucrose, arbinose, trehalose utilization, acetoin production (Voges proskauers test), nitrate reduction and all were found negative for H2S production, Arginine utilization and phenyl alanine deaminase (Table-3).
Table (1):
Plant growth promoting attributes and Mechanism of pathogen Inhibition of Bacillus sp. isolated from rice rhizosphere
Bacillus strains |
Phosphate solubilization |
HCN production |
Tryptophan Utilization (IAA Production) |
Protease activity |
Chitinase activity |
|---|---|---|---|---|---|
B-1 |
+ |
– |
+ |
+ |
+ |
B-2 |
+ |
– |
+ |
+ |
+ |
B-3 |
+ |
– |
+ |
+ |
+ |
B-4 |
+ |
– |
+ |
+ |
+ |
B-5 |
+ |
– |
+ |
+ |
+ |
B-6 |
+ |
– |
+ |
+ |
+ |
B-7 |
+ |
– |
+ |
+ |
+ |
B-8 |
+ |
– |
+ |
+ |
+ |
B-9 |
+ |
– |
+ |
+ |
+ |
B-10 |
+ |
– |
+ |
+ |
+ |
B-11 |
+ |
– |
+ |
+ |
+ |
B-12 |
+ |
– |
+ |
+ |
+ |
B-13 |
+ |
– |
+ |
+ |
+ |
B-14 |
+ |
– |
+ |
+ |
+ |
B-15 |
+ |
– |
+ |
+ |
+ |
B-16 |
+ |
– |
+ |
+ |
+ |
B-17 |
+ |
– |
+ |
+ |
+ |
Table (2):
Morphological and biochemical characters of Bacillus sp. isolated from rice rhizosphere
Colony shape |
Round |
|---|---|
Color |
White |
Appearance |
Rough |
Margin |
lobate |
H2S production |
Negative |
Phenyl alanine deaminase |
Negative |
Gram reaction |
+ ve |
Table (3):
Different Carbon Utilization Test (Hi carbokit)
Test |
Original colour of the medium |
Colour of the medium change |
Reaction |
|---|---|---|---|
Malonate |
Bluish green |
No change |
negative |
Voges proskauer’s |
Light yellow |
Pinkish red |
positive |
Citrate utilizing |
Light green |
Dark blue |
positive |
ONPG (Detects Beta galactosidase) |
Colourless |
Yellow |
positive |
Nitrate Reduction |
Light yellow |
Pinkish red |
positive |
Catalase activity |
Colourless |
Effervescence coming out from the loop |
positive |
Arginine utilization |
Light purple |
No colour change |
negative |
Sucrose utilization |
Red |
Yellow |
positive |
Mannitol utilization |
Red |
Yellow |
Positive |
Glucose utilization |
Red |
Yellow |
Positive |
Arabinose utilization |
Red |
Yellow |
Positive |
Trehalose utilization |
Red |
Yellow |
Positive |
Maltose utilization |
Red |
Yellow |
Positive |
Antagonistic effect of Bacillus spp. and mechanisms
Study reveals that the six isolates of Bacillus sp. inhibited the growth of R. solani, S. rolfsii and S. oryzae significantly. The results obtained were presented in the Plate-1 (Fig-1, 2, 3). The results obtained showed that all six bacilli spp showed that inhibited the growth of these pathogens most by 90%. The mechanism of antagonism of the six Bacillus isolates was investigated and the mechanism results are presented in table-1.
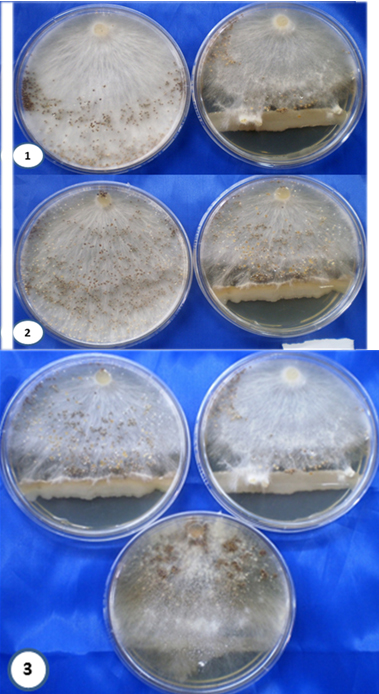
Plate 1. Dual culture assay of Bacillus sp. isolated from rice rhizosphere against rice plant pathogen S. oryzae (1) S. rolfsii (2) (3) R. solani for growth inhibition assays
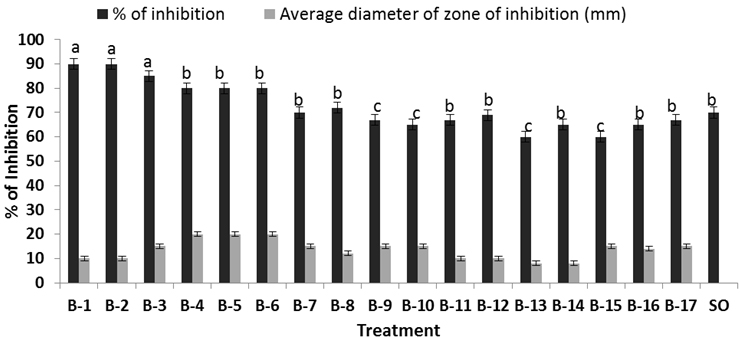
Fig. 1. Inhibitory effect of the six bacillus isolates against S. oryzae in vitro. Percent of inhibition of Bacillus against pathogen, while SO is the growth of Schelortium oryzae alone in the Petri dish. Each value is average of ten replicates. For each bar value are followed by a different lower case letters indicates LSD at p<0.05
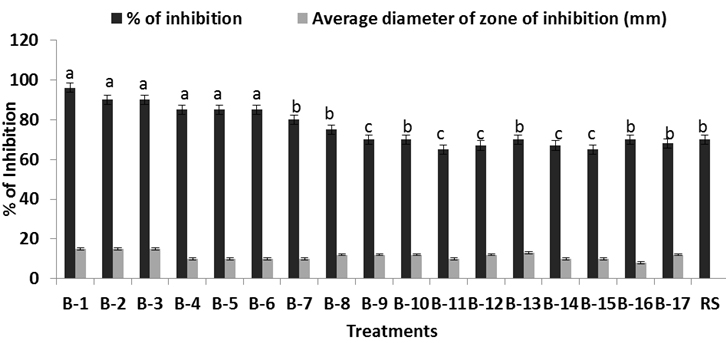
Fig. 2. Inhibitory effect of the six bacillus isolates against R. solani in vitro. Percent of inhibition of Bacillus against pathogen, while RS is the growth of Rhizoctonia solani alone in the Petri dish. Each value is average of ten replicates. For each bar value are followed by a different lower case letters indicates LSD at p<0.05
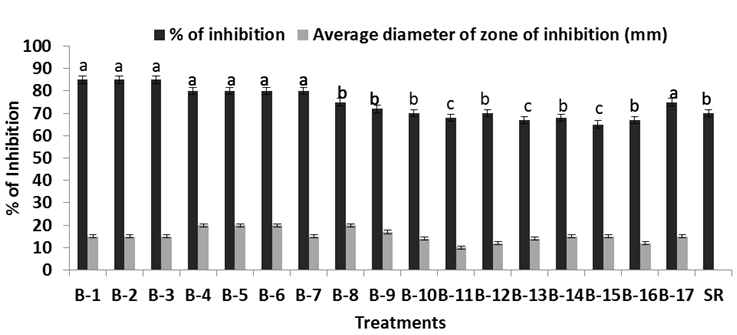
Fig. 3. Inhibitory effect of the six bacillus isolates against S. rolfsii in vitro. Percent of inhibition of Bacillus against pathogen, while SR is the growth of Schelortium rolfsi alone in the Petri dish. Each. value is average of ten replicates. For each bar value are followed by a different lower case letters indicates LSD at p<0.05
Phosphate solubilisation, HCN and IAA production
Bacillus isolates were examined for their ability to solubilize phosphate as an indirect mechanism of biocontrol by modifying the environmental condition. In order to examine the Bacillus isolates for their ability to solubilize phosphate, a standard agar medium; (pH 6-7) containing 5 g of tricalcium phosphate (TCP) as a sole source of phosphorus was prepared and used. All Bacillus isolates formed clear halos around on pikovskayas medium having bromothymol blue changed its colour from blue to yellow due to increase in pH of the growth medium. In case of IAA production test, all the six isolates of bacillus showed IAA production. Development of pink colour with and without addition of tryptophan in culture broth was also observed in to their cell free supernatant using spectrophotometer absorbance at 530 nm. Maximum IAA production was recorded in B-1, 2 and 3. It was found that none of the bacillus strains found produces HCN. (Table-1)
Cell wall degrading enzymes (chitinase and protease)
All bacillus strains showed chitinase and protease activities. Chitinase activity was confirmed due to the formation of purple colored zone. Colour intensity and diameter of the purple colored zone were taken as the criteria to determine the chitinase activity after three to seven days of incubation. All Bacillus sp. which showed the protease activity and zone confirmed the production of protease enzyme (Plate-2 Fig-1). The degree of protease activities was measured based on the diameter of clearance zone.
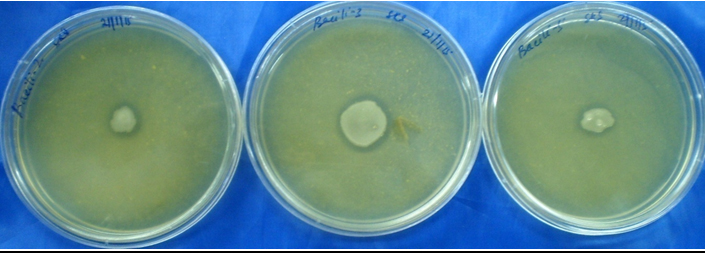
Plate 2. (Fig-1): Protease activities of Bacillus sp. showed clearance zone after 48 hr inoculation
Efficacy of Bacillus sp. on pathogen control, vegetative growth and yield of rice crop
As a result of the in vitro screening performances of the six isolates in antagonising the growth of these three pathogens. Out of the six isolates 3 strains were screening and studied the efficacy in the net house test on rice using RBD (randomized block design) as follows:
Rice planted without any treatment (control); Rice planted with different Bacillus sp.; Rice planted with each of R. Solani, S. rolfsii, S. Oryzae; Rice planted with both Bacillus and each of R. Solani, S. rolfsii, S. Oryzae.
Response to Disease control using Bacillus Sp. as treatments
Disease control caused by these pathogens was achieved using Bacillus sp. in the net house trial with pot experiments. The treatment with highest disease control activity was significant. The isolate B-1 was found with 82, 86 and 82% disease control against R. Solani, S. rolfsii, S. Oryzae and BAC-2&3 was showed 72, 70, 82% and 65, 70 66% against R. Solani, S. rolfsii, S. Oryzae in. This result was quite significantly different from the control which was having no disease control.
Response to plant growth parameters using Bacillus sp. as treatments
All resulted in a significant increase in vegetative growth like length and weight of root and shoot, yield, 1000 grain weight over than plants not infected with pathogens but treated with bacillus sp. (Fig: 4-6). Among the treatments, isolate B-1 displayed the highest growth promoting activity in all of the parameters evaluated B-1 treated plants showed significant increase in shoot and root biomass respectively (Fig-5). Chlorophyll content was measured active tillering and panicle initiation stage and found the highest in Bacillus treated plants with a significant difference from the non-inoculated control. There was no significant variation in the chlorophyll content of rice plants among the bacterial treatments (Fig-7) however, non-inoculated (control) plants were found less chlorophyll (Fig-7).
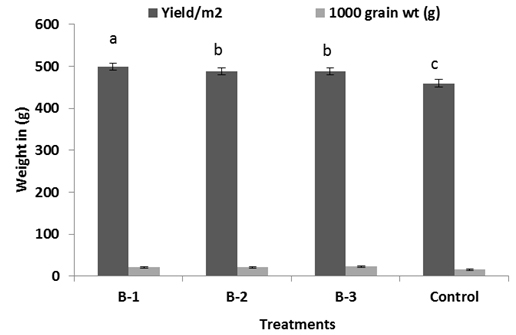
Fig. 4.
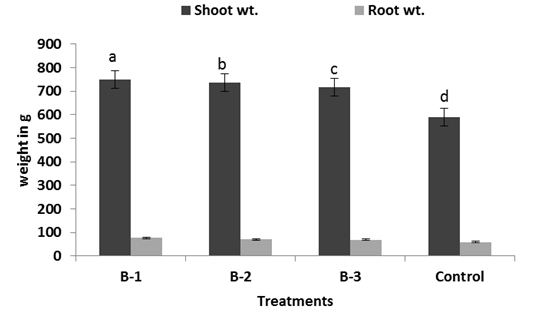
Fig. 5.
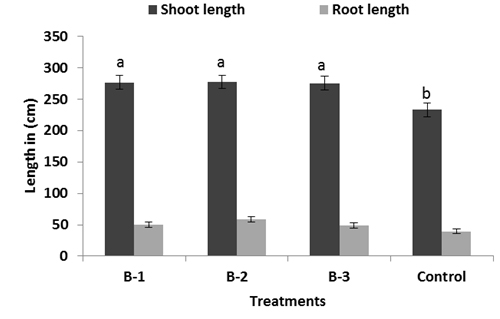
Fig. 6.
Fig. 4, 5 & 6. Plant growth promotion effect on rice of Different Bacillus sp. under net house trials. For each bar value are followed by a different lower case letters indicates LSD at p<0.05
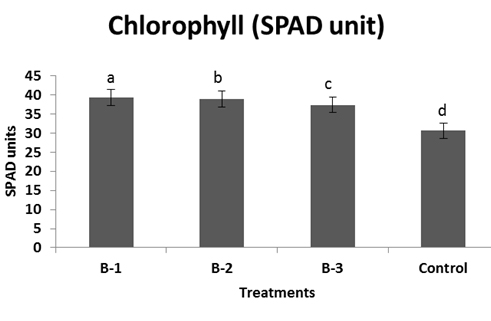
Fig. 7. Chlorophyll estimation of leaf samples after treated with Bacillus sp. and un-inoculated control. For each bar value are followed by a different lower case letters indicates LSD at p<0.05.

Fig. 8. Growth promotion of rice plant in the pot culture experiment under net house by inoculation of different Bacillus isolates (B-1, B-2 and B-3 is of Bacillus sp. isolates and Control-non-inoculated).
Note that the non-inoculated control resulted in retarded shoot and root growth compared with the treatments with Bacillus isolates
The application of Bacillus sp. which was already reported that act as PGPR (plant growth promoting rhizobacteria) is a potentially attractive approach to disease management and improved crop productivity in sustainable agriculture (Kloepper et al. 2004; Lamsal et al. 2013).
It has been well reported that the isolation of bacillus sp. from different cultivated lands cultivating different crops including cereals and vegetables having their plant growth promoting activities (IAA, Siderophore production, Phosphate solubilization) and biocontrol efficiency such as chitinase, protease, ACC deaminase, oxalate oxidase has been well reported (Kumar et al. 2012; Mehta et al. 2010; Singh et al. 2008; Chung et al. 2008). The results showed that all six strains isolated from rice rhizosphere showed good plant growth promoting activities as well as better antagonistic effects against three pathogens like R. solani, S.rolfsii and S. oryzae respectively.
Bacillus strains were identified based on morphological, phenotypic characters and biochemical parameters as per Bergys manual of systematic Bacteriology. It was grown and maintained on NA medium at 300C. The isolate upon gram staining was gram positive, rod shaped, spore forming, motile and biochemical feature such as starch hydrolysis, utilization of phosphates and production of cell wall specific extracellular enzymes (such as chitinase, protease). In vitro inhibition of various phytopathogens by Bacillus sp. has also been reported (Chung et al. 2008; Ashwini and Srividya 2012). In vitro IAA production by Bacillus spp. in significant amount has also been reported by (Mehta et al. 2010; Singh et al. 2008). Production of IAA with or without tryptophan by all Bacillus isolates is similar to above finding.
Bacillus sp. produced a significant amount of ACC deaminase. It has been reported earlier that rhizospheric bacteria producing á-ketobutyrate show plant growth promoting activities (Penrose and Glick 2003). Some of the isolates produced mycolytic enzymes viz., chitinase which probably degrade the components of fungal cell wall such as chitin, There are many reports on the production of lytic enzymes by microorganisms (Gupta et al. 2006; Huang and Chen 2004).
The action of lytic enzymes BPR7 was quite effective on all six test pathogens. Therefore, it is likely that cell wall lysis would have been due to concerted action of chitinase (Chaiharn et al. 2008). Positive colour change of filter paper to reddish brown indicated the production of HCN. None of the six Bacillus isolates produced HCN which is similar to in whose research the Bacillus isolate were all negative for HCN (Singh et al. 2008). Cyanide is a toxic and dreaded chemical produced by many rhizobacteria. Some bacteria synthesis it, others excrete it and yet others metabolize it in other to avoid predation and competition (Zeller et al. 2007).
Formation of inhibition zones due to reduction in radial growth of target phytopathogens by bacillus sp. may be due to the production of antimicrobial substances such as chitinolytic enzymes, HCN, antibiotics, siderophore and nutrient competition (Singh et al. 2008; Glick 1995; Chung et al. 2008). However, role of other inhibitory metabolites such as toxins and proteolytic enzymes in the inhibition process of fungal pathogens may not be ruled out (Hu et al. 2008). It may be concluded that Bacillus sp. B-1, 2 &3 being as a good PGPR and a potential biocontrol agents, may be used as potential bioinoculants for rice. Further study on field experiments related to growth promotion, biocontrol and induced systemic resistance to pathogens is in progress.
It was also found that use of microorganisms to control plant diseases offers a most alternative to the use of commercially available chemicals (Emmert and Handelsman 1999; Shoda 2000; Bais et al. 2004). The presence of the most potential strains of microorganism in the soil and associated with plant roots which may suppress plant pathogens without producing lasting effects on the rest of the soil microbial and plant communities (Osburn et al., 1995; Howarth, 1991). In accordance, the diversity of microbial communities provides a rich source of potential biocontrol agents. The method of specific isolation, characterization and their use as biocontrol agent described here, it should be possible to isolate and characterization of new Bacillus sps are effective in biocontrol of microbial pathogens.
Six different sp. of Bacillus were isolated from Odisha (as indeginous) for screened and compare for the antifungal against R. Solani, S. Rolfsii and S. oryzae fungal pathogen species of rice which causes the stem rot, seedling blight and sheath blight disease. Their plant growth promotion activities like IAA production, phosphate solubilisation ability, plant growth parameters like vegetative growth and yield were tested. Mechanism of antifungal activities against pathogens was also studied by Chitinase, protease activity and dual culture assays against pathogens in terms of growth inhibition of pathogens. All the strains showed positive in IAA and phosphate solubilising efficiency, biochemical characterization was showed and confirmed that all the isolates were Bacillus subtilis.
All the six strains were showed the positive against pathogens, B-1, B-2, B-3 showed strong inhibitory potential on the mycelia growth of the studied fungi and showed 90% inhibit the growth of pathogens and also showed significant in vegetative growth and yield of crops then control. As per the antifungal and IAA and phosphate solubilising efficiency out of six strains the indigenous strains BAC-1, BAC-2, BAC-3 was further selected for plant growth promotion assays.
We consider the selected indigenous bacterial strains BAC-1, BAC-2, BAC-3 isolated from Odisha soil are feasible to be used for development of potential biocontrol inoculants for these three pathogens and may also used for biofertilizer and biocontrol programme successful.
ACKNOWLEDGMENTS
We are gratefully acknowledged the University Grant Commission, Govt. of India for financial support through a Post Doctoral research grant to SKS. We also Thanks to successive Heads, Division of Crop Protection and Directors, National Rice Research Institute for permission and provide the laboratory facilities.
- Ashwini N, Srividya S. Optimization of Chitinase produced by a biocontrol strain of B. subtilis using Plackett-Burman design. Eur J of Exp Biol, 2012; 2(4):861-865.
- Ashwini N, Srividya S. Potentiality of Bacillus subtilis as biocontrol agent for management of Anthracnose disease of chilli caused by Collettotrichum gloeosporioides OGC1. 3 Biotech, 2014; 4: 127-136.
- Ajilogba CF, Babalola OO, Ahmed F. Antagonistic Effects of Bacillus Species in Biocontrol of Tomato Fusarium Wilt. Ethno Med., 2013; 7(3):205-216
- Azad P. Screening of native Rhizobium strains for sustainable crop production. In: pulse-rice cropping system. En: Yadav AK, Chaudhary SR, Talukdar NC, editors. Biotechnology in Sustainable and Organic Farming, Shree Publishers and Distributors, New Delhi, 1994; pp 244-248
- Adebayo OS, Ekpo EJA. Efficiency of fungal and bacterial biocontrol organisms for the control of fusarium wilt of tomato. Nigerian J Hort Sci, 2005; 9: 63-68
- Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of Arabidopsis Roots by Pseudomonas syringae is facilitated by Biofilm formation and Surfactin Production1. Plant Physiol, 2004; 134: 307–319
- Berg G, Krechel M, Ditz R, Sikora A, UlrichHallmann J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol, 2002; 2: 215-229
- Chaiharn M, Chunhaleuchanon S, Kozo A, Lumyong S. Screening of rhizobacteria for their plant growth promoting activities. KMITL Sci Tech J, 2008; 8(1):18–23
- Chung S, Kong H, Buyerm JS, Lakshman DK, Lydon J, Kim SD. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl Microbiol Biotech, 2008; 80(1):115–23
- Compant S, Duffy B, Nowak J, Clement C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects, Appl Environ Microbiol, 2005; 71: 4951-4959
- Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, de Bruijn FJ, O’Gara F. Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiol, 1997; 143: 3921–3931
- Emmert EAB, Handelsman J. Biocontrol of plant disease: a Grampositive perspective. FEMS Microbiol Lett, 1999; 171: 1–9
- Foldes T, Banhegyi I, Herpai Z, Varga L, Szigeti J. Isolation of Bacillus strains from the rhizosphere of cereals and in vitro screening for antagonism against phytopathogenic, food-borne pathogenic and spoilage microorganisms. J. Appl Microbiol, 2000; 89: 840–846.
- Gomez KA, Gomez AA. Statistical Procedures for Agri cultural Research. A. Lviley. Interscience Publication, New York, 1984; 678 pp.
- Gordon SA, Weber RP. Colorimeteric estimation of indole acetic acid. Plant Physiol, 1951; 26: 192–5
- Gupta CP, Kumar B, Dubey RC, Maheshwari DK. Chitinase mediated destructive antagonistic potential of Pseudomonas aeruginosa GRC1 against Sclerotinia sclerotiorum causing charcoal rot of peanut. BioControl, 2006; 51: 821–35
- Glick BR. The enhancement of plant growth by free-living bacteria. Can J Microbiol, 1995; 41: 109–17
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Bergey’s manual of determinative bacteriology. Baltimore, USA: Williams and Wilkins Press 1994.
- Howarth FG. Environmental impacts of classical biological control. Annu Rev Entomol, 1991; 336: 485–509
- Huang CJ, Chen CY. Gene cloning and biochemical characterization of chitinase CH from Bacillus cereus 289. Annals of Microbiol, 2004; 53(3):289–97
- Hu QP, Xu JG, Song P, Song JN, Chen WL. Isolation and identification of a potential biocontrol agent Bacillus subtilis QM3 from Qinghai yak dung in China. World J Microbiol Biotechnol, 2008; 24: 2451–2458
- Jacobsen BJ, Zidack NK, Larson BJ. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. In: Symposium- The nature and application of biocontrol microbes: Bacillus sp. Phytopathol, 2004; 94:1272-1275
- Joshi PK, Acharya SS, Chaand R, Kumar A. Agricultural sector, status and performance In: State of Indian Agriculture, En. Rai M National Academy of Agricultural Sciences, New Delhi, India, 2009; pp 1-34
- Karlidag H, Esitken A, Turan M, Sahin F. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Sci Horticul, 2007; 114:16-20
- Kumar P, Dubey RC, Maheswari DK. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res, 2012; 167: 493– 499
- Kloepper JW, Ryu CM, Zhang SA. Induced systemic resistance and promotion of plant growth by bacillus spp. Phytopathol, 2004; 94: 1259-1266
- Lamsal K, Kim SW, Kim YS, Lee YS. Biocontrol of late blight and plant growth promotion in tomato using rhizobacterial isolates. J Microbiol Biotechnol, 2013; 23(7):1-8
- Leelasuphakul W, Hemmanee P, Chuenchitt S. Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillum digitatum Sacc.) of citrus fruit. Post Harvest Biol Techno, 2008; 48:113-121
- Lorck H. Production of hydrocyanic acid by bacteria. Physiol. Plant, 1948; 1: 142–146
- Mehta P, Chauhan A, Mahajan R, Mahajan PK, Shirko CK. Strain of Bacillus circulans isolated from apple rhizosphere showing plant growth promoting potential. Curr Sci, 2010; 98(4):538–42
- Osburn RM, Milner JL, Oplinger ES, Smith RS Handelsman J. Effect of Bacillus cereus UW85 on the yield of soybean at two field sites in Wisconsin. Plant Dis, 1995; 79: 551–556
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase containing plant growth-promoting rhizobacteria. Physiol Plant, 2003; 118: 10–5
- Pikovskaya RI. Mobilization of P in soil in connection with vital activity by some microbial species. Microbiologica, 1948; 17: 362-370
- Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology, 2010; 91(12):3463–3470
- Ramirez KS, Craine JM, Fierer N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Global Change Biol, 2012; 18(6):1918-1927
- Roberts WK, Selitrennikoff CP. Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol, 1988; 134: 169-176
- Sethi SK, Adhikary SP. Efficacy of region specific Azotobacter strain on vegetative growth and yield of Solanum melongena, Lycopersicon esculentum and Capsicum annum. J Pure Appl Microbiol, 2009a; 3(1):331-336
- Sethi SK, Adhikary SP. Vegetative growth and yield of Arachis hypogea and Vigna radiata in response to region specific Rhizobium biofertilizer treatments. J Pure Appl Microbiol, 2009b; 3(1):295-300
- Sethi SK, Adhikary SP. Growth response of region specific Rhizobium strains isolated from Arachis hypogea and Vigna radiata to different environmental variables. African J Biotechnol, 2014; 13(34): 3496-3504.
- Sethi SK, Adhikary SP. Growth response and protein profile of Region specific Rhizobium strains isolated from Vigna mungo from different localities of Odisha state, India at different Temperature, pH, Salinity and Iron. J Adv Microbiol, 2015; 2(1):64-75
- Shoda M. Bacterial control of plant diseases. J Biosci Bioeng, 2000; 89: 515–521
- Singh N, Pandey P, Dubey RC, Maheshwar DK. Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (Sarg.) by rhizosphere competent Bacillus subtilis BN1. World J Microbiol Biotechnol, 2008; 24: 1669-1679
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature, 2002; 418: 671-677
- Zeller SL, Brandl H, Schmid B. Host-Plant Selectivity of Rhizobacteria in a Crop/Weed Model System. Plos One, 2007; 9:1-7.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


