ISSN: 0973-7510
E-ISSN: 2581-690X
Folate represents an essential nutritional component in human diet and involved in many metabolic pathways, its deficiency results in disorders like megaloblastic anemia and neural tube defects. The present study reports screening of lactic acid bacteria (LAB) with additional benefit towards folate production and their characterization for probiotic potential. 64 LABs isolated from human colostrums were subjected for further screening for extracellular folate production. Fourteen LAB isolates belonging to species L. plantarum(12) and L. rhamnosus(2) had folate production beyond 40µg/L, with highest being in L. plantarum CKR26 (74.2±2.8µg/L). These isolates were further characterized for the probiotic properties. Four L. plantarum isolates namely CKR5, CKR8, CKR12 and CKR28 confirmed to posses good probiotic potential. All four isolates exhibited broad spectrum of antibacterial activity towards eight bacterial pathogens tested and two among them CKR8 and CKR12 also had antifungal activity. Both CKR5 and CKR12 strains had relatively high folate production (58.6±3.8µg/L and 56.5±3.4µg/L) but CKR12 and CKR28 had additional antifungal activity. So, all the four folate producing L. plantarum strains can find their application as food supplement for adults and can also be included in weaning foods for infants, which aid in utilizing novel food products to provide natural folate.
Lactic acid bacteria, Colostrum, Folate, Probiotic properties
Folate is an important water soluble vitamin, also termed as vitamin B9, vitamin M or folic acid. The major difference between folate and folic acid is that, folate is the natural form where as folic acid is the synthetic form that is found in major supplements and fortified foods. It is mainly involved in one-carbon mediated metabolic pathways such as synthesis, repair and methylation of DNA, as well as acts as cofactor in certain biological reactions (LeBlanc et al. 2007). The Recommended Daily Allowance (RDA) of folate for adults is 0.4mg and for pregnant women is 0.6mg. Deficiency of this vitamin causes disorders like Alzheimer’s, osteoporosis, poor cognitive performance, increased risk of colorectal cancer, megaloblastic anemia, and neural tube defects (NTD) in newborns. Globally each year more than 300,000 babies are born with NTD (NCBDDD. 2012). According to Bhide et al. (2013) the highest burden of NTD’s is found in India i.e. 4.1/1000 births. It is reported that intake of folic acid during preconception can help to reduce 62-70% of NTDs (Mills and Signore 2004)
Even though the folic acid is easily absorbed by the body when compared to folate, it has some undesirable effects such as excess level of folic acid reported to stimulate the growth of neoplasm that can ultimately escort to cancer by decreasing natural killer cytotoxicity, which helps in destruction of tumor cells. The unmetabolized folic acid in blood masks the deficiency of vitamin B12. Therefore natural folate is potential alternative for folic acid (Iyer et al. 2009). The main sources of natural folate are green leafy vegetables and dairy products. The use of vitamin producing microorganisms is more economical, as once this bacteria resides, and dislodges from gastro intestinal tract, they serves as continuous source of folate.
Lactic acid bacteria (LAB) are diverse group of Gram positive, saccharolytic, beneficial microbes for host, certain strains are capable to produce and release specific beneficial compounds such as vitamins, exopolysaccharides and amino acids. These bacteria acidifies large intestine, restrict pathogens, stimulate immune response, exert anti-inflammatory activity and reduce the risk of colon cancer, because of such beneficial properties LAB are considered as probiotics (Rossi et al. 2011). Several researchers have reported LAB such as Lactococcus lactis, Streptococcus thermophilus, Lactobacillus spp. such as L. plantarum, L. rhamnosus, L. helveticus and some of the species of Bifidobacterium like B. adolescentis and B. pseudocatenulanum have the ability to produce folate both extracellularly and intracellularly in varying amount (Iyer et al. 2009). In L. lactis, Leuconostoc spp, Propionibacteria spp, and Bifidobacteria spp, folate production is mainly intracellular; hence the bioavailability of folate is less (Iyer et al. 2011). Therefore LAB with extracellular folate production is more advantageous over intracellular producers as bioavailability of folate will be supplementary.
Colostrum (human milk) is considered as the preeminent source of nutrients for growth and development of newborn. It contains many important protective molecules such as carbohydrates, nucleotides, fatty acids, immunoglobulins and immunomodulatory factors. In addition, it also serves as continuous source of commensal, mutualistic and potentially probiotic bacteria, which prevent pathogen adherence in the intestine of newborn (Diba et al. 2013). L. gasseri, L. rhamnosus, L. plantarum, L. fermentum, E. faecium and some of Bifidobacterium spp. have been isolated from human milk for their probiotic characterization (Mehanna et al. 2013, Nuraida et al. 2012). Further the research into folate producing LAB from colostrums and their potential as probiotics have been overlooked. Therefore the present study aims to isolate the lactic acid bacteria from human colostrum which has high probiotic potential along with extracellular folate producing ability.
Sample collection
Colostrum was voluntarily bequeathed by 10 healthy mothers during their early period of lactation i.e. within 8 days of birth in Krishna Rajendra hospital, Mysore, Karnataka, India. The samples were stored on ice until it was remitted to laboratory for screening of lactic acid bacteria.
Isolation and identification of LAB
The samples were serially diluted in physiological saline and pour plate technique was carried out on selective Man-Rogosa-Sharp (MRS) agar for isolation. The plates were incubated at 37ºC for 24-48h. The individual colonies were randomly isolated and sub-cultured in MRS broth. The isolates were tested for catalase, oxidase, Gram-staining, cell morphology, motility, spore formation and ability to grow in 6.5% of NaCl for confirmation of LAB (Abbas et al. 2014).
The genomic DNA was isolated for molecular identification by phenol-chloroform method and DNA integrity was tested on 1% agarose gel electrophoresis. PCR was performed by using reported genus and species specific primers designed by Byun et al. (2004) and Markiewicz et al. (2005) and were synthesized by Eurofins Bangalore.
Initial screening for folate producers
Initially all the isolates were evaluated for their ability to grow in folic acid-free media, where there growth in this media indicates their ability to produce folate. The isolates which were able to grow were only taken for evaluation for extracellular folate production using HPLC. S. thermophilus 177 procured from NCDC (National Dairy Research Institute, Karnal) was taken as a positive control for this study (13).
Screening for folate producing LAB by HPLC
Initially all the isolates were evaluated for their ability to grow in folic acid-free media, where there growth in this media indicates their ability to produce folate. The isolates which were able to grow were only taken for evaluation for extracellular folate production using HPLC. S. thermophilus 177 procured from NCDC (National Dairy Research Institute, Karnal) was taken as a positive control for this study (13). The 16-18h cultures were centrifuged at 10,000xg for 10min at 4ºC (14). Cell free supernatant (CFS) was diluted (1:1) in 0.1M Sodium acetate buffer (pH 4.8) containing 1% ascorbic acid (prevents oxidation of folate). All the samples were filtered through 0.22µm filter, and used directly or stored at -20ºC until use. The JASCO HPLC(Japan) system consisting of quaternary gradient controller with two pumps 1258 and manual Rheodyne injector, C18 column (JASCO Japan), 5µm particle size 4.6 Id X 250mm and Photo diode array detector was used. Mobile phase of 2:8 ratios of acetonitrile and 0.05M phosphate buffer with pH-2.0 used was, at constant flow rate of 0.8 ml/min at room temperature (27±2ºC) at 280nm. Peaks were identified using the retention time of standard folic acid.
Probiotic characterization
Tolerance to low pH and high bile salts concentration
Acid and bile tolerance of LAB was evaluated according to Chiu et al. 2007. Survival ability of LAB isolates in acidic conditions was screened by using MRS broth with different pH stipulations, i.e. pH 2 and pH 2.5. Bile salt tolerance was assayed using MRS broth supplemented with ox-bile to makeup the bile concentration in the media to 0.5%, 1% and 1.5%. The log phase culture (6log cfu/ml) was inoculated in media with different pH and bile concentrations, respectively for 4h at 37ºC. Percentage of survival was evaluated by using equation,
% of survival = (log B/log A) x100 …(1)
Where, log A – initial cfu and log B – cfu at 4thh.
Survivability in synthetic gastric juice
Survivability of LAB in Synthetic gastric juice (SGJ) was assayed according to Huang and Adams (2004). GJ artificially prepared was used to scrutinize the survivability of isolates in gastric environment. The log phase culture was resuspended in SGJ consisting of D-glucose – 3.5g/l, NaCl – 2.5g/l, KH2PO4 – 0.6g/l, CaCl2 – 0.11g/l, KCl – 0.37g/l, porcine bile – 0.05g/l, lysozyme – 0.1g/l and pepsin – 13.3 mg/l with pH adjusted to 2.0 and incubated at 37ºC for 4h. Samples drawn at 0, 0.5, 1, 2, 3 and 4h, were plated on MRS agar and incubated at 37ºC for 48 h. Survivability percentage was evaluated using the above mentioned formula (1).
Autoaggregation and Coaggregation
For autoaggregation assay, log phase LAB cultures were centrifuged at 3,000xg for 10 min at 4ºC. The pellets were washed thrice and resuspended in PBS (pH7) to give optical density of 1 at 600nm and incubated at 37ºC for 5h. Autoaggregation was evaluated by monitoring the absorbance for every 1h intervals for 5h. The percentage of autoaggregation was measured using the equation,
% Aggregation = (1-At /Ao) x 100, Where, At – final absorbance and Ao – initial absorbance.
For Coaggregation the bacterial suspension was prepared as above, the equal amount of different probiotics and pathogens were mixed thoroughly by vortex and the absorbance were read at 600nm and incubated for 5h at 37ºC. The pathogens used were S. typhimurium, E. coli and B. subtilis. Autoaggregation of each bacteria used were separately evaluated for 0 and 5thh. Percentage of coaggregation was evaluated according to Collado et al. (2008).
% Coaggregation = {[(Ax-Ay)/2 – A (x+y)] / [(Ax-Ay)/2]} x100
Where A represents absorbance, x and y represents two strains individually and x+y represents mixture of two strains.
Cell surface hydrophobicity of LAB
Cell surface hydrophobicity was determined by bacterial adherence to hydrocarbon (BATH) assay, which is a common method to assess the ability of the cells to adhere to epithelial cells. LAB isolates of 18h old were centrifuged at 7000xg for 4 min at 4ºC and pellets were washed twice with PBS and resuspended in the same. 3ml of cell suspension was added to 1ml of Xylene and vortexed for 2min. The tubes were incubated for phase separation at 37ºC for 1h. O.D of aqueous phase was measured at 600nm (Presti et al. 2015). Cell surface hydrophobicity was expressed as percentage hydrophobicity using the equation:
% Hydrophobicity = [(A0-A1)/A0x100], where A0 and A1 are initial and final absorbance.
Adherence to Caco-2 cell line
Human colonic adenocarcinoma (Caco-2) cell lines (National Center of Cell Sciences, Pune) were used to study the adhesion of LAB isolates. 1×105 Caco-2 cell were seeded on to coverslip in 6-well plate with Minimal Essential Media (MEM) with antibiotics and incubated at 37ºC in 5% CO2. After cells reaching 80% confluence, media was replaced with MEM without antibiotic and used for the experiment. 18h old LAB culture was washed twice with PBS (pH-7) and suspended in MEM without antibiotics and ~ 1×106 cells was added to each well. MEM alone was taken as control. After incubation for 2h at 37ºC, the cells were washed twice with PBS. Adhesion was determined by enumerating bacteria adhered to the cells in 25 random microscopic fields.
Enumeration
Cells grown on cover slips were fixed by adding 1ml of methanol to each well and incubated for 10min at room temperature. Methanol was removed completely and stained with Giemsa stain and incubated for 20 min. Excess stain was removed by washing with ethanol, cover slips were air dried and used for microscopic study. The number of bacteria adhered was counted in 25 random microscopic fields. Depending on the number of bacteria, result was interpreted as non-adhesive (< 60), adhesive (61-100) and strong adhesive (>100) (Re et al, 2000).
Antibacterial activity of probiotic LAB
The ability of probiotic LAB to inhibit pathogens was perceived by well diffusion assay, against eight pathogens such as S. aureus, B. subtilis, S. flexneri, S. typhimurium, B. cereus, L. monocytogenes, E. coli, and C. freundii as described by Presti et al, 2015. The log phase LAB cultures were centrifuged at 10,000xg for 10 min at 4ºC and supernatant was passed through 0.22µm filters. This cell free supernatant (CFS) was used directly or stored at -20ºC for later use. The target bacteria were spread on Mueller-Hinton agar, wells of 6mm were made on the plate. These wells were filled with 100µl of CFS and plates were incubated at 37ºC for 24h.
Antifungal activity of probiotic LAB
Antifungal activity was assessed according to Magnusson et al. (2003) by confrontation assay with some modifications. Antifungal activity was performed against eight fungal species such C. perpurea, F. graminarium, F. sporotrichioides, P. expansum, A. parasiticus, A. flavous and A. ochraceous. Fungal spore suspension of 1×102 spores was spread on to potato dextrose agar and incubated for 2h at 30ºC. The log phase probiotic LAB isolates were streaked parallelly onto the same plates and incubated at 30ºC for 72h and checked for the zone of inhibition. The zone of inhibition was interpreted as follows: ‘’–’’ ’! no visible inhibition, ‘’+’’ ’! less inhibition (1–3 % of plate area), ‘’++’’ ’! moderate inhibition (3–8 % of plate area) and ‘’+++’’ ’! greater inhibition (>8 % of plate area).
Isolation and identification of LAB
Isolation of LABs from ten colostrum samples was carried out on selective MRS medium, 6-8 colonies were randomly selected from each plate and purified on MRS agar (Chiu et al. 2007). 64 isolates were selected on the basis of morphological, physiological and biochemical characteristics i.e., Gram’s positive, catalase negative, oxidase negative, non-sporulating, non-motile and resistant to 6.5% NaCl. Molecular identification according to Byun et al (2004) and Markiewicz et al. (2005) confirmed that the 64 isolates belong to genera Lactobacillus of which 14.2% were L. acidophillus, 67.1% were L. pantarum and 18.7% were L. rhamnosus. These selected isolates were stored at -80ºC in MRS broth with 10% glycerol.
Initial screening for folate producers
64 lactobacilli colostrum isolates were initially screened for their ability to grow in folic acid free media. 34 isolates were found to be auxotropic, indicating they were able to produce folate either intracellularly or extracellularly which is important for the cell growth and metabolism.
Screening for folate producing LAB by HPLC
Extracellular folate production by the above obtained isolates was evaluated using HPLC. In HPLC program of 30min run time, the peak retention time for folic acid was 16.4±0.2min. The chromatogram obtained by HPLC of each isolate revealed that 22 isolates had the ability to produce folate, which had the same retention time as standard Folic acid used. There was a significant difference in peak area which indicates the difference in folate production by these isolates. The extracellular folate concentration of screened isolates was in the range of 6-74.2±4µg/L. Reported folate producing S. thermophillus NCDC177 produced 34.2±0.3µg/L was used as reference strain. Based on the amount of folate produce by the isolates, there were 18.3% least producers (below 20µg/L), 18.1% were moderate producer (21-40µg/L) and 63.6% of good producer (more than 40µg/L). Out of 22 folate producing isolate, only 14 had ability to produce more than 40µg/L of extracellular folate of which 12 were L. plantarum and 2 were L. rhamnosus (Table 1). Isolate L. plantarum CKR26 showed the highest extracellular folate production of 74.2µg/L
Table (1):
Results of HPLC analysis for Folate: 22 LAB isolates produced folate in varying amounts
S No. |
Isolate Id |
Amount of folate produced (µg/L) |
|---|---|---|
1 |
L.plantarum CKR1 |
45.53 |
2 |
L.rhamnosus CKR2 |
43.76 |
3 |
L.plantarum CKR3 |
25.37 |
4 |
L.plantarum CKR5 |
52.41 |
5 |
L.plantarum CKR7 |
10.2 |
6 |
L.plantarum CKR8 |
66.52 |
7 |
L.plantarum CKR9 |
35.92 |
8 |
L.plantarum CKR11 |
53.26 |
9 |
L.plantarum CKR12 |
63.24 |
10 |
L.plantarum CKR15 |
31.92 |
11 |
L.rhamnosus CKR18 |
55.24 |
12 |
L.plantarum CKR19 |
15.23 |
13 |
L.plantarum CKR22 |
45.64 |
14 |
L.plantarum CKR26 |
74.2 |
15 |
L.plantarum CKR28 |
62.82 |
16 |
L.plantarum CKR31 |
53.77 |
17 |
L.plantarum CKR36 |
50.37 |
18 |
L.plantarum CKR37 |
46.82 |
19 |
L.plantarum CKR41 |
50.26 |
20 |
L.rhamnosus CKR42 |
30.11 |
21 |
L.rhamnosus CKR51 |
15.82 |
22 |
L.plantarum CKR56 |
13.26 |
Probiotic characterization
Tolerance to low pH and high bile salts concentration
The ability of isolates to resist and survive at low pH condition was examined by using MRS medium attuned with pH 2.0 and pH 2.0. At pH 2.5, 8 isolate of 14 tested showed 52-84% of survival rate, whereas L. plantarum CKR32 isolate had high degree of resistance of 84%. Significant variation in survival percentage was seen when the isolates were subjected to pH 2.0, which is considered as lethal for most of the bacteria. 5 isolate among tested had survival percentage of 26-59% and isolate L. plantarum CKR8 had highest survivability of 73% at pH 2. Isolates L. plantarum CKR5 and L. plantarum CKR12 showed increase in their cfu/ml even after 4 hour of incubation, this indicates that these isolates could resist and multiply at low pH.
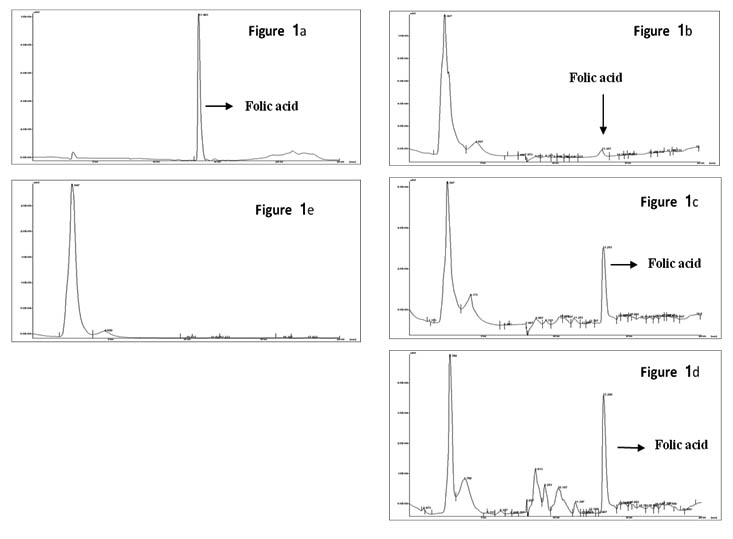
Figure 1a – chromatogram of standard folic acid;
Figure 1b, 1c, and 1d – chromatograms of different isolates pro-ducing folate in different concentration.
Figure 1e – chromatrogram of non folate producing isolate
Fig. 1. Chromatoragram of HPLC analysis for Folate
Tolerance and survival percentage of 14 isolate to bile salts was assessed at varying bile salt concentration (0.5%, 1% and 1.5%). Most of all the isolates showed good tolerance to 0.5% bile with 60-88% of survivability, where as L. rhamnosus CKR2 and L. plantarum CKR26 were bile sensitive. At 1% bile only three isolates L. plantarum CKR5, L. plantarum CKR8 and L. plantarum CKR12 were able to tolerate with survival rate of 42%, 50% and 56% respectively. Most interestingly none of the isolates were able to tolerate 1.5% bile, which was lethal to all cultures including reference strain.
Survivability in synthetic gastric juice
Eleven isolates were evaluated for their ability to survive in SGJ with pH 2.0. Isolates were selected on their recital in acid and bile tolerance. Survival curve of these 11 isolate during five hour incubation of 1hour interval in SGJ showed significant variation in their survivability. Initially till 2h there was significant decrease in viability of about 20-40% in most of the isolates. After 3h some isolates showed much reduction in viability, but, six isolates (L. plantarum isolates CKR5, CKR8, CKR11, CKR12 and CKR31 and L. rhamnosus CKR2) shown partial multiplication indicating their ability to resist and survive in this harsh condition. After 4h of incubation in SGJ, 9 isolate had 32-97% of survivability, where L. plantarum CKR5 had highest survival percentage (Fig 2).
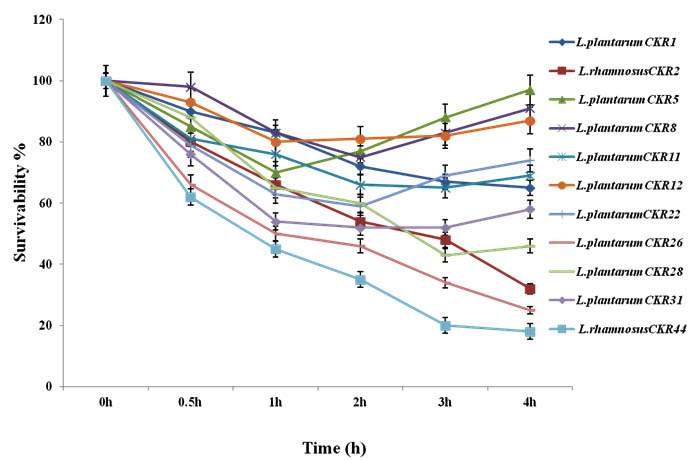
Fig. 2. Survival curve of 11 Lactic acid bacteria isolates synthetic gastric juice
Autoaggregation and Coaggregation
During adhesion to epithelial cells of intestine and in situation where there is less residence time for lactobacilli, the autoaggregation property can increase their adhesion. Nine isolate were initially tested for autoaggregation, each isolate was spectrometrically monitored each time at intervals of 1h for 5h. The auto aggregation curve obtained by this experiment showed the gradual increase in aggregation percentage along with incubation time. Isolate L. plantarum CKR8, L. plantarum CKR12 and L. plantarum CKR28 demonstrated the high rate of increase in their percentage of aggregation; at the end of 5h these isolates had 98%, 94% and 92% of autoaggregation respectively (Fig 3).
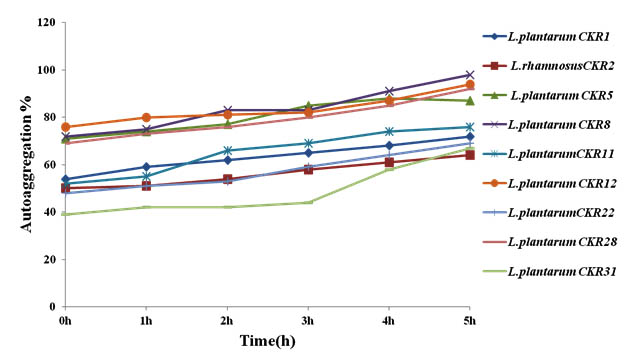
Fig. 3. Percentage of Autoggregation of nine Lactic acid bacteria
Coaggregation of seven isolates was tested against three pathogens E. coli, S. typhimurium and B. subtilis. Coaggregation of LAB is advantageous to host, because it interferes with the pathogen adherence to intestinal epithelial cells and prevent colonization of pathogenic bacteria. The Percentage of coaggregation varied among each isolate with each pathogen (Fig 4). Most of the isolates showed more co-aggregation with E. coli compare to other pathogens used. Isolates L. plantarum CKR5 and L. plantarum CKR12 had high percentage of coaggregation with E. coli (38% and 32%) and B. subtilis (28% and 31%), whereas L. plantarum CKR8 isolate showed highest coaggregation percentage with S. typhimurium (27%).
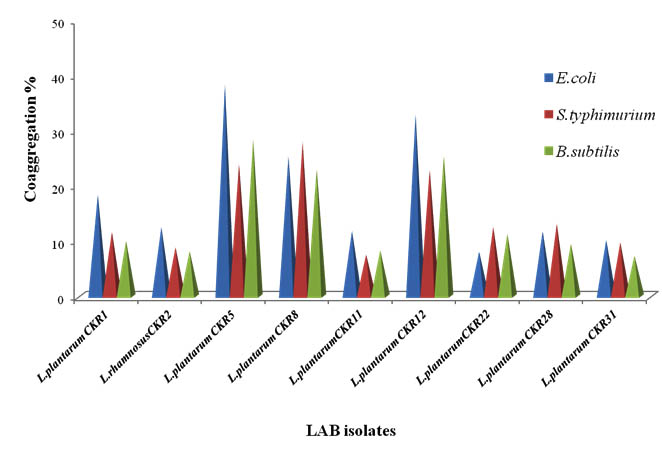
Fig. 4. Percentage of Coaggregation of nine Lactic acid bacteria
Cell surface hydrophobicity
Cell surface hydrophobicity (CSH) play an important role in LAB adhesion to intestinal epithelial cell and to some extant for the development of biofilm (Presti et al, 2015). To examine the hydrophobic nature of bacterial cell surface, xylene a non-polar solvent was used. Nine LAB isolates showed varying degree of hydrophobicity towards xylene (Fig 5). Four isolates had least hydrophobicity of less than 50%, three isolates L.plantarum CKR5, L. plantarum CKR8 and L. plantarum CKR22 had high affinity towards xylene of 65%, 67% and 74% respectively and isolate L. plantarum CKR28 showed highest of 76% of hydrophobicity.
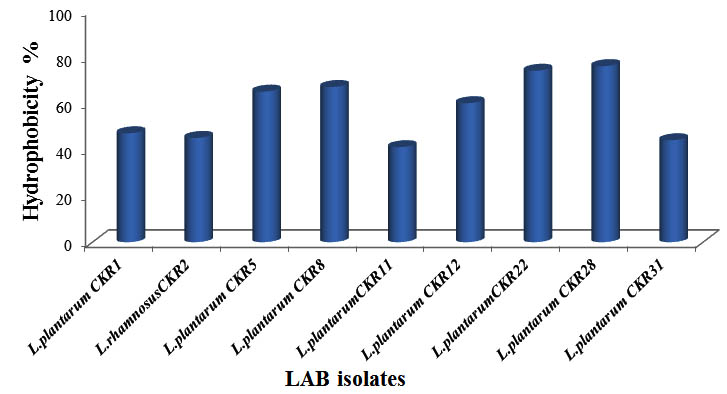
Fig. 5. Percentage of Cell surface hydrophobicity of nine Lactic acid bacteria isolates
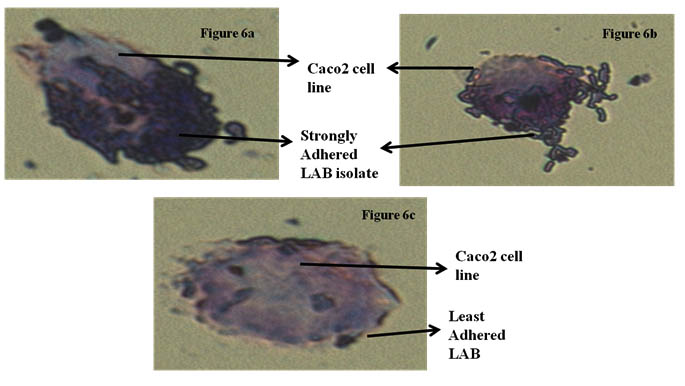
Fig 6(a) and 6(b) showing stron adherence LAB,
Fig 6(c) showing least adherence of LAB isolates
Fig. 6. Adherence of LAB isolates of Caco-2 cell line
Adherence to Caco-2 cell line
Caco-2 cell line is widely used as in vitro model for the assessment of adhesion ability to human intestinal epithelial cells. For Lactobacillus to colonize and to execute their optimal functionality, adhesion to intestinal cells is an imperative property. Adherence ability of nine isolates was microscopically examined. Depending on the number of bacteria adhered to Caco-2 cell line in 25 random microscopic fields. Isolate L. rhamnosus CKR2 had less adherence to Caco-2 cell line (28). Three isolate L. plantarum CKR11, L. plantarum CKR22, L. plantarum CKR31 had moderate adhesion, whereas four putative isolates L. plantarum CKR5, L. plantarum CKR8, L. plantarum CKR12 and L. plantarum CKR28 proved to have strong adherence ability.
Antibacterial activity of probiotic LAB
Inhibitory spectra of LAB against eight pathogenic bacteria were screened using agar well diffusion assay. Four isolates which had good acid-bile tolerance and adherence to caco-2 cell lines were screened for inhibitory activity against Salmonella typhimurium, Shigella flexneri, Citrobacter freundii, Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes and Bacillus cereus. Based on the result obtained by measure of zone of inhibition, isolates L. plantarum CKR8 and L. plantarum CKR28 had inhibitory activity towards Gram negative bacteria than Gram positive ones and isolate L. plantarum CKR5 had inhibitory activity against Gram positive bacteria (Table 2). Isolate L. plantarum CKR12 had wide range of inhibition towards all the pathogens tested.
Table (2):
Antibacterial activity of Lactic acid bacteria isolate against pathogens.
LAB Isolates Pathogen |
L.plantarun CKR5 |
L.plantarun CKR8 |
L. plantarun CKR8 |
L. plantarun CKR8 |
|---|---|---|---|---|
S.aureus |
+++ |
++ |
+++ |
+ |
B.subtilis |
+++ |
+ |
+++ |
++ |
B.cereus |
+++ |
++ |
+++ |
+ |
L.monocytogenes |
++ |
++ |
+++ |
+ |
S.flexneri |
+ |
++ |
+++ |
+++ |
S.typhimurium |
++ |
+++ |
++ |
++ |
E.coli |
++ |
+++ |
+++ |
+++ |
C.freundii |
++ |
+++ |
+++ |
+++ |
Zone of inhibition in mm: + -1-10 mm, ++-11-20 mm, +++-<20mm
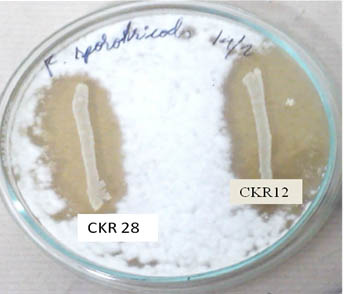
Fig. 7. Antifungal activity of probiotic lactic acid bacteria: Probiotic LAB isolates L.plantarum CKR28 and L.plantarum CKR12 showing inhibition against F. sporotrichioides
Antifungal activity of probiotic LAB
Confrontation assay was performed accordingly for seven isolates, which had conceded antibacterial activity, out of which three isolates had no effect on fungal growth, but two isolates L. plantarum CKR5 and L. plantarum CKR8 had mycelial inhibitory activity for 5 days against C. perpurea and F. graminarium. Isolates L. plantarum CKR28 and L. plantarum CKR12 showed wide range of inhibition against F. sporotrichioides (Fig 7), P. expansum, F. graminearum and A. parasiticus, these isolates not only inhibited the mycelial growth but also inhibited the spore formation even after 2 weeks of incubation. The effect of isolates L. plantarum CKR12 on other fungi such as A. flavous and A. ochraceous were also studied, but no significant effect was noted.
Folate is present in variety of foods like vegetables, fruits and dairy products, however their concentrations are not adequate to overcome folate deficiency (Iyer et al. 2009a). Hence products that are fermented by folate producing microorganism will be an effective alternative (Laino et al. 2013). In this concern an attempt was made to isolate a potent probiotic LAB from colostrums with high extracellular folate production. The scientific evidences illustrate colostrum as one of the best source for isolation of probiotic LAB (Mehanna at al. 2013), as it initiates and develop the neonatal gut microflora, which append for the benefit of newborn. To best of our knowledge, there are no reports on isolation of folate producing probiotic LAB from human colostrum.
Many researchers report that Lactococcus lactis, Streptococcus thermophilus, some Lactobacillus spp such as L. plantarum, L. bulgaricus, L. rhamnosus, L. crustorum, L. acidophilus, L. amylovorus and few Bifidobacterium spp like B. adolescentis, B. longum, and B. breve have the ability to produce folate both extracellularly and intracellularly in varying amounts, isolated from various sources such as dairy products, vegetables, milk and traditional fermented foods (Iyer et al. 2009), but, none of them have been used in product development, even though some of them have proved as probiotic like L. crustorum, B. longum, B. breve and S. thermophilus. Laino et al. (2013) developed yogurt using combination of folate producing L. bulgaricus and S. thermophilus isolates and claimed as, they are the first to develop yogurt bio-enriched with folate. Hence, there is a necessity for potential microbes for development of product enriched with natural folate.
In view of this, colostrum samples were collected from 10 healthy mothers within 2-8days after delivery and good number of LAB were isolated. Initially, all the isolated LAB were screened for their ability to grow in folic acid-free media, which indicates their competence for folate production, divulging 34 folate-auxotrophic isolates. Extracellular folate concentration in LABs was assayed in folic acid free medium using HPLC, which is highly sensitive than microbiological assay. The data obtained from HPLC (Table 1) illustrated that 22 isolated LABs were able to produce extracellular folate in the range of 6-74.2±4µg/L. This means, 22 of 34 folate-auxotroph isolates, were able to produce extracellular folate and remaining 12 might have produced folate intracellularly. Finally we found that only few strains of L. plantarum and an L. rhamnosus strain were able to produce high amount of extracellular folate and L. plantarum CKR26 was the highest producer when compared with standard strain S. thermophilus NCDC 177 (Iyer et al. 2011), which were later screened for their probiotic potentials.
The survivability of selected LAB was evaluated at high acidic pH for selection of potent strains, because according to Chiu et al. (2007) and Huang and Adams (2009) a significant number of LAB are lysed during transit through the GI tract. L. plantarum CKR5 and L. plantarum CKR12 were the best acidophiles screened. It is reported that when probiotics are consumed along with food, the food matrix are known to protect LAB from high acidity, hence the isolates which had moderate tolerance to low pH were also considered for further studies. All acid tolerant strains selected had good survival rate at standard bile concentration, except L. plantarum CKR26, a high folate producer. At high bile concentration L. plantarum CKR12 showed the highest resistance, which was found to be lethal to few isolate. Isolates which were able to resist both acid and bile salt were screened further for their survival in synthetic gastric juice. Even thought, all the isolates showed rapid drop in their viability initially (till 1.5h), few isolates slowly recovered and maintained good viability till 4h (Fig 2), where L. plantarum CKR5 had highest survivability and L. rhamnosus CKR44 showed least survivability. In order to offer their protection in a specific habitat, LAB must have ability to colonize well to intestinal surface, hence adhesion is considered as an important property that enables probiotic bacteria to colonize (Huang and Adams 2004). The adhesion abilities of these strains were strain dependent and showed high variability, even though all were belonging to same species. Many strain showed high hydrophobicity which indicates their adhesion strength. Although hydrophobicity contributes to explain the adhesion to some extent, the influence of other factors such as surface charge and cell surfaces molecules also contribute to adhesion. Collado et al. (2007) reported that autoaggregative capacity correlates with adherence; the high autoaggregation indicates the ability of LAB to form biofilm, which is required for its colonization. The autoaggregation abilities of L. plantarum isolates CKR5, CKR8, CKR12 and CKR28 were found to be very high (Fig 3). The strains with good autoaggregation ability showed good aggregation with E. coli when compared to two other pathogens tested i.e. B. subtilis and S. typhimurium. Theoretically, this aggregation ability is an important factor that interferes with the pathogens to adhere to receptors on the epithelial surface (Watson et al. 2008). L. plantarum isolates CKR5, CKR8, CKR12 and CKR28 showed strong adherence ability to Caco-2 cell line correlating with the results of cell surface hydrophobicity and aggregation abilities, which was similar to the study of Wang et al. (2010) on bifidobacteria. Processing of food is bound to kill bacteria, so these probiotics with strong adhesion ability will be more advantageous as the survived bacteria from processing will have high potential to adhere and colonize in the intestinal.
Probiotic LAB must have to compete with other microbes for existence in gut and one of the means of survival is through antimicrobial activity. Hence the screened folate producing probiotic L. plantarum isolates CKR5, CKR8, CKR12 and CKR28 were screened for antimicrobial activity. The inhibitory activity of L. plantarum CKR8 and CKR28 against Gram-negative bacteria (S. Typhimurium, S. flexneri, C. freundii and E. coli) was higher than that for Gram-positive bacteria (S. aureus, B. subtilis, L. monocytogens and B. cereus) Setyawardani et al. (2014). However, a different result was obtained for L. plantarum CKR5, which was correlating with Anas et al. (2008) where the Lactobacillus isolates from Algerian goat’s milk had a higher inhibition against Gram-positive than Gram-negative bacteria. Isolate L. plantarum CKR12 had wide range antibacterial activity against both Gram positive and Gram negative bacteria tested (Table 2). Inhibitory activity of L. plantarum against various fungal species is previously reported by Yang et al (2008). Therefore, the confrontation assay performed for antifungal activity which showed, L. plantarum CKR5 and L. plantarum CKR8 could only inhibit C. perpurea and F. graminarium of six fungi tested, but L. plantarum isolates CKR28 and CKR12 had inhibitory activity against F. sporotrichioides, P. expansum, A. parasiticus and F. graminearum. Generally, this antimicrobial activity of LAB is due to the production of organic acids, hydrogen peroxide, and protein or specific protein complex compound which is called as bacteriocin. The result of this study showed that L. plantarum CKR5, CKR8 CKR12 and CKR28 are having antibacterial and antifungal activity, so if these probiotics are consumed, the consumer will have additionally benefited as they offer protection against pathogens.
In this study, it is demonstrated that four isolates from human colostrum CKR5, CKR8, CKR12 and CKR28 belonging to L. plantarum, proved their probiotic potential, with high extracellular folate production in range of 52.4 – 66.5µg/L. The right combination of these folate producing strains and the optimization of fermentation conditions could lead to the development of foods with increased concentrations of folate without using genetic engineering techniques or chemical fortification. The consumers would be obviously benefited with such product, which are part of their normal diet and it is also true that before drawing any conclusion, animal and clinical trials has to be done, to prove their potential under in vivo condition. These potent probiotic vitamin B9 producers can be used in foods to provide natural folate both in weaning food for infants as its origin is colostrums and novel bio-enriched foods for adults as well as for pregnant woman, who require high RDA of folate.
ACKNOWLEDGMENTS
Authors are thankful to the Director, DFRL, Mysore, for providing the facility to carry out the work successfully.
- LeBlanc JG, Giori GSD, Smid EJ, Hugenholtz J and Sesma F. Folate production by lactic acid bacteria and other food-grade microorganisms. Communicating Current Research and Educational Topics and Trend in Applied Microbiology 2007; 1: 329–39.
- National Center on Birth Defects and Developmental Disabilities (NCBDDD), Neural Tube Defects. Annual Report, Fiscal Year. 2012.
- Bhide P, Sagoo GS, Moorthie S, Burton H and Kar A. Systematic review of birth prevalence of neural tube defects in India. Birth Defects Research Part A: Clinical and Molecular Teratology 2012; 97: 437– 443.
- Mills JL and Signore C. Neural tube defect rates before and after food fortification with folic acid. Birth Defects Research Part A: Clinical and Molecular Teratology 2004; 70(11), 844-5.
- Ramya Iyer and Tomar S.K. Folate: A Functional Food Constituent. Journal of Food Science 2009; 74: 9.
- Maddalena Rossi, Alberto Amaretti and Stefano Raimondi. Folate Production by Probiotic Bacteria. Nutrients 2011; 3: 118-134.
- Diba FS, Hossain KM, Azim MA and Hoque M. Isolation, characterization and determination of antimicrobial properties of lactic acid bacteria from human milk. Jordan Journal of Biological Sciences 2013; 6: 111–116.
- Nayra S.H. Mehanna, Nabil F. Tawfik, Moussa M.E. Salem, Baher A.M. Effat and D.A. Gad El-Rab. Assessment of Potential Probiotic Bacteria Isolated from Breast Milk. Middle-East Journal of Scientific Research 2013; 14(3): 354-360.
- Iiss Nuraida, Susanti, Nurheni SPalupi, hana, Rizka R Bastomi, Dhieta Priscilia and Siti Nurjahan. Evalialtion of probiotics properties of lactic acid bacteria isolated from breast milk and their potency as starter culture for yogurt fermentation. Interenational journal of food, nutrition and public health 2012; 5, 1/2/3.
- Abbas, M. and Mahasneh, A. Isolation of Lactobacillus strain with probiotic potential from camel’s milk. Academic journals 2014; 8(15): 1645-1655.
- Roy Byun, Mangala A. Nadkarni, Kim-Ly Chhour, F. Elizabeth Martin, Nicholas A. Jacques, and Neil Hunter. Quantitative Analysis of Diverse Lactobacillus Species Present in Advanced Dental Caries. Journal of clinical microbiology 2004; 3128–3136.
- Lidia Markiewicz, El¿bieta Biedrzycka. Identification of Lactobacillus and Bifidobacterium species with pcr applied to quality control of fermented dairy beverages. Polish Journal of Food and Nutrition Sciences 2005; 14(55) 4: 359–365.
- Ramya Iyer, Sudhir Kumar Tomar, Ashok Kumar Mohanty, Prashant Singh and Rameshwar Singh. Bioprospecting of strains of Streptococcus thermophilus from Indian fermented milk products for folate production. Dairy Science and Technology 2011; 91: 237–246.
- Chudar Kodi, K.M. Gothandam and Geetha Prabakaran. Identification and Characterization of Folic Acid Producing Potential Starter for Curd Fermentation. International journal of Current Microbiology and Applied Science 2015; 4(6): 118-130.
- Chiu HH, Tsai CC, Hsih HY and Tsen HY. Screening from pickled vegetables the potential probiotic strains of lactic acid bacteria able to inhibit the Salmonella invasion in mice. Journal of Applied Microbiology 2007; 104(2): 605-12.
- Huang J & Adams MC. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. International Journal of Food Microbiology 2004; 91: 253-260.
- Collado M.C, Surono I, Meriluoto J and Salminen S. Indugenous dadih lactic acid bacteria: cells-surface properties and interaction with pathogens. Journal of food Science 2007; 72: 89-93.
- Presti I, D’Orazio G, Labra M, La Ferla B, Mezzasalma V, Bizzaro G, Giardina S, Michelotti A, Tursi F, Vassallo M, Di Gennaro P. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Applied Microbiolgy and Biotechnologyc 2015; 99: 5613–5626.
- Del Re B, Sgorbati B, Miglioli M and Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Letters in Applied Microbiology 2000; 31: 438-442.
- Magnusson J, Ström K, Roos S, Sjögren J, and Schnürer J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiology Letters 2003; 219: 129–135.
- Laino JE, Valle MJ, Giori GS and LeBlanc JGJ. Development of a high folate concentration yogurt naturally bio-enriched using selected lactic acid bacteria. LWT – Food Science and Technology 2013; 54: 1-5.
- Kesarcodi-Watson A, Kaspar H, Lategan MJ and Gibson L. Probiotics in aquaculture: the need principles and mechanisms of action and screening processes. Aquaculture 2008; 274: 1-14.
- Wang L, Meng X, Zhang B, Wang Y and Shang Y. Influence of cell surface properties on adhesion ability of bifidobacteria. World Journal Microbiology Biotechnology 2010; 26: 1999–2007.
- Setyawardani T, Rahayu W. P, Maheswari R. R and Palupi, N. S. Antimicrobial activity and adhesion ability of indigenous lactic acid bacteria isolated from goat milk. International Food Research Journal 2014; 21(3): 959-964.
- Anas, M., Eddine, H. J. and Mebrouk, K. Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat’s milk against Staphylococcus aureus. World Journal of Dairy Food Science 2008; 3(2): 39-49.
- Yang, Eun-Ju, Chang and Hae-Choon. Antifungal Activity of Lactobacillus plantarum isolated from Kimchi. Microbiology and Biotechnology Letters. 2008; 36(4): 276-284.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


