ISSN: 0973-7510
E-ISSN: 2581-690X
Sputum microscopy is the primary diagnostic tool for screening pulmonary tuberculosis (PTB) suspects in TB prevalent countries. Our study had evaluated the ‘front loading’ or ‘same day’ sputum microscopy feasibility to screen clinically suspected PTB cases, in comparison to standard method of smear microscopy. In this hospital based cross-sectional study, three sputum specimens i.e., two consecutive spot samples collected in one hour interval on the first day of visit and a single sputum sample was collected on the next day early morning from 312 randomly selected adults suspected for PTB. Sputum samples were cultured on Lowenstein-Jensen (LJ) medium and stained by auramine O method and examined under LED-fluorescence microscopy. Out of 312 presumptive PTB patients, 43 (13.8%) were smear-positive by front loading method and 46 (14.7%) by standard method respectively. Considering LJ media culture as the gold standard test, the sensitivity was 83.7% and 89.8% respectively for front loading and the standard sputum microscopy and specificity was 99.2% by both methods. The statistical difference was insignificant between two methods of sputum microscopy (p-value > 0.05 by McNemar’s test). In health care settings of high burden countries same day sputum microscopy could be an acceptable method to screen the suspects of pulmonary tuberculosis and complete the diagnosis procedure on the first day of visit, which will decrease patients’ drop-out from the diagnostic procedure and initiate treatments as soon as possible.
Front-loading, LED fluorescence microscopy, pulmonary tuberculosis, sputum smear, diagnosis, same day.
Tuberculosis (TB), an ancient disease still remains as greatest public health challenge in many parts of the world1. WHO reported TB as topmost disease caused from a single infectious agent and also marked as the ninth leading cause of death worldwide. According to the World Health Organization (WHO) 2017 report, 6.3 million cases of TB were reported in 2016 (up from 6.1 million in 2015) equivalent to 61% of the estimated incidence of 10.4 million, and an estimated 1.7 million deaths resulted from TB in 2016 globally. Among top ten high burden countries India accounted 25% of TB cases2. Despite recent advances in diagnostic techniques, smear microscopy is the frequently used technique for screening pulmonary tuberculosis (PTB) suspects in TB prevalent and low-income countries1. In TB prevalent countries, microscopic technique is highly specific, relatively simple, rapid and inexpensive for detecting Mycobacterium tuberculosis (MTB)3. According to RNTCP guidelines, whenever pulmonary TB is suspected, at least two sputum specimens should be collected over two consecutive days, i.e. one spot sputum sample at first day of visit and a single early morning sputum sample brought by patient next day to health-care center4. It takes multiple visits to health care facilities and further delayed in start the treatment. This makes the diagnostic process costly and more inconvenient for patients, which leads to high drop-outs and spreads the disease. The undiagnosed patients often give rise to newer TB cases with high mortality5.
For diagnosis of PTB, “same day” or “front loading” method of sputum specimen collection has been suggested by WHO. This method recommends to provide two spot sputum samples in one hour interval at first day of visit (spot 1-spot 2) by all the PTB suspects5. It may reduce patients’ drop-outs and help to initiate early treatment. Our study had evaluated the diagnostic accuracy of “same day” sputum microscopy method using Light emitting diode-fluorescence microscopy (LED-FM) to screen clinically suspected PTB cases and it’s outcome compared with standard method of sputum microscopy (spot1- early morning) and conventional culture on Lowenstein-Jensen medium.
This study was approved by the Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee. This cross-sectional study was conducted at the Department of Microbiology, Kasturba Medical College, Manipal, in Karnataka, India from February – April 2018. It is a tertiary care hospital with referral centre for DOTS. The study included pulmonary TB suspects aged ³ 18 years with history of cough ³ 2 weeks and excluded old PTB patients and none of the patients had received medication of anti-tubercular drugs for at least one month before sputum sample collection. None of the patients had HIV and other immune-compromising diseases. Informed consent was taken from the participants before samples were collected. Patients’ relevant clinical details were filled in a standardized Performa.
The participants were asked to give 3-5 ml sputum samples in a wide mouth, sterile, leak proof, appropriately labelled disposable container. Three sputum samples were collected from all the individuals: on first day of visit two sputum samples were collected at the interval of one hour (spot1-spot2) and the third sputum sample, which was collected early morning in pre-labelled container at home and bought to laboratory next day. Samples were examined on daily basis.
Sputum smear microscopy followed RNTCP standard protocol6. Sputum smears were prepared on clean, grease-free slides using mucopurulent portion (2cm x 3cm) and stained using the Auramine O fluorescence technique.
The smears were stained using primary stain 0.3% auramine-O solution (7-10 minutes), decolourizer 1% acid-alcohol (2 minutes) and counterstained with 0.1% potassium permanganate (30 seconds) and read at 400x magnification and graded according to the WHO/IUATLD system7. Tubercular bacilli appear yellowish-green colour, Long, slender or slightly curved, some are granular and arrange in single, small groups or in large clumps.
The diagnosis of presumptive TB were further confirmed with the ‘gold standard’ culture method on Lowenstein-Jensen (LJ) medium. Conventional culture technique followed RNTCP standard protocol8. For conventional culture, patient’s sputum samples were pooled and decontaminated by Petroffs’ method (4% NaOH). Then the decontaminated sediment was inoculated on two slopes of LJ medium. After inoculation appropriately labelled LJ medium bottles were incubated at 37°C and the growth on LJ slopes were examined weekly up to 8 weeks. Positive growths on LJ medium slopes were confirmed by examining acid fast bacilli and further strain identification was done using colony texture, morphology, and pigment production and SD BIOLINE TB Ag MPT64 Rapid kit to identify MTB complex or Non Tuberculosis Mycobacteria .
In this study, the culture is considered as “gold standard”, to compare the results of standard and front loading smear microscopy method.
Data were entered, validated and analysed using IBM SPSS Statistics var. 16 (Chicago, IL, USA). Patients were considered as “smear positive” as the 1-9 bacilli is seen in any of the samples.
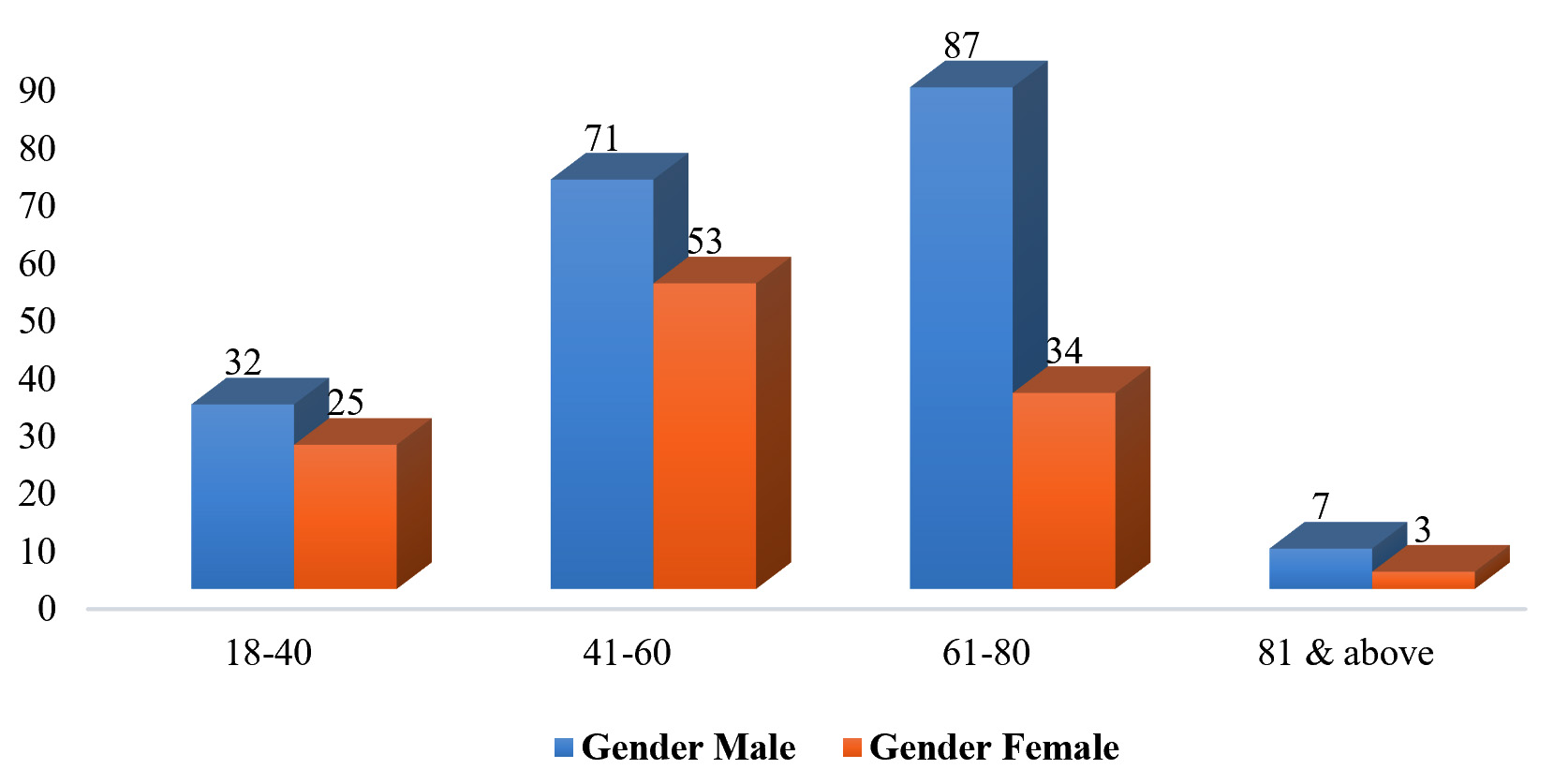
Out of 312 enrolled TB suspects 197 (63.1%) were males and 115 (36.9%) were females. The male: female ratio was 1.71:1. Mean age of male patients were 56.94 years and female 52.45 years. The distribution based on age and gender of the enrolled patients is given in Fig. 1.
Patients clinical records showed history of fever 120 (38.5%), breathlessness 229 (73.4%), haemoptysis 23 (7.4%), chest pain 48 (15.4%) and loss of appetite 31 (9.9%). Patients had history of tobacco smoking 71 (22.8%), alcoholism 17 (5.4%) and diabetes 79 (25.3%). Majority of the patients had preferred same day submission of sputum specimens 295 (94.8%).
Out of 312 TB suspects, 42 (13.5%), 41 (13.1%) and 46 (14.7%) were smear positive and 270 (86.5%), 271 (86.9%) and 266 (85.3%) were smear negative for acid fast bacilli from spot 1, spot 2 and early morning sputum respectively. Both spot 1 and spot 2 sputum smears were positive for 40 patients. Only spot 1 sputum smear was positive for 2 PTB suspects and 1 patients was positive in spot 2 sputum smear. Spot 1 sputum showed 0.4% more positivity than spot 2 sputum specimen. For 42 PTB suspects, both spot 1 and early morning sputum smears showed AFB positive and only early morning sputum smears of 4 patients detected AFB positive.
By front loading method of sputum microscopy 43 (13.8%) patients were AFB positive, taking any of the spot 1 and spot 2 smear as AFB positive or both. Standard sputum microscopy had detected 46 (14.7%) patients, taking any of the spot 1 and early morning smear as AFB positive or both positive. Standard method had detected 3 presumptive PTB patients, who were not detected by front loading method.
A total 46 (14.7%) sputum smears were AFB positive and 266 (85.3%) sputum smears were AFB negative among 312 presumptive PTB patients. In comparison with the standard method sensitivity (93.47%), specificity (100%), positive predictive value (100%) and negative predictive value (98.89%) of the front loading sputum microscopy was detected [Table 1]. The statistical difference was insignificant for this two methods (P= 0.250 by McNemar’s test).
Table (1):
Comparison of standard method and front loading method of sputum microscopy
|
Sputum microscopy |
Smear |
Standard method | Sensitivity
(%) |
Specificity
(%) |
PPV
(%) |
NPV
(%) |
|
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| Front-loading
method |
Positive | 43 | 0 |
93.47 |
100 |
100 |
98.89 |
| Negative | 3 | 266 | |||||
In early morning sputum samples showed a predominance of 3+ [20 (43.5%)] and 2+ [15 (32.6%)] positive smears for AFB. In contrast second spot sputum samples showed predominance of scanty [14 (34.1%)] positive smears for AFB, as shown in Table 2.
Table (2):
Grading of AFB positive sputum smears by WHO/IUATLD system
|
Sputum microscopy |
Samples |
Smear grading (by RNTCP AFB grading ) |
Total
|
|||
|---|---|---|---|---|---|---|
|
Scanty |
1+ |
2+ |
3+ |
|||
|
Front-loading Method |
Spot 1 |
7
(16.7 %) |
9
(21.4 %) |
10
(23.8 %) |
16
(38.1 %) |
42 |
|
Spot 2 |
14
(34.1 %) |
7
(17.1%) |
9
(22.0%) |
11
(26.8 %) |
41 | |
|
Standard method |
Spot 1 |
7
(16.7 %) |
9
(21.4 %) |
10
(23.8 %) |
16
(38.1 %) |
42 |
| Early morning | 6
(13.0 %) |
5
(10.9 %) |
15
(32.6 %) |
20
(43.5 %) |
46 | |
The sputum culture of 312 PTB suspects on Lowenstein Jensen (LJ) Medium showed 49 (15.7%) positive growth for M. tuberculosis. Culture was negative for M. tuberculosis growth in 263 (84.3%) PTB suspects. Majority of M.tuberculosis growth on LJ media was observed [36 (73.5%)] during 5–7 weeks of incubation.
In comparison with the culture on LJ media, the ‘gold standard test’ for MTB, the sensitivity (83.7%), specificity (99.2%), positive predictive value (95.3%) and negative predictive value (97%) of the front loading method of sputum microscopy was calculated. Also the sensitivity (89.8%), specificity (99.2%), positive predictive value (95.7%) and negative predictive value (98.1%) of the standard method of sputum microscopy was calculated, in comparison to the culture. The statistical differences between two sputum microscopy methods and conventional culture method were insignificant (front-loading method P = 0.109 and standard method P = 0.453 by McNemar’s test).
Sputum smear microscopy is a widely used method, especially low-income and middle-income countries for diagnosing pulmonary tuberculosis. Microscopy is rapid, inexpensive, relatively simple, and highly specific for MTB in the prevalent areas10.
In this study 295 (94.8%) patients had preferred same day sputum microscopy. The study by Firdaus et.al. and Myneedu et al. in which 91.1% and 93.03% of patients had preferred same day sputum collection respectively9,10. However, a study carried out by Nayak et.al. in Chhattisgarh where higher patients drop-out was observed, 7% with same-day approach compare to 5% with standard method11. A study conducted in South Western Uganda where 22% of the participants failed to bring the second day specimens12. Rural health-care setup could be the reason of higher number of dropouts, where front loading method is highly recommendable.
In this study, sputum smears from spot 1, spot 2 and early morning specimen were positive for AFB 13.5% (42), 13.1% (41), 14.7% (46) respectively. Studies conducted by Firdaus et. al. showed 12.7%, 12% and 13.8% of smears from spot 1, spot 2 and early morning specimen were AFB positive and Myneedu et al. showed the smear positivity of spot 1, spot 2 and early morning sputum samples were 12.72%, 11.8% and 18.48% respectively9,10. The total sputum smear positivity rate in present study was 14.7% in contrast to above mentioned studies9,10 i.e. 14.1% and 18.48% respectively. Early morning specimens detected higher number of individuals with pulmonary TB compare to spot samples. As per observation, the 4 PTB cases not detected by front loading method had a lower bacillary load and were scanty positive by standard method.
Also, early morning samples showed predominantly of 3+ sputum smears (43.5 %), whereas second spot samples showed predominantly of scanty smears (34.1%). A slight increase in positivity in third sputum samples reported from various studies9,11. Study conducted in Chhattisgarh by Nayak et.al. observed 4% progressive yield of second sputum sample by same day microscopy, in contrast to standard method which was 20%13. The reason behind lower positivity in second spot samples may be poor quality of spot sputum samples or intermittent shedding of tubercular bacilli in respiratory secretion. In this study, the statistical difference between two methods were insignificant. Similar results have been observed by various other studies, where higher positivity showed by standard method compare to front loading method but it was not statistically significant9,14.
In this study the culture positivity rate was 15.7%, comparing with the sensitivity of smear examination by front loading and standard method were 83.7% and 89.8% respectively, while specificity was 99.2% for both methods. Our study had found a better sensitivity for both microscopic methods using LED-fluorescence microscopy. The sensitivity could be increased due to higher bacillary load in sputum samples and proper samples collection from the participants. The front loading and standard method were unable to detect 8 and 5 TB positive cases respectively. Implementing culture method for diagnosis of tuberculosis can detect further new cases, which were not detected by smear microscopy.
In this study, two smear positive cases showed no growth on LJ medium. This difference can depend on a wide variety of factors such as bacterial load, method of decontamination, heat produced during centrifugation, quality of LJ medium8,9.
Apparently, the cases unable to detect by front loading method of sputum microscopy were paucibacillary. Front loading method could identify most of the smear positive cases and this method could be implemented in high prevalence and low income countries.
In high burden countries sputum smear microscopy remains the backbone of tuberculosis control programmes for diagnosis of pulmonary TB. Front loading sputum microscopy could be implemented in National Tuberculosis Control Programmes to screen the suspects of pulmonary tuberculosis and complete the diagnosis procedure on the first day of visit, decrease patients drop-out and initiate treatment as soon as possible.
Acknowledgements
We would like to express our heartfelt thanks to Ms Suraksha for providing technical assistance for out this study. We also like to thank MAHE for providing all the support in conducting this study.
Conflicts Of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contribution
All authors have made substantial, direct and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by Microbiology Department KMC Manipal, MAHE under student’s research support scheme.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
- Ryu Y.J. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc. Respir. Dis., 2015; 78(2): 64-71.
- World Health Organization. Global Tuberculosis Report 2017 (WHO/HTM/TB/2017.23). Geneva: World Health Organization, 2017.
- Steingart K.R., Ramsay A., Pai M. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert. Rev. Anti Infect. Ther., 2007; 5(3): 327–331.
- Revised National tuberculosis Control Programme (RNTCP). Training module for medical practitioners. Central TB Division, Directorate General of Health Services. New Delhi India: Ministry of Health and Family Welfare, 2010.
- World Health Organization. Same-Day Diagnosis of Tuberculosis by Microscopy: WHO Policy Statement. Geneva: World Health Organization, 2011.
- Revised National tuberculosis Control Programme (RNTCP). Manual for Sputum Smear Fluorescence Microscopy. Central TB Division, Directorate General of Health Services. New Delhi India: Ministry of Health and Family Welfare.
- Lumb R., Deun A.V., Bastian I., Fitz-Gerald M. Laboratory diagnosis of tuberculosis by sputum microscopy: The handbook Adelalde: SA Pathology, 2013.
- Revised National TB Control Programme (RNTCP). Training Manual for Mycobacterium tuberculosis Culture & Drug susceptibility testing. Central TB Division, Directorate General of Health Services. New Delhi India: Ministry of Health and Family Welfare, 2009.
- Firdaus S., Kaur I.R., Kashyap B., Avasthi R., Singh N.P. Front loading sputum microscopy–an alternative approach for diagnosis of pulmonary tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis., 2017; 8: 6-12.
- Myneedu V.P., Verma A.K., Sharma P.P., Behera D. A pilot study of same day sputum smear examination, its feasibility and usefulness in diagnosis of pulmonary TB. Indian J. Tuberc., 2011; 58(4): 160-167.
- Nayak P., Kumar A.M., Agrawal T.K., Chandraker S., Nair S.A. Same-day light-emitting diode fluorescence microscopy for the diagnosis of tuberculosis in Chhattisgarh, India. Int J. Tuberc Lung Dis., 2014; 18(6): 666-670.
- Bazira J., Mwambi B. and Muyindike W. Feasibility for Same Day Tuberculosis Diagnosis Using the Smear Microscopy Approach in Rural South Western Uganda. Adv. in Res., 2015; 4(3): 151-155.
- Nayak P., Kumar A.M., Claassens M., Enarson D.A., Satyanarayana S., Kundu D., Khaparde K., Agrawal T.K., Dapkekar S., Chandraker S., Nair S.A. Comparing Same Day Sputum Microscopy with Conventional Sputum Microscopy for the Diagnosis of Tuberculosis–Chhattisgarh, India. PloS one, 2013; 8(9): 74964.
- Chandra T.J., Raj R.S., Sharma Y.V. Same day sputum smear microscopy approach with modified ZN staining for the diagnosis of pulmonary tuberculosis in a microscopy centre at Rajahmundry. Indian J. Med. microbiol., 2014; 32(2): 153.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.