ISSN: 0973-7510
E-ISSN: 2581-690X
Human papillomavirus (HPV) can be transmitted sexually and causes cervical malignancies in women. Among the risk factors, sexually transmitted infections (STIs) caused by bacteria have raised concerns because they are asymptomatic and persistent and can increase the risk of HPV infection. This study assessed the correlation among Ureaplasma urealyticum, Ureaplasma parvum, Chlamydia trachomatis, and Mycoplasma hominis infections towards abnormal cervical cells positive for HPV. Seventy outpatients at the Colposcopy Outpatient Clinic, Dr. Cipto Mangunkusumo Hospital in Jakarta, Indonesia, were enrolled in this cross-sectional study. Viruses and bacteria were detected using polymerase chain reaction and compared with liquid-based cytology results for cervical cytology. Of the 70 patients, 24 (34.28%) showed abnormal cervical cytology. Among those with abnormal cervical cytology, C.trachomatis was reported in 1 patient (4.2%), M.hominis in 6 patients (25%), U.urealyticum in 13 patients (54.2%), and U.parvum in 10 patients (41.7%). Statistical analysis demonstrated an association between U.urealyticum and U.parvum infections and HPV infection (U.urealyticum p = 0.012; U.parvum p = 0.022). U.urealyticum and U.parvum were more prevalent than C.trachomatis and M.hominis in HPV-positive women, suggesting their role in HPV infection.
Cervical Cytology, Cervical Malignancy, Ureaplasma Urealyticum, Ureaplasma Parvum, Chlamydia Trachomatis, Mycoplasma Hominis
Human papillomavirus (HPV) is an etiological agent of cervical malignancy in women.1-3 HPV has been classified based on specific genomic regions, such as the E7, E6, and L1 protein-coding regions. More than 200 HPV types have been identified to date,4,5 which have been grouped into low-risk HPV (LR-HPV) and high-risk HPV (HR-HPV) based on the malignant transformation of infected cells.4,6 HR-HPV has oncogenic features and includes types 16,18,31,33,35,39,45,51,52,56,58,59,68,73 and 82.2,4,7 Most HPV infections are cleared by cell-mediated immunity within 2 years of exposure.1,4 However, certain types of HPV show persistent infections, such as HPV-16 and HPV-18.2,4,8 Long-term persistence of HR-HPV causes a decline in viral clearance and indicates the change of cervical cells to pre-cancerous forms or even cancer.4,8
Besides the persistence of HPV infection, other risk factors also play an essential role in the progression of cervical precancer and cancer, including the early onset of sexual activity, high parity, smoking, hormonal contraception, immunosuppression, and bacterial sexually transmitted infections (STIs).9,10Among these, bacterial STIs have raised concern because they are asymptomatic and persistent and can increase the risk of HPV infection.10-12 Several bacterial STIs have been reported as risk factors, including Ureaplasma urealyticum,Ureaplasma parvum, Chlamydia trachomatis, and Mycoplasma hominis.3,12-14
A study showed that C.trachomatis infection was reported more frequently in HPV-positive women than that in HPV-negative women.3,14,15 A study on genital mycoplasma infections and cervical cytology abnormalities showed that M.hominis and U.urealyticum were found in samples with abnormal cervical cytology.16-18 There are no established data from Indonesia showing a correlation among U.urealyticum, U.parvum, C.trachomatis, and M.hominis infections with abnormal HPV-positive cervical cytology. Therefore, a cross-sectional study was conducted to assess the relationship among U.urealyticum, U.parvum, C.trachomatis, and M.hominis infections and abnormal HPV-positive cervical cytology.
Patient and Specimen Collection
This was a cross-sectional study with a minimum sample size of 30 participants. Seventy women were recruited from the Colposcopy Outpatient Clinic of Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia, between June 2016 and June 2017. Respondents were recruited through consecutive sampling. All participants were sexually active, 20–67 years old, nonpregnant, and provided written informed consent prior to enrollment in the study. An obstetrician obtained a cervical sample and placed it in a Liqui-PREP liquid-based cytology (LBC) vial (LGM International Inc., Melbourne, FL, USA). The sample was subsequently divided into 5 mL portions for cytological examination and polymerase chain reaction (PCR). The samples were stored at a temperature of 4°C and were processed within 2 days of collection.
Liquid-Based Cytology Pap Smear
Liquid-based cytology Pap smears were performed based on the corporation’s protocols under the supervision of a pathologist.19 The cervical cytology was categorized using the 2014 Bethesda system: high-grade squamous intraepithelial lesions (HSIL); low-grade squamous intraepithelial lesions (LSIL); atypical squamous cells, cannot exclude HSIL (ASCH); atypical squamous cells of undetermined significance (ASCUS); and normal cytology or negative for intraepithelial lesion or malignancy (NILM).20
DNA Extraction
A 1-mL aliquot of a 5-mL LBC sample was centrifuged (Sorvall Biofuge Primo R, Thermo-Fisher Scientific Inc., Waltham, MA, USA) for 30 min. at 13,000 rpm. The pellet was then resuspended in 200µL phosphate-buffered saline after removing the supernatant. DNA isolation was conducted with a High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany), based on the corporation’s protocols, with 50µL of final elution volume.21 Purified DNA was kept at -20oC for ≤1 week prior to being used for PCR.
DNA Positive Control
A positive control was obtained from the Laboratory of Virology and Molecular Biology DNA Collection, Microbiology Department, Medical Faculty, Universitas, Indonesia. The positive control was detected using PCR and confirmed via DNA sequencing.
PCR for Detecting HPV
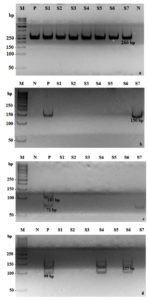
A 266-bp fragment of human b-globin was amplified to validate the sample collection and/or the DNA extractions (Figure 1a) using specific primers (Forward/Reverse: 5′ -AAG AGC CAA GGA AAG GTA-3′ / 5′-AAC TTC ATC CAC GTT CAC-3′).22 The PCR was conducted in 10µL total volume:1x PCR buffer, 1.2mM MgCl2, 0.2mM dNTP mix, 5x Q Solution, 0.25 µM primer mix, 1.25 U HotStar Taq DNA Polymerase (Qiagen, Hilden, Germany), and 2µL DNA sample. HPV was detected using consensus primers GP5+/GP6+: 5’-TTT GTT ACT GTG GTA GAT ACT AC-3’ / 5’-GAA AAA TAA ACT GTA AAT CAT ATT C-3’,23 in a 20µL total volume:1x PCR buffer, 1.2mM MgCl2, 0.2mM dNTP mix, 5x Q Solution, 0.25µM primer mix, 1.25 U HotStar Taq DNA Polymerase (Qiagen, Hilden, Germany), and 5 µL DNA isolate. Thermal cycler settings were 95°C for 15 min followed by 40 amplification cycles at the following conditions: 30s at 94°C, 90s at 49°C,45s at 72°C, and final elongation 10 min at 72°C.
PCR for Detecting U. urealyticum, U.parvum, C.trachomatis, and M.hominis
Conventional duplex PCR was used to identify U.urealyticum, U.parvum, C.trachomatis, and M.hominis. Duplex PCR was carried out twice using primers according to previous studies, with modifications.24,25 The first duplex was performed to detect M.hominis (101 bp; Forward/Reverse: 5’-TTT GGT CAA GTC CTG CAA CGA-3’ / 5’-CCC CAC CTT CCT CCC AGT TA-3’ and C.trachomatis (71 bp; Forward/Reverse 5’-CAT GAA AAC TCG TTC CGA AAT AGAA-3 / 5’-TCA GAG CTT TAC CTA ACA ACG CATA-3’), in 20µLtotal volume:0.2mM dNTP mix, 1.2 mM MgCl2, 1x PCR buffer, 5x Q Solution, 0.05 µM of M.hominis primer mix, 0.45µM of C.trachomatis forward primer, 0.15µM of C.trachomatis reverse primer, 1.25 U Taq polymerase (Qiagen, Hilden, Germany), and 4 µL DNA isolate. Thermal cycler conditions were set up for 15 min at 95°C followed by 40 amplification cycles at the following conditions: 30s at 94°C, 30s at 60°C, 30s at 72°C, and a final elongation of 7 min at 72oC. The second duplex PCR was performed to detect U.urealyticum (127 bp; Forward/Reverse: 5’-GAT CAC ATT TTC ACT TGT TTG AAG TG-3’/ 5’-CAC GAG CAG ATT GCA TTA AGT CAG-3’)26 and U.parvum (99 bp; Forward/Reverse: 5’-GAT CAC ATT TTC ACT TGT TTG AAG TG’3 / 5’-AAC GTC GT CCA TAA GCA ACT TTG-3’).27 Duplex PCR was performed according to previous studies, with modifications.26,27 A 20 µL PCR reaction was performed with the following reagents:1x PCR buffer, 0.2 mM dNTP mix, 1.2 mM MgCl2, 5x Q Solution, 1.25 U HotStar Taq DNA Polymerase (Qiagen, Hilden, Germany), 0.3 µM of U.urealyticum primer mix, 0.4 µM of U.parvum primer mix, and 4 µL DNA isolate. Thermal cyclers were set up for 15 min at 95oC followed by 40 amplification cycles at the following conditions: 30s at 94°C, 30s at 60°C, 30s at 72°C, and a final elongation of 7 min at 72°C. All PCR amplifications were performed using an Applied Biosystems GeneAmp PCR System 2700 (Thermo Fisher Scientific, Waltham, MA, USA). Polyacrylamide gel electrophoresis (9%) was performed to analyze the PCR products, and UV transillumination for DNA band documentation was performed using a GelDoc system (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
Data were inputted into Microsoft Excel 2019 v16.0 (Microsoft, Redmond, WA, USA) and subsequently analyzed using IBM SPSS Statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA). A comprehensive descriptive analysis was conducted to generate summary statistics, including mean, median, standard deviation, and 95% confidence intervals, pertaining to baseline demographic and behavioral attributes. Data are presented as numbers and percentages with 95% confidence intervals. Pearson’s chi-square test was used to examine the associations between categorical variables, whereas Fisher’s exact test was implemented in instances where one or more cells exhibited an expected frequency ≤5. Statistical significance was determined by associations with P values <0.05.
The patient characteristics are shown in Table 1. Of the 70 patients who participated in this study, 24 (34.3%) had abnormal cervical cells. Of these 24 patients, 9 (37.5%) had ASCUS, 5 (20.8%) had ASCH, 2 (8.3%) had LSIL, 7 (29.2%) had HSIL, and only 1 (4.2%) had cervical carcinoma (data not shown). HPV PCR for these 24 patients identified 11 (45.8%) HPV-positive patients (Figure 1b), predominantly those with HSIL. Statistical analysis revealed a significant correlation between HPV infection and abnormal cervical cytology results (p = 0.007; Table 2).
Table (1):
Colposcopy Outpatient Clinic Patients Characteristics.
Normal cytology n (%) |
Abnormal cytology n (%) |
p-value |
|
|---|---|---|---|
Early onset of sexual activity |
|||
≤19 years old |
6 (13) |
6 (25) |
0.176** |
>19 years old |
40 (87) |
18 (75) |
|
Parity (children) |
|||
3–5 |
13 (28.3) |
7 (29.2) |
0.937* |
0–2 |
33 (71.7) |
17 (70.8) |
|
Smoking |
|||
Yes |
5 (10.9) |
4 (16.7) |
0.368** |
No |
41 (89.1) |
20 (83.3) |
|
Contraception |
|||
Yes |
23 (50) |
13 (54.2) |
0.741* |
No |
23 (50) |
11 (45.8) |
|
Immunosuppression status |
|||
Yes |
0 (0) |
2 (8.3) |
0.114** |
No |
46 (100) |
22 (91.7) |
Abnormal cytology: cervical carcinoma, HSIL, LSIL, ASCH, and ASCUS. *Pearson Chi-squared test. **Fisher exact test.
Table 1 shows that most respondents were sexually active at >19 years of age, had 0–2 children, did not smoke, used contraception, and were immunocompetent. Most respondents used an intrauterine device (12/36), followed by injections (10/36), pills (7/36), condoms (4/36), tubectomies (2/36), and implants (1/36) for their contraceptive control. Two respondents had HIV infections and systemic lupus erythematosus.
Identification of bacterial STIs via conventional duplex PCR showed that 35 (50%) patients were U.urealyticum-positive, 25 (25.7%) were U.parvum-positive, 9 (12.9%) were M.hominis-positive, and 2 (2.9%) were C.trachomatis-positive (Figure 1c and 1d). Of the 24 patients with abnormal cervical cytology, 13 (54.2%) were U.urealyticum-positive, 10 (41.7%) wereU.parvum-positive, 6 (25%) were M.hominis-positive, and only 1 (4.2%) was C.trachomatis-positive. Statistical analysis revealed a significant correlation between cervical abnormalities and M.hominis infection (p = 0.037), whereas other infections showed no association (Table 2).
Table (2):
Association of LBC cytology results with HPV, Ureaplasma urealyticum, Ureaplasma parvum, Chlamydia trachomatis, and Mycoplasma hominis infection
Normal cytology n (%) |
Abnormal cytology n (%) |
p-value |
|
|---|---|---|---|
Human papillomavirus |
|||
Negative |
40 (87) |
13 (54.2) |
0.007* |
Positive |
6 (13) |
11 (45.8) |
|
Chlamydia trachomatis |
|||
Negative |
45 (97.8) |
23 (95.8) |
0.453* |
Positive |
1 (2.2) |
1 (4.2) |
|
Mycoplasma hominis |
|||
Negative |
43 (93.5) |
18 (75) |
0.037** |
Positive |
3 (6.5) |
6 (25) |
|
Ureaplasma urealyticum |
|||
Negative |
24 (52.2) |
11 (45.8) |
0.615* |
Positive |
22 (47.8) |
13 (54.2) |
|
Ureaplasma parvum |
|||
Negative |
31 (67.4) |
14 (58.3) |
0.453* |
Positive |
15 (32.6) |
10 (41.7) |
Abnormal cytology: cervical carcinoma, HSIL, LSIL, ASCH, and ASCUS. *Pearson Chi-squared test. **Fisher exact test.
Figure 1. Detection using conventional PCR. Detection of: (a) Human β-globin (266 bp), (b) HPV (150bp), (c) Chlamydia trachomatis (71 bp) and Mycoplasma hominis (101 bp), (d) Ureaplasma urealyticum (127 bp) and Ureaplasma parvum (99 bp). M: marker; N: negative control; P: positive control; S1–S7: samples; bp: base pairs.
The association between HPV and bacterial STIs was also analyzed. Of the 17 patients with HPV, 13 (76.5%) were U.urealyticum-positive, 10 (58.8%) were U.parvum-positive, 4 (30.8%) were M.hominis-positive, and 1 (5.9%) was C.trachomatis-positive. There were significant associations between HPV infection and U.urealyticum and U.parvum infections, with p-values of 0.012 and 0.022, respectively (Table 3).
Table (3):
Association of HPV with Ureaplasma urealyticum, Ureaplasma parvum, Chlamydia trachomatis, and Mycoplasma hominis infections
Negative HPV n (%) |
Positive HPV n (%) |
p value |
|
|---|---|---|---|
Chlamydia trachomatis |
|||
Negative |
52 (98.1) |
16 (94.1) |
0.429** |
Positive |
1 (1.9) |
1 (5.9) |
|
Mycoplasma hominis |
|||
Negative |
48 (90.6) |
13 (76.5) |
0.138** |
Positive |
5 (9.4) |
4 (23.5) |
|
Ureaplasma urealyticum |
|||
Negative |
31 (58.5) |
4 (23.5) |
0.012* |
Positive |
22 (41.5) |
13 (76.5) |
|
Ureaplasma parvum |
|||
Negative |
38 (71.7) |
7 (41.2) |
0.022* |
Positive |
15 (28.3) |
10 (58.8) |
*Pearson Chi-squared test. **Fisher exact test.
HPV infection has been reported as the primary etiological agent of precancerous and cancerous lesions on the cervix.2,5 Several studies have demonstrated that other risk factors also play a role in cervical precancerous and cancerous lesion development, such as early onset of sexual activity, high parity, smoking, hormonal contraception, immunosuppression, and STIs other than HPV.9,10,28
According to the current study, there was no association between early onset sexual activity, high parity, smoking, contraception, or immunosuppression, and cervical precancerous lesions (Table 1). Pap smear results showed that 24 of 70 patients had abnormal cervical cytology, classified as ASCUS (37.5%), ASCH (20.89%), LSIL (8.33%), HSIL (29.17%), or cervical carcinoma (4.2%). Of the 24 patients with abnormal cervical cytology, 11 (45.8%) were positive for HPV. Abnormal cervical cytology with negative HPV was predominantly found in atypical squamous cell types (ASCUS and ASCH), whereas abnormal cervical cytology with positive HPV was predominantly found in HSIL 45.5% (5, 11). The current study revealed a significant correlation between HPV infection and cervical dysplasia (p = 0.007; Table 2).HPV is a sexually transmitted infectious agent that plays an important role in cervical carcinogenesis. Approximately 90% of cervical cancer cases are dominated by HPV infection.4,5,8,29 Primary viral E6 and E7 oncoproteins are produced as the malignancy progresses by integrating the HPV genome into host cells. Their overexpression aids in the development of cervical carcinoma by impairing the function of the tumor suppressor p53 and degrading the retinoblastoma protein, respectively.30 A limitation of the current study was that HPV genotyping was not performed; therefore, there was no differentiation of HR and LR-HPV infection.
Although HPV is the primary causative agent of precancerous and cancerous lesions of the cervix, other STIs have been discovered to play a role in the pathogenesis, although the specific mechanisms remain unclear.3,10,11 C.trachomatis and M. hominis increase the susceptibility of cervical cells to HPV infection, which could be implicated in cervical dysplasia.3,12 The current study showed that there was a significant correlation between abnormal cervical cytology and M.hominis (p = 0.037), while C.trachomatis, U.urealyticum, and U.parvum showed no significant association. The results for C.trachomatis were consistent with those of another study.31 By contrast, another study showed a significant association between U. urealyticum and U. parvum with abnormal cervical cytology.18,32, 33
M.hominis was found predominantly in cervical dysplasia (6 out of 9 positive patients) and predominantly in HSIL. Cervical dysplasia development appears to be influenced by M.hominis by initiating inflammation of cells and might be involved in increasing cervical cell susceptibility to HPV infection.34,35 Furthermore, establishing persistent infections could promote abnormal cell growth by altering host cell life cycles, elevating the inflammatory cytokines linked to DNA breakdown, and affecting apoptotic pathways.34-36
The direct relationship between HPV and M.hominis in cervical cytology is shown in Figure 2, which also shows a significant association between U.urealyticum and U.parvum with HPV infection, with p-values of 0.012 and 0.022, respectively, whereas other organisms showed no association (Table 3).U.urealyticum and U.parvum are commensal and pathogenic bacteria found in the human urogenital tract.11,13,18U.urealyticum and U.parvum play a role in HPV infection, implying an association with cervical dysplasia.12,34,37 Previous studies have shown similar results to those of the current study.11,12,32,37 Ureaplasma infections are implicated in increased susceptibility of inflamed cervical cells to HPV infection and persistence.3,32 U.urealyticum and U.parvum infection could suppress cell-mediated immunity by affecting the balance between cellular and humoral immune responses, which could affect the protection of cells against HPV infection.3 Furthermore, U.urealyticum stimulates the expression of HPV oncoprotein E6 mRNA by 4.8 fold in SiHa cells.13 This suggested that U.urealyticum plays a role in the pathogenesis of cervical precancerous and cancerous lesions by increasing the expression of oncoprotein E6. Figure 2 shows an indirect relationship among U.urealyticum, U.parvum, and cervical cytology through HPV infection.
This study demonstrated an association among U.urealyticum, U.parvum, and HPV infection. U.urealyticum and U.parvum presumably play roles in HPV infection and persistence, which can lead to cervical dysplasia. In addition, M.hominis was associated with abnormal cervical cytology. However, further studies need to be conducted with larger populations to reveal the role of bacterial coinfection with high-risk HPV and to serotype U.urealyticum and U.parvum.
ACKNOWLEDGMENTS
The authors would like to thank Department of Gynaecology, RSCM Hospital, for providing the clinical samples. Authors are also thankful to DIPA of Universitas Tanjungpura 2016 and BOPTN Universitas Indonesia 2014 for partial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors have made a direct, substantial, and intellectual contribution to the work and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (No: 98/UN2.F1/ETIK/2016).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Hu SY, Tsang SH, Chen F, et al. Association between common vaginal infections and cervical non-human papillomavirus (HPV) 16/18 infection in HPV-vaccinated women. J Infect Dis. 2021;223(3):445-451.
Crossref - Scarth JA, Patterson MR, Morgan EL, Macdonald A. The human papillomavirus oncoproteins: A review of the host pathways targeted on the road to transformation. J Gen Virol. 2021;102(3):001540.
Crossref - Liang Y, Chen M, Qin L, Wan B, Wang H. A meta-analysis of the relationship between vaginal microecology, human papillomavirus infection and cervical intraepithelial neoplasia. Infect Agent Cancer. 2019;14:29.
Crossref - Viarisio D, Gissmann L, Tommasino M. Human papillomaviruses and carcinogenesis/ : well- established and novel models. Curr Opin Virol. 2017;26:56-62.
Crossref - Okunade KS. Human Papillomavirus and Cervical Cancer. J Obstet Gynaecol. 2020;40(5):602-608.
Crossref - Zafari E, Soleimanjahi H, Samiee S, Razavinikoo H, Farahmand Z. Comparison of methylation patterns of E6 gene promoter region in the low-risk and high-risk human papillomavirus. Iran J Microbiol. 2019;10(6):441-446. PMID: 30873273
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527.
Crossref - Wilting SM, Steenbergen RDM. Molecular events leading to HPV-induced high grade neoplasia. Papillomavirus Res. 2016;2:85-88.
Crossref - Wei ZT, Chen HL, Wang CF, Yang GL, Han SM, Zhang SL. Depiction of Vaginal Microbiota in Women With High-Risk Human Papillomavirus Infection. Front Public Heal. 2021;8:587298.
Crossref - Carneiro FP, Daros AC, Daros ACM, et al. Cervical Cytology of Samples with Ureaplasma urealyticum, Ureaplasma parvum, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma hominis, and Neisseria gonorrhoeae Detected by Multiplex PCR. Biomed Res Int. 2020;1-10.
Crossref - Camporiondo MP, Farchi F, Ciccozzi M, et al. Detection of HPV and co-infecting pathogens in healthy Italian women by multiplex real-time PCR. Le Infez Med. 2016;24(1):12-17 PMID: 27031891.
- Kim S Il, Yoon JH, Park DC, et al. Co-infection Of Ureaplasma urealyticum and Human Papilloma Virus In Asymptomatic Sexually Active Individuals. Int J Med Sci. 2018;15(9):915-920.
Crossref - Szostek S, Zawilinska B, Malgorzata B-S, et al. Differences in the Expression of Human Papillomavirus Type 16 (HPV-16) E6 Oncogene mRNA in SiHa Cell Line Inoculated with CMV, HSV or Ureaplasmas. Folia Biol. 2014;62(2):135-142.
Crossref - Martinelli M, Musumeci R, Rizzo A, et al. Prevalence of chlamydia trachomatis infection, serovar distribution and co-infections with seven high-risk HPV types among Italian women with a recent history of abnormal cervical cytology. Int J Environ Res Public Health. 2019;16(18):3354.
Crossref - Naldini G, Grisc C, Chiavarin M, Fabiani R. Association between human papillomavirus and chlamydia trachomatis infection risk in women/ : a systematic review and meta- analysis. Int J Public Health. 2019;64(6):943-955.
Crossref - Ye H, Song T, Zeng X, Li L, Hou M, Xi M. Association between genital mycoplasmas infection and human papillomavirus infection, abnormal cervical cytopathology, and cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2018;297(6):1377-1387.
Crossref - Lima LDM, Hoelzle CR, Simoes RT, et al. Sexually transmitted infections detected by multiplex real time PCR in asymptomatic women and association with cervical intraepithelial neoplasia. Rev Bras Ginecol e Obstet. 2018;40(9):540-546.
Crossref - Noma IHY, Shinobu-Mesquita CS, Suehiro TT, et al. Association of Righ-Risk Human Papillomavirus and Ureaplasma parvum Co-Infections with Increased Risk of Low-Grade Squamous Intraepithelial Cervical Lesions. Asian Pacific J Cancer Prev. 2021;22(4):1239-1246.
Crossref - LGM. Liqui-PREP TM Cytology Processing Procedur.; 2011. https://liquiprepreagents.com/wp-content/uploads/2018/02/Cytology-Processing-Procedure-WPO5019-0811.pdf
- Nayar R, Wilbur DC, Solomon D. The Bethesda System for Reporting Cervical Cytology. 2008.
Crossref - Roche Diagnostics. High Pure PCR Template Preparation Kit. 2020. https://elabdoc-prod.roche.com/eLD/api/downloads/c4c8ae2d-dc11-eb11-0091-005056a71a5d?countryIsoCode=id
- Janezic SA. Genetic variation in the bioactivation of arylamineprocarcinogens in bladder cancer. Univ Toronto. 1997. https://tspace.library.utoronto.ca/handle/1807/10995.
- Venceslau EM, Bezerra MM, Lopes ACM, et al. HPV detection using primers MY09/MY11 and GP5+/GP6+ in patients with cytologic and/or colposcopic changes. J Bras Patol e Med Lab. 2014;50(4):280-285.
Crossref - Dhawan B, Rawre J, Ghosh A, et al. Diagnostic efficacy of a real time-PCR assay for chlamydia trachomatis infection in infertile women in north india. Indian J Med Res. 2014;140(2):252-261.
- Pascual A, Jaton K, Ninet B, Bille J, Greub G. New diagnostic real-time PCR for specific detection of Mycoplasma hominis DNA. Int J Microbiol. 2010;317512.
Crossref - Frolund M, Bjornelius E, Lidbrink P, Ahrens P, Jensen JS. Comparison between culture and a multiplex quantitative real-time polymerase chain reaction assay detecting Ureaplasma urealyticum and U. parvum. PLoS One. 2014;9(7):e102743.
Crossref - Mallard K, Schopfer K, Bodmer T. Development of real-time PCR for the differential detection and quantification of Ureaplasma urealyticum and Ureaplasma parvum. J Microbiol Methods. 2005;60(1):13-19.
Crossref - Foster E, Malloy MJ, Jokubaitis VG, et al. Increased risk of cervical dysplasia in females with autoimmune conditions-Results from an Australia database linkage study. PLoS One. 2020;15(6):1-14.
Crossref - Luckett R, Painter H, Hacker MR, et al. Persistence and clearance of high-risk human papillomavirus and cervical dysplasia at 1 year in women living with human immunodeficiency virus: a prospective cohort study. BJOG An Int J Obstet Gynaecol. 2021;128(12):1986-1996.
Crossref - Sammarco ML, Tamburro M, Pulliero A, Izzotti A, Ripabelli G. Human Papillomavirus Infections, Cervical Cancer and MicroRNAs: An Overview and Implications for Public Health. MicroRNA. 2019;9(3):174-186.
Crossref - Smelov V, Gheit T, Sundstrom K, et al. Lack of significant effects of Chlamydia trachomatis infection on cervical adenocarcinoma risk: Nested case-control study. PLoS One. 2016;11(5):e0156215.
Crossref - Ji Y Il. Co-infections with human papillomavirus and mycoplasma/ureaplasma spp. in women with abnormal cervical cytology. Res Rep Gynecol Obstet. 2017;1(1):1-3.
- Zhu H, Shen Z, Luo H, Zhang W, Zhu X. Chlamydia Trachomatis Infection-Associated Risk of Cervical Cancer. Medicine (Baltimore). 2016;95(13):e3077.
Crossref - Klein C, Samwel K, Kahesa C, et al. Mycoplasma co-infection is associated with cervical cancer risk. Cancers (Basel). 2020;12(5):1-13.
Crossref - Borchsenius SN, Vishnyakov IE, Chernova OA, Chernov VM, Barlev NA. Effects of Mycoplasmas on the Host Cell Signaling Pathways. Pathogens. 2020;9(4):308.
Crossref - Adebamowo SN, Ma B, Zella D, Famooto A, Ravel J, Adebamowo C. Mycoplasma hominis and Mycoplasma genitalium in the Vaginal Microbiota and Persistent high-risk human Papillomavirus infection. Front Public Heal. 2017;5:140.
Crossref - Alotaibi HJ, Almajhdi FN, Alsaleh AN, et al. Association of sexually transmitted infections and human papillomavirus co-infection with abnormal cervical cytology among women in Saudi Arabia. Saudi J Biol Sci. 2020;27(6):1587-1595.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.