Ritika Pathak1, Abhishek Sharma1, Anurup Adak2,
Satyawati Sharma1* and Rajendra Prasad1
1Centre for Rural Development and Technology, Indian Institute of Technology Delhi, Hauz Khas, New Delhi – 110 016, India.
2Division of Microbiology, Indian Agricultural Research Institute, New Delhi – 110 012, India.
ABSTRACT
Present study is focused on utilization of Jatropha curcas deoiled cake (JDC, a by-product of biodiesel industry), as potential source for various value added products. To produce cellulases and xylanase enzymes efficiently, JDC was used as nutrient substrate for Paecilomyces variotii in solid state fermentation. Under the optimized conditions viz., initial moisture content 70%, incubation temperature 30°C and inoculum dose 10%, maximum CMCase (383.95 IU/g), FPase (27.37 IU/g) and xylanase (4842.93 IU/g) were produced. Zymograms of crude enzyme extracts showed six active bands ranging from 35 kDa to 74 kDa for cellulases and four active bands ranging from 45-64 kDa for xylanase. Simultaneously, the addition of 10% JDC (w/w) in kitchen waste during its vermicomposting with and without P. variotii was studied. The data revealed that the inoculation of P. variotii in feedstocks with JDC not only improved the quality of vermicompost with higher nutritional status but also enhanced the growth and reproduction of earthworms manyfold.
Keywords: Jatropha deoiled cake; solid state fermentation; cellulases; xylanases; vermicomposting.
INTRODUCTION
In India there is a rising consensus over biofuel production utilizing non-edible oil seeds and Jatropha curcas in particular for providing energy security1. Consequently, a huge quantity of residual Jatropha deoiled cake (JDC) will be generated as a by-product2. The toxic or anti-nutrient compounds isolated from JDC include curcin, phorbol esters, phytate, curcalonic acids, flavanoids, vitexine and isovitexine3, 4. The presence of such bioactive compounds restrict the use of JDC as animal feed5 and its disposal could also create serious environmental pollution problem6. This encompasses the need for the value addition of JDC as substrate in various biotechnological processes.
Lignocellulolytic enzymes such as cellulases and xylanases play a critical role in depolymerization of structural carbohydrate polymer into monomeric sugars. Cellulase is a multienzyme complex that consists of three major enzymes, endo-1, 4 glucanase, cellobiohydrolase and cellobiase7. These enzymes synergistically hydrolyze the crystalline structure of cellulose8. Xylanase breaks down ²-1, 4-glycosidic bonds in a xylan, one of the main components present in hemicelluloses9. Cellulases and xylanases play significant role in winery, laundry, brewery, textiles, paper and pulp industries10, 11, 12. Besides, the utility of lignocellulolytic enzymes in recycling of waste via composting is also well reported13. Consequently, the increased demand of cellulases and xylanases in agro-industrial applications has put a pressure on the production system of these enzymes14. It is therefore imperative to utilize low cost organic wastes to make bioconversion processes more economical and feasible.
The kitchen waste produced from hostels, hotels and canteens ends up in landfill sites or putrefied along roadsides in various developing countries 15. Vermicomposting of kitchen waste could be an alternative option for its sustainable management. It is a simple biotechnology to convert complex organic substances into stable and homogeneous humus-like vermicompost employing earthworms and microorganisms16, 17. However, the high moisture content of kitchen waste makes unacceptable conditions for the earthworms and requires additives (bulking agents) for the successful vermicomposting 18. The use of nutrient rich additives in the kitchen waste would not only provide favorable environment to earthworms and microbes but also contemplated to improve the quality of the end product19
The objective of present study was to investigate the potential of JDC for the production of cellulases and xylanase under optimized conditions by solid state fermentation (SSF) using ascomycetic fungus, Paecilomyces variotii. We have earlier reported the prospects of P. variotii in rapid degradation of municipal solid waste during vermicomposting20. In the present investigation, we evaluated the role of JDC as additive in the vermicomposting of kitchen waste with and without P. variotii. To the best of the authors’ knowledge, this is the novel attempt to address the management issues of biodiesel and kitchen waste through a very effective and sustainable approach.
Materials and methods
Substrate analysis
The cold press JDC obtained from Udaipur, India, was further deoiled by extracting with petroleum ether using a soxhlet chamber5. The cake was dried and powdered prior to subsequent chemical analysis (Table 1). Total Kjeldahl nitrogen (TKN) and total organic carbon (TOC) were estimated by using a Micro-Kjeldahl method21 and partial-oxidation method22 respectively. Flame photometer and spectrophotometer were used for the determination of exchangeable potassium (Kex) and available phosphorous (Pav), respectively. EC and pH were determined using digital conductivity and pH meter (Eutech Instruments) respectively. Cellulose was estimated by the method of23. Hemicelluloses were calculated by determining neutral detergent fiber and acid detergent fibre24. All the chemicals and reagents, of analytical grade, were purchased from Merck (India).
Table 1: Composition of Jatropha deoiled cake (JDC) used in this study
Parameters |
Value |
pH |
6.38 ± 0.3 |
EC (mS/cm) |
1.83 ± 0.6 |
TOC (%) |
34.67 ± 1.9 |
TN (%)
Kex (%) Pav (%) |
3.93± 0.4
1.26 ± 0.2 1.43 ± 0.12 |
Cellulose (%) |
18.53 ± 0.37 |
Hemicelluloses (%) |
22.56 ± 0.42 |
Values are means of triplicates.
Kex: Exchangeable potassium; Pav: Available phosphorous
Culture and inoculum
The pure culture of P. variotii was collected from Indian Type Culture Collection (ITCC), Indian Agriculture Research Institute, New Delhi. The spores of the fungal strain were harvested from the slant surface by pouring sterile 0.1 % Tween 80 to wash off the spores. The spore suspension (1 x 106spores/ml) was used immediately to inoculate the subsequent flasks containing fermentation media. P. variotii was routinely maintained on PDA slants at 4°C by regular sub-cultivation (no longer than 3 months).
Enzyme production under solid state fermentation (SSF)
SSF of JDC was carried out in 250 ml Erlenmeyer flask having five grams of JDC moistened with sterile deionized water to maintain the initial moisture of 50%. The contents of the flask were autoclaved at 121 °C for 20 min, cooled and inoculated with 10% (v/w) of P. variotii spore suspension followed by incubation at 28°C for 10 days under static condition. The enzymes were extracted by adding 25 ml citrate buffer (0.05 M, pH 4.8) followed by constant shaking at 200 rpm for 30 min after which it was centrifuged at 10,000 rpm for 20 min at 4 °C. The clear supernatant was analyzed for FPase [25], CMCase and xylanase activity [26]. One International unit (IU) of enzyme activity was defined as the quantity of enzyme required to liberate 1 µmol of reducing sugar (glucose/xylose) per ml of crude filtrate per minute under standard assay conditions.
Optimization of cellulases and xylanase production
The cellulases and xylanase production by the fungus was optimized following one factor at a time (OFAT) method. The effect of various factors such as initial moisture (40-80%), incubation temperature (20-40°C) and inoculum dose (5-25% on FPase, CMCase and xylanase production was studied. All experiments were conducted in triplicates.
Zymography
For the detection of cellulases and xylanase, 30 µl of supernatant was taken and boiled with 20 µl SDS sample buffer (without ²-mercaptoethanol) and loaded on a zymogram gel. The gel was prepared using 12% (w/v) SDS-PAGE gel containing 0.1% CMC or brich wood xylan27. Following electrophoresis, the zymogram gel was socked in 2.5% (v/v) Triton-X for an hour and wash thoroughly in distilled water prior to incubation at 50oC for 30 min with 50 mM citrate buffer (pH-5.0). After completion of incubation, the gel was stained with 0.1% Congo red for 15 min and then destained with 5% NaCl until the clear bands appeared.
Procurement of earthworms and kitchen waste
The earthworms, Eisenia fetida, were procured from an earthworm bank (pit) in the micromodel complex, IIT Delhi where it has been cultured for the last 15 years. The wet kitchen waste (KW), consisting of 55% uneaten cooked vegetables, 30% vegetable peels and 15 % fruit peels, was collected from hostels of IIT Delhi.
Vermicomposting
The experiment was conducted in earthen pots, each with capacity to handle 15 kg waste, with a small hole at the bottom. The details of substrate compositions in different pots were as follows: T1: 10 kg KW; T2: 9 Kg KW + 1 Kg JDC; T3: 10 kg KW + P. variotii and T4: 9 Kg KW + P. variotii + 1 Kg JDC. It is worthwhile mentioning here that the higher percentage of JDC in the substrate mixtures retarded the growth and fecundity of worms and hence only 10% JDC (w/w dry weight basis) was included in the present experiment. Each substrate composition was mixed with 2 kg of cow dung to provide an initial favorable environmental condition for the worms28. Pure culture of P. variotii was inoculated, at 500 g mycelium/ton substrate, in the respective pots29. After 10 days, 100 healthy earthworms of the same size were introduced in each earthen pot. Moisture was maintained to about 60% of the water holding capacity. The duration of experiments was two months. The experiment was set up in a randomized complete block design with five replications of each type of substrate combinations.
The adult earthworms, juveniles and cocoons were removed manually and counted at the end of vermicomposting for their number and weight determination. The mature vermicompost was analyzed for various physic-chemical characteristics by the methods described above.
Statistical analysis
The data have been expressed by their mean values and standard deviations (SD). The results were subjected to one way analysis of variance (ANOVA) using SPSS for windows (version 18.0). The significance of difference has been determined according to Duncan’s multiple range test (DMRT). P values < 0.05 have been considered to be statistically significant.
Results and discussion
Time course of cellulases and xylanase production
Experiments were conducted to study the time-course profile of cellulases and xylanase production by P. variotii under SSF using JDC as substrate. The CMCase activity was found to be maximum (133.64 IU/g of substrate) on 6th day of fermentation while maximum FPase activity occurred on 4th day with the yield of 12.86 IU/g of substrate (Fig. 1a). Under the same conditions, maximum xylanase production (3218.26 IU/g of substrate) was observed on 6th day (Fig. 1b). Further increase in respective incubation period caused the decrease in the activity of CMCase, FPase and xylanase. The chemical composition of JDC (Table 1) justified its suitability as a potential substrate for the production of lignocellulolytic enzymes30. The SSF conditions were optimized to further enhance the production level of cellulases and xylanase.
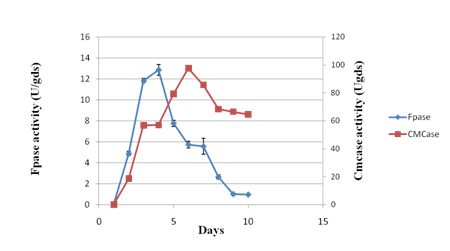
Fig 1 a. Time course profile of CMCase and FPase activity during solid state fermentation of Jatropha deoiled cake. Values are means of triplicates. Bars indicate standard deviation.
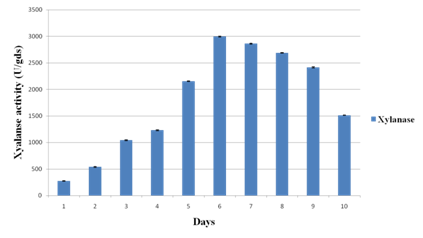
Fig. 1b. Time course profile of xylanase activity during solid state fermentation of Jatropha deoiled cake. Values are means of triplicates. Bars indicate standard deviation
Optimization of physiological parameters for enzyme production
The physiological parameters viz., initial moisture content, temperature and inoculum dose are key factors influencing enzyme production by a fungus (Table 2). The moisture content of the medium changes during SSF because of evaporation and metabolic activities, and thus the optimum moisture level of the substrate become very important31. The optimization of initial moisture content of the substrate revealed that the moisture content significantly (p < 0.5) affected cellulases and xylanase production by P. variotii. Maximum production of CMCase (133.60 IU/g), FPase (17.62 IU/g and xylanase (3797.29 IU/g) was achieved at 70% initial moisture content and thereafter, the production declined sharply. High moisture caused the substrate sticky and agglomerated, which might have restricted the diffusion of oxygen within the substrate32. Similarly, lower moisture content also affected the growth of microorganism due to reduced solubility of nutrients and substrate swelling33.
Incubation temperature is another important factor which affects the growth and enzyme production during SSF. Highest yields of CMCase (154.97 IU/g), FPase (23.62 IU/g) and xylanase (4440.26) by P. variotii were observed at 30°C reflecting the mesophilic nature of the microorganism. At 40°C, the production of FPase, CMCase and xylanase declined by 74.57, 79 and 41.73% respectively. The optimum temperature for cellulases and xylanase production varies with the fungal strain. Ncube et al26 reported maximum xylanase and cellulases production by Aspergillus niger FGSCA733 at 25°C and 40°C. In a study by Jain et al34 Thermoascus aurantiacus RCKK produced maximum cellulases and xylanase at 45°C while various Penicillium species produced maximum xylanase and cellulases activity near about 30°C35, 36.
Inoculum dose also plays a vital role in enzyme production. In the present study, SSF was carried out with an inoculum dose ranging between 5 and 25% (v/w). It was observed that P. variotii produced maximum CMCase (383.95 IU/g), FPase (27.37 IU/g) and xylanase (4842.93 IU/g) yield at the inoculum dose of 10%. The lower inoculum dose could have resulted in longer growth time (extended lag phase) while a higher inoculum dose might have increased the moisture content of the substrate resulting in poor growth of fungus and ultimately lower enzyme production37. Another possible reason for low enzymatic production at higher inoculum dose could be the increased competition for the substrate and thereby causing a rapid depletion of macro and micro nutrients necessary for growth and enzyme production38.
Table 2: Optimization of different physiological parameters for cellulases and xylanase production on jatropha cake
Physiological parameters |
Fpase (IU/g) |
CMCase(IU/g) |
Xylanase(IU/g) |
Moisture (%) |
|||
40 |
6.78 ± 0.84c |
57.94 ± 0.69e |
2377.06 ± 3.07e |
50 |
14.42 ± 0.8b |
96.34 ± 1.19d |
2894.88 ± 7.12d |
60 |
17.62 ± 0.81a |
122.17 ± 3.17b |
3661.89 ± 6.11b |
70 |
17.81 ± 0.78a |
133.60 ± 2.62a |
3797.29 ± 8.29a |
80 |
13.42 ± 0.87b |
102.79 ± 1.31c |
3414.58 ± 8.58c |
Temperature (°C)
(At best moisture %) |
|||
20 |
9.23 ± 0.25d |
86.720 ± 2.46d |
1947.49 ± 3.43e |
25 |
17.82 ± 0.20b |
149.03 ± 2.33b |
3560.32 ± 10.74b |
30 |
23.62 ± 0.58a |
154.97 ± 1.73a |
4440.25 ± 11.82a |
35 |
11.86 ± 0.56c |
92.66 ± 1.92c |
3488.73 ± 10.23c |
40 |
6.006 ± 0.27e |
32.54 ± 0.89e |
2587.96 ± 8.63d |
Inoculums dose (%)
(at best temperature and moisture content) |
|||
5 |
26.42 ± 0.60b |
371.54 ± 0.85b |
3811.74 ± 5.76d |
10 |
27.37 ± 0.32a |
383.95 ± 1.37a |
4842.9 3 ± 9.01a |
15 |
19.39 ± 0.05e |
341.32 ± 1.05c |
4255.74 ± 7.85b |
20 |
25.04 ± 0.48c |
256.09 ± 1.34d |
4227.99 ± 9.49c |
25 |
24.72 ± 0.48d |
154.97 ± 1.00e |
2957.04 ± 4.78e |
In each column, data followed by the same letter are not significantly different at P < 0.05 by DMRT.
Zymography of cellulases and xylanase
The zymograms of cellulases and xylanase showed multiple bands (Figure 2) indicating that P. variotii was a producer of multiple forms of cellulases and xylanases. The cellulase zymogram showed six active bands (Cel 1of 74kDa, Cel 2 of 63 kDa, Cel 3 of 62 kDa, Cel 4 of 60 kDa, Cel 5 of 48 kDa and Cel 6 of 35 kDa). On the other hand xylanase zymogram showed four bands (Xyl 1of 64, xyl of 63kDa, xyl of 52kDa, and xyl of 45 kDa). P. variotii produces wide variety of extracellular hydrolytic enzymes including cellulases and xylanase39. Some researchers explained that depending upon the culture conditions, variations in the amidation and glycosylation could occur resulting in the production of multiple cellulases and xylanases by the fungus40, 26
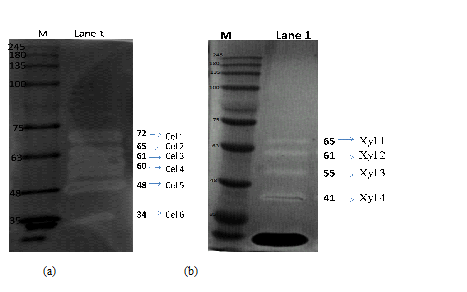
Fig. 2. Zymograms for (a) cellulases and (b) xylanase crude enzyme extracts produced by P. variotii on J. curcas deoiled cake. Lane M: molecular weight marker; Lane 1: enzyme extract from P. variotii culture grown on J. curcas deoiled cake. Cel 1 to Cel 6 indicates the different cellulase bands. Xyl 1 to Xyl 4 indicates the different xylanase bands
Vermicomposting
All the matured composts appeared granular and dark grey after 30 days except treatment T1, where the appearances of initial feed stocks were recognizable even after 45 days of vermicomposting. No foul odor was found in any of the treatments at the end of vermicomposting.
The changes in physico-chemical parameters during vermicomposting of kitchen waste using JDC and P. variotii were also studied. All the treatments had an initial pH ranging between 6.3- 6.9 and final pH of all treatments shifted to neutral (Figure 3a). As the microbial decomposition of organic matter results in the formation of ammonium ions and organic acids, the combined effect of both could have caused the neutrality of the final vermicompost 41. The EC of a vermicompost reflects the presence of different mineral salts in available (ionic) forms28. In present study, the EC of T4 vermicompost was 22.48% higher than that of T1 vermicompost (Figure 3b). This may be attributed to the enhanced mineralization of feed stocks i.e. kitchen waste and JDC by P. variotii. It is noteworthy that none of the vermicompost had the EC > 3 ms/cm suggesting that all the treated vermicomposts are safe for the plant growth42.
There was a decrease in TOC content in all the treatments after 60 days of vermicomposting. Highest reduction was seen in T4 (65.99%) followed by T3 (54.03%), T2 (51.05%) and T1 (36.38%). Our results are also supported by Elvira et al43 who reported 20- 43 % loss of TOC after vermicomposting of paper mill and dairy sludges. We believe that the addition of JDC might have enhanced the production of lignocellulolytic enzymes by P. variotii causing maximum breakdown of complex organic matter compared to other feedstocks.
TKN of final vermicompost increased in all the treatments compared to initial organic substrates. The increase of TKN in the vermicompost might be attributed to loss of organic carbon as CO2 and water loss by evaporation44. TKN content in the vermicompost also depends upon the initial nitrogen content of the feedstock45, 46. In the present investigation, addition of JDC significantly (p < 0.5) increased the TKN content (25.84 – 92.44%) of the vermicompost in different treatments. TKN content was highest (2.29%) in vermicompost obtained from kitchen waste supplemented with JDC and P. variotii. Final C/N ratios of vermicomposts were in between 5.33 and 16.92 indicating a stable and matured end product47. The T4 vermicompost had the least C/N ratio (5.33). The decrease in C/N ratio in current study might be attributed to increase in the earthworm population (Table 3) leading to reduction in organic carbon by earthworm mediated microbial decomposition48.
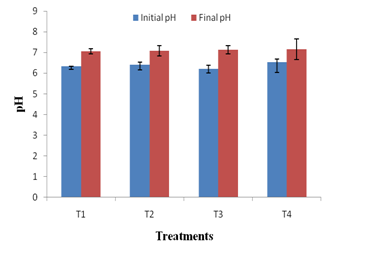
Fig. 3 a. Changes in pH in different treatments after vermicomposting. Values are means of triplicates. Bars indicate standard deviation
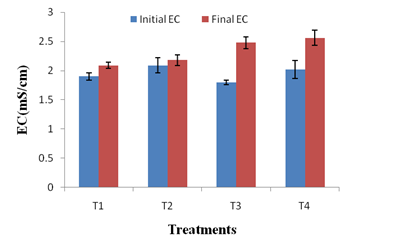
Fig. 3 b. Changes in EC in different treatments after vermicomposting. Values are means of triplicates. Bars indicate standard deviation
Table 3: Changes in total organic carbon (TOC), total kjeldahl nitrogen (TKN), C/N and available P (Pav) and exchangeable K (Kex) in different treatments after vermicomposting
In each column, data followed by the same letter are not significantly different at P < 0.05 by DMRT.
Value in parenthesis depict percentage decrease in TOC and C/N ratio and increase in TKN, Kex and Pav with respect to their respective initial values
Pav and Kex in all the treated vermicomposts increased compared to initial feedstocks. The Pav content of initial feedstocks was in the range of 0.62 – 0.73%, while, in final vermicompost it was 0.91- 1.51%. The Kex content was 41.77- 107.69% higher than initial feedstocks. T4 vermicompost recorded the highest amount of Pav (1.51%) and Kex (1.89%). It has been well documented that earthworms accelerate the microbial growth, which in turn solubilize the nutrients making them easily available to the plants [49]. In T4, the availability of nutrient rich JDC might have helped P. variotii in enhancing the mineralization that would have led to increase the final Pav and Kex content in the end product. Our results in this work are in accordance with the works of Sangwan et al50 who reported a significant (p < 0.5) decrease in C/N ratio and increase in total kjeldahl nitrogen and total available phosphorus contents during vermicomposting of horse dung mixed with 10% sugar mill filter cake.
Table 4: Growth of earthworms in vermicompost produced from jatropha cake (10%) amended substrate
Treatments |
Mean initial weight earthworm -1 (mg) |
Maximum
weight achieved earthworm -1 (mg) |
weight achieved in (weeks) |
Net weight gain earthworm -1 (mg) |
T1 |
357±15.14 |
749.7±15.52 |
8th |
392.7±17.96 |
T2 |
367±11.51 |
954.2±19.23 |
7th |
587.2±17.52 |
T3 |
371±19.63 |
853.3±20.52 |
6th |
482.3±28.96 |
T4 |
360±17.52 |
1123.2±24.52 |
6th |
763.2±21.56 |
In each column, data followed by the same letter are not significantly different at P < 0.05 by DMRT.
Earthworm growth and cocoon production during vermicomposting
Table 4 revealed that the maximum weight achieved per earthworm (1123.2 mg) was recorded in treatment T4 followed by T2 (954.2 mg), and the lowest was in T1 (749.7 mg). Similarly, maximum number of cocoons (58), juveniles (132) and adult earthworms (412) were found in T4 vermicompost. It has been studied that the survival growth rate and reproduction of earthworms depend on the palatability and quality of food51. We believe that JDC as additive helped in improving the texture and moisture retention capabilities of feed stocks and thus providing better environment for the growth of worms. The presence of fungal cell wall as source of protein and polysaccharide after degradation might have contributed to the increased number and weight of earthworms41.
Conclusions
The present study proposed an economical and sustainable approach for the management of JDC as suitable substrate for the production of cellulases and xylanase and additive in vermicomposting of kitchen waste. The culture conditions for P. variotii were optimized to get maximum CMCase (383.95 IU/g), FPase (27.37 IU/g) and xylanase (4842.93 IU/g) under SSF. Vermicomposting study highlighted that addition of JDC in kitchen waste mixed with P. variotii culture not only produced high quality vermicompost but also provided better environment for promoting population growth of earthworms.
Acknowledgement
First author is thankful for the financial assistance provided by Indian Institute Of technology, Delhi for carrying out the research work.
References
- Rakshit, K.D., Darukeshwara, J., Rathina, R.K., Narasimhamurthy, K., Saibaba, P., Bhagya, S. Toxicity studies of detoxified J. curcas meal (J. curcas) in rats. Food Chem Toxicol., 2008; 46: 3621-3625.
- Sharma, S., Verma, M., Sharma, A. Utilization of Non Edible Oil Seed Cakes as Substrate for Growth of Paecilomyces lilacinus and as Biopesticide Against Termites. Waste Biomass Valor., 2013; 4:325–330.
- Makkar, H.P.S., Aderibigbe, A.O., Becker, K. Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem., 1998; 62: 207–215.
- Kumar, V., Chandrashekhar, K., Sidhu, O.P. Synergistic action of neem and karanja to aphids and mites. J. Entomol. Res., 2007; 31: 121–124.
- Sharma, A., Sharma, S., Yadav, S., Naik, S.N. Role of Karanja deoiled cake based medium in production of protease and fatty acids by Paecilomyces lilacinus 6029, J. Biosci. Bioeng., 2014; 118: 270-271.
- Sadaf, A., Khare, S.K. Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiled Jatropha curcas seed cake and its application in xylooligosachharide synthesis. Bioresour. Technol., 2014; 153: 126–130.
- Prasad, S., Singh, A., Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycling., 2007; 50: 1–39.
- Dutta, T., Sahoo, R., Sengupta, R., Ray, S.S., Bhattacharjee, Ghosh, S. Novel cellulases from extremophilic filamentous fungi Penicillium citrium: production and characterisation. J. Ind. Microbiol. Biotechnol., 2008; 35: 275–282.
- Maciel, G.M., Vandenberghe, L.P., Winson, I.H., Fendrich, R.C., Bianca, B.E.D., Brandalize, T.Q.S., Pandey, A., Soccol, R.C. Xylanase production by Aspergillus niger LPB 326 in solid state fermentation using experimental design. Food Technol. Biotechnol., 2008; 46: 181–187.
- Howard, R.L., Abotsi, E., Van, Renberg, E.L., Howard, S. Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr. J. Biotechnol., 2003; 2: 602–619.
- Polizeli, M.L.T.M., Rizzatti, A.C.S., Monti, R., Trrenzi, H.F., Jorge, J.A., Amorim, D.S. Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol., 2005; 67: 577–591.
- Ferreira, G., Boer, C.G., Peralta, R.M. Production of xylanolytic enzymes by Aspergillus tamarii in solid state fermentation. FEMS Microbiol. Lett., 1999; 173: 335-339.
- Mondini, C., Fornasier, F., Sinicco, T. Enzymatic activity as a parameter for the characterization of the composting process. Soil Biol Biochem., 2004; 36: 1587-1594.
- Song, J.M., Wei, D.Z. Production and characterization of cellulases and xylanases of Cellulosimicrobium cellulans grown in pretreated and extracted bagasse and minimal nutrient medium M9. Biomass and Bioenerg., 2010; 34: 1930- 1934.
- Nair, J., Sekiozoic, V., Anda, M. Effect of pre-composting on vermicomposting of kitchen waste. Bioresour. Technol., 2006; 97: 2091–2095.
- Lazcano, C., Gómez-Brandón, M., Domínguez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere., 2008; 72: 1013–1019.
- Bhat, S.A., Singh, J., Vig, A.P. Vermiremediation of dyeing sludge from textile mill with the help of exotic earthworm Eisenia fetida Savigny. Environ Sci Pollut R. 2013; 20: 5975–5982.
- Adi, A.J., Noor, Z.M. Waste recycling: Utilization of coffee grounds and kitchen waste in vermicomposting. Bioresour. Technol., 2009; 100: 1027–1030.
- Singh, J., Kaur, A., Vig, A.P., Rup, P.J. Role of Eisenia fetida in rapid recycling of nutrients from bio sludge of beverage industry. Ecotoxicol Environ Saf., 201;. 73: 430–435.
- Sharma, S., Arora, K. Role of Paecilomyces in waste management. Journal of solid waste technology and management., 2012; 38: 73-81.
- Singh, R., Pradhan, K.: Determination of nitrogen and protein by Kjeldahl method. In: Forage Evaluation Science. New Delhi: Pvt. Publishers Ltd., 1981; , pp 23
- Walkey, J.A., Black, J.A. Estimation of organic carbon by the chromic acid titration method Soil Sci., 1934; 37: 29–31.
- Updegraff, D.M. Semi-micro determination of cellulose in biological materials. Anal Biochem., 1969; 32: 420–424.
- Thimmaiah, S.K.: Standard methods of biochemical analysis, Kalyani publishers, India, Noida, 1997; pp 49–77.
- Ghose, T.K. Measurements of cellulase activities. Methods Enzymol., 1987; 59: 257–268.
- Ncube, T., Howard, R.L., Abotsi, E.K., Rensburg, E.L.J.V., Ncube, I. Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Ind Crops and Prod., 2012; 37: 118– 123.
- Peterson, R., Grinyer, J., Joss, J., Khan, A., Nevalainen, H. Fungal proteins with mannanase activity identified directly from a Congo Red stained zymogram by massspectrometry. J Microbiol Methods., 2009; 79: 374–7.
- Kaviraj, Sharma, S. Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour. Technol., 2003; 90: 169–173.
- Singh, A., Sharma, S. Composting of a crop residue through treatment with microorganisms and subsequent vermicomposting. Bioresour. Technol., 2002; 85: 107–111,
- Mahanta, N., Gupta, A., Khare, S.K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa PseA in solid-state fermentation using Jatropha curcas seed cake as substrate. Bioresour. Technol., 2008; 99: 1729–1735.
- Baysal, T., Icier, F., Ersus, S., Y1ld1z, H. Effects of microwave and infrared drying on the quality of carrot and garlic. Eur Food Rres and Technol., 2003; 218: 68–73.
- Jenkins, N.E., Heviefo. G., Langewald, J., Cherry, A.J., Lomer, C.J. Development of mass production technology for aerial conidia for use as mycopesticides. Biocontrol News and Info., 1998; 19: 21N–31N.
- Joshi, C., Khare, S.K. Utilization of deoiled Jatropha curcas seed cake for production of xylanase from thermophilic Scytalidium thermophilum. Bioresour. Technol., 2011; 102: 1722–1726.
- Jain, K.K., Dey, T.B., Kumar, S., Kuhad, R.C. Production of thermostable hydrolases (cellulases and xylanase) from Thermoascus aurantiacus RCKK: a potential fungus. Bioprocess Biosyst Eng. 2015; 38: 787-796.
- Cui, F., Zhao, L. Optimization of Xylanase Production from Penicillium sp.WX-Z1 by a Two-Step Statistical Strategy: Plackett-Burman and Box-Behnken Experimental Design. Int. J. Mol. Sci., 2012; 13: 10630-10646.
- Liu, Y.T., Luo, Z.Y., Long, C.N., Wang, H.D., Long, M.N., Hu, Z. Cellulase production in a new mutant strain of Penicillium decumbens ML-017 by solid state fermentation with rice bran. New Biotechnol., 2011. 28: 733-737.
- Irfan, M., Nadeem, M., Syed, Q. One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride-IR05 in solid-state fermentation. J. Radiat. Res. and Appl. Sci., 2014; 7: 317-326.
- Omojasola, P., Folakemi, Jilani, Priscilla O., Ibiyemi S.A. Cellulase Production by some Fungi Cultured on Pineapple Waste Nature and Science., 2008; 6: ISSN: 1545-0740.
- Laguna, I.H.B.D., Marante, F.J.T., Mioso, R. Enzymes and bio products produced by the ascomycete fungus Paecilomyces variotii. J Appl Microbiol., 2015; 1-12.
- Freitas, A.C., Castro, R.J.S., Fontenele, M.A., Egito, A.S., Farinas, C.S., Pinto, G.A.S. Canola Cake as a Potential Substrate for Proteolytic Enzymes Production by a Selected Strain of Aspergillus oryzae: Selection of Process Conditions and Product Characterization. 2013; Article ID 369082: 1-8.
- Garg, V.K., Gupta, R. Effect of Temperature Variations on Vermicomposting of Household Solid Waste and Fecundity of Eisenia fetida. Biorem. J., 2011; 15: 165-172.
- Yuan, J., Yang, Q., Zhang, Z., Li, G., Luo, W.H., Zhang, D. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. J Environ Sci., 2015; 37: 83 – 90.
- Elvira, C., Sampedro, L., Benitez, E., Nogales, R. Vermicomposting of sludges from paper mill and dairy industries with Eisenia Andrei : a pilot scale study. Bioresour. Technol., 1998; 63: 205-211.
- Pramanik, P., Ghosh, G.K., Ghosal, P.K., Banik, P. Changes in organic – C, N, P and K and enzyme activities in vermicompost of biodegradable organic wastes under liming and microbial inoculants. Bioresour. Technol., 2007; 98: 2485–2494.
- Crawford, J.H. Review of composting. Process Biochem., 1983; 18: 14–15.
- A.C. Gaur, G. Singh, In: Tandon, HLS, editors, Recycling of rural and urban wastes through conventional and vermicomposting, Recycling of Crop, Animal, Human And Industrial Waste in Agriculture. Fertilizer Development and Consultation Organization, New Delhi, 1995, p. 31–49.
- Gupta, R., Garg, V.K. Stabilization of primary sewage sludge during vermicomposting. J. Hazard. Mater., 2008; 153: 1023–1030.
- Garg, V.K., Gupta, R. Effect of Temperature Variations on Vermicomposting of Household Solid Waste and Fecundity of Eisenia fetida. Biorem., 2011; J. 15: 165-172.
- Parthasarathi, K., Ranganathan, L.S. Aging effect on enzyme activities in pressmud vermicasts of Lampito mauritii (Kinberg) and Eudrilus eugeniae (Kinberg). Biol. Fertil. Soils., 2000; 30: 347–350.
- Sangwan, P., Kaushik, C.P., Garg, V.K. Feasibility of utilization of horse dung spiked filter cake in vermicomposters using exotic earthworm Eisenia foetida. Bioresour. Technol., 2008; 99: 2442–2448.
- Ndegwa, P.M., Thompson, S.A., Das, K.C. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol., 2000; 71: 5–12.

