ISSN: 0973-7510
E-ISSN: 2581-690X
This study was designed to identify some fungi responsible for damage of fruits in storage and sale outlet markets. Results declared the role of CMC-ase and poly galacturonase (PG) enzymes secreted by three fungi of Alternaria alternata, Penicillium italicum and Rhizopus stolonifer in decomposition of tomato, lemon and cucumber fruits at the grocery markets in Sakaka, Saudi Arabia. CMC-ase and PG were produced after 2 days for P. italicum, R. stolonifer whereas very little quantity was detected for A. alternata. Maximum CMC-ase and PG production were seen after 8-10 days of incubation at 28ºC. Enzyme production started at a temperature of 5°C but in small quantities, then the production began to increase steadily until the temperature of 28°C, which produced the highest amount of enzymes. The production began to decline starting at 35ºC to be at the lowest rates at 40ºC. Enzymes were produced at pH values of 4.5-9.5, with optima between 5.5-6.5 for the three tested fungi. Acidic medium of pH 4.5, and alkaline medium (above pH 6.5) affected negatively on the production of enzymes, and this effect was more evident in the exo CMC-ase and exo PG than in endo enzymes. Results showed that the mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate oil-rich air inhibited growth of R. stolonifer and A. alternata by 100% and inhibited the growth of P. italicum by approximately 50%, compared to the control sample. Camphor and mint oils gave negative effect. Application of devices to emit the odor and quench this mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate to fumigate the air of stores and outlets for selling vegetables and fruits are recommended.
Alternaria alternata, Enzymes, Essential oil, Penicillium italicum, Rhizopus stolonifer, Rotted Fruits
Errors of Fruit picking, lack of suitable storage and poor sales outlets and markets lead to an increase in sum of waste, which amounts to 20% of the total quantity of any crop of vegetables or fruit. Agricultural experts pointed out that this percentage is the result of several factors ranging from pre-harvest to post-harvest, which are often microbiological causes of infection mainly bacteria and fungi. All of these factors lead to decomposition, together with other reasons that occur during process of collection of fruit and the method of storage and cooling, during the period before and after harvest as well as the lack of clean markets and outlets1-5.
Fruit losses are estimated to be between 20 and 30%, and in vegetables it can be as high as 50% in some cases, but for example it occurs in some varieties only, such as tomatoes for manufacturing, which is characterized by rapid maturity, this type is not collected in stages but combines once, and here is likely to happen lost in one way or another. It also happens in fruit due to the abundance of water in it. It is worth to mention that modern machines use the best method of harvesting fruits6-7.
It should be noted that causes of losses in fruit and vegetable crops are many, including biological causes, in the sense that birds, small animals and mice can cause damage to fruit or vegetables by eating some of its parts leading to loss, as well as goats and livestock that walk in the land. The second reason is microbes which infect fruits, whether by fungi, bacteria or virus that leads to its decay. Mechanical damage of fruits occurs during process of harvesting such as shaking the trees to drop the fruit on the Earth. Other causes in places and outlets and is contaminated with microbes and the lack of ways to control pollution and damage in those places8-10.
Cellulolytic and pectinolytic ability of some fungi is of great importance to explain how that these microorganisms analyze and corrupt fruits. Some enzymes that dissolve cellulose and pectin are already present inside the fruit cells and are responsible for maturity of fruits as they age. When the fruit is infected with a fungus and fungal hyphae spread in intercellular spaces and inside parenchyma cells, the molds secrete enzymes that cause tissue maceration which eventually leads to corruption of the fruit11-14.
It is noteworthy that we have bad habits so far, such as packing fruits in plastic cages and white foam, and this leads to destruction of large quantities of the crop as a result of the cages placed on top of each other during transport and pressure on the fruits, which leads to the injury and mechanical destruction. This case facilitates growth of fungi and therefore is not used for sale except in the local market. High temperature in the outlets and places of sale accelerate pollution and damage caused by the action of fungi in general. It is also noted that among causes of loss is also the changes that occur to the fruit in pre-mature stage due to injury of the fruit, it appears outwardly from the outside well, but from the inside is corrupt or infected, knowing that the injury occurred at the beginning. The full know-how of some farmers in the manner of collecting and harvesting fruits may lead to a loss, and this is contrary to the factors that must be taken into account, such as the existence of places for cooling and packaging and the right method of cutting15.
It is emphasized here that there is a bad need of places for conservation and cooling agricultural crops after collection. This is in order to absorb the heat of fruits resulting from exposure to high temperatures. The high temperature lead to rapid breathing of the fruit, because the faster the breathing of the fruits the more vulnerable to infection by fungi and collapse or Maturity is faster. The stage after fruit ripening period is damaged, but during process of cooling the fruit is reduced breathing to increase its validity and age lasts longer. This is the importance of the initial cooling, and then the process of packing and sorting of fruits becomes safer16.
Various microbes attack fruit and vegetable crops during stages of their formation on the plant throughout harvesting, storage, transport and marketing. Before the fruit is mature and collected, it may be damaged by some fungi and bacteria. Fruits before their maturity contain some organic acids and inhibitory substances as well as strong fruit envelopes that inhibit microbial activity, and this can keep the contents of internal fruits intact3,9.
PH determines the nature and type of microbes that cause damage to vegetables and fruits. In fruits, pH ranges between 2.5-5. Fungi and yeast are responsible for their corruption and are often sourced from soil. They require monosaccharide’s and disaccharides. It is difficult for fungi to consume complex sugars because they do not have the necessary enzymes. In addition, yeast and fungi can grow with high sugary concentrations ranging from 65 – 70%. Most bacteria cannot grow in these concentrations while bacteria are responsible for damage to vegetables because their pH ranges between (4.5-7). Therefore, bacteria are responsible for 36% of vegetable corruption17.
Sources of Corrosive Microorganisms
- Pathogenic microorganisms of the plant itself.
- Microorganisms found in fertilizers, some of which are human or animal.
- Soil, irrigation water and air.
- Field workers, tools, transport and storage.
The aim of this study is to isolate fungi that cause decay and rotting of cucumber, lemons and tomatoes in some markets selling fruit and vegetable in Sakaka city, Jouf, Saudi Arabia. This work is also intended to clarify the role of CMC-ase and polygalacturonase (PG) enzymes in corruption of these fruits. The possibility of using the effect of three types of volatile oils [(1) camphor, (2) mint, (3) mixture of mint, camphor and methylsalcilate] in preventing spoil of those fruits and increase the life of the products offered in the markets will be investigated.
Collecting samples of rotten fruits from different outlet markets in the city of Sakaka and isolate the causal fungi
Rotten cucumber, lemons and tomatoes were collected separately each in sterile bags and immediately taken to the laboratory in a refrigerated box to perform fungal isolation. If the fungi were not isolated on the same day, preservation in a refrigerator takes place at a temperature of 5°C until the isolation. For each fruit type, a piece of the rotted fruit was placed on the surface of Potato Dextrose Agar (PDA) medium containing Rose – Bengal to suppress bacteria, under aseptic conditions. Dishes were placed upside down, after sealing with paraffin, at 28±2°C, until emergence of fungal colonies.
Purification of the mixed fungal colonies
In the case of emergence of colonies in a variety of dishes, each colony (containing one fungal type) is transferred in a process called purification, to a Petri dish containing a sterilized PDA, and incubated at 27°C for 7 days, or till appearance of the fungal growth18.
Fungal identification
Isolated genera and species were identified, according to the identification key of Ainsworth and Bisby19.
Tested fungal isolates
Isolate JU366 of Alternaria alternata, isolate JU466 of Penicillium italicum and isolate JU466 of Rhizopus stolonifer were used in this work. All of them were maintained on PDA medium18.
Enzyme production
Czapek Dox medium was used for testing enzyme production by the studied fungi which contained the following ingredients [2g NaNO3, 1g K2HPO4, 0.5g MgSO4, 0.5g KCl, and 0.01g FeSO4 (initial pH was adjusted to 6.5±0.2 at 25°C)] per 1L distilled H2O. This medium was amended with 2% cellulose powder for CMC-ase production and apple Pectin for PG production.
The rate of enzymes production was performed, over a period of 12 days, from the beginning of the incubation period at 28°C with pH 6.5±0.2. Effect of incubation temperature (5-40°C) on enzyme production in the cultural medium of the three tested fungi after 8 days of incubation in the dark was done. Different initial pH (4.5,5.5,6.5,7.5,8.5,9.5) values were used to study the effect of them on enzyme production at 25°C in the dark.
Endo CMC-ase assay
The method was done using viscometric trial20. Reaction mixture of 10 ml, 2% carboxymethylcellulose (CMC, Na salt), 3 ml of 0.1 M acetate buffer (pH 5) and 2 ml of fungal cultural filtrate, this mixture was incubated at 40°C (in water bath) for 30 min. Control was done using reaction mixture included boiled cultural filtrate.
Endo PG assay
The method was done using viscometric trial20. Reaction mixture of 10 ml, 1% sodium polypectate, 3 ml of 0.1 M acetate buffer (pH 5) and 2 ml of fungal cultural filtrate, this mixture was incubated at 30°C (in water bath) for 30 min. Control was done using reaction mixture included boiled cultural filtrate.
Measurement of endo CMC-ase and PG was calculated after prepared in Viscometer (Assi company, Inc, Osaka, Japan) as (%) reduction in viscosity of the reaction mixture, after 30 min of incubation (as described previously) and was counted according to the equation:
Relative CMC-as or PG activity = (T0-T30/T0-Tw) x 100
Whereas, T0 = Run time immediately after preparing reaction mixture
T30 = Run time after 30 min of incubation
Tw = Run time of distilled H2O
Exo CMC-ase assay
It was performed by measuring the increase in liberated reducing groups (sugars). Reaction mixtures of 0.4 ml of 1% CMC, 0.4 ml of 0.1 M acetate buffer (pH 5) and 0.2 ml of culture filtrate, and incubated at 40°C for 60 min20. Control was done using fungal culture filtrate of 0.2 ml which was inactive by boiling it in water bath for 30 min.
Exo PG assay
It was also performed by measuring the increase in liberated reducing sugars. Reaction mixtures of 0.4 ml of 1% sodium polypectate, 0.4 ml of 0.1 M acetate buffer (pH 5) and 0.2 ml of culture filtrate, and incubated at 30°C for 60 min20. Control was done using fungal culture filtrate of 0.2 ml which was inactive by boiling it in water bath for 30 min.
For the two exo-enzymes, the quantities of reducing sugar were determined by adding 0.5 ml of the reaction mixture with 0.5 ml of Fehling’s solution, boiling the mixture for 20 min and measuring absorbances at 520 nm using Spectrophotometer. Standard solutions of different concentrations (0-100 µg/ml) of D-glucose were prepared and their absorbance’s at 520 nm were determined using the method modified by20. Readings of the standard solutions were collected to prepare a chart of absorbance as related to µg of glucose per ml. The quantities of reducing sugar formed by 1 ml of fungal filtrate acting on either CMC or sodium polypectate at 40 and 30ºC, respectively, were calculated from the glucose standard curve. Exo CMC-ae and PG activities of the filtrate were set in terms of total reducing groups per ml per hour.
Study the effect of three volatile oils [(1) camphor – (2) mint – (3) a mixture of menthol crystals, Eucalyptus oil, camphor and methyl salicylate] on mycelium growth and production of spores and conidia of A. alternata, P. italicum and R. stolonifer:
Inoculate a Petri dish (9 cm diam) in the centre with each of A. alternata, P. italicum and R. stolonifer.
Put the opened 9 cm diam Petri dish in another 15 cm diam dish and add 5 ml of each volatile oil in the large dish (around the small one). Close the large dish and wrapped with paraffin to prevent Aviation Oil. Dishes were incubated at 27°C for 7 days in the dark. Control dishes were applied to each treatment by adding sterile distilled water only.
Isolation and purification of fungi from rotten fruits
A. alternata, P. italicum and R. stolonifer were isolated from fruits of rotten tomato, lemon and cucumber, respectively. The apparent growth of each fungus gave distinct color and texture. Fungal colony color of R. stolonifer was white cottony with black pinch heads, growth of P. italicum showed a pale green color with velvety texture, while growth of A. alternata was velvety olive green.
Fungal identification
Isolated fungi were identified using the identification key19. The isolated fungi were classified under the following sections:
- Ascomycotina of the genus Penicillium; one species of P. italicum from rotten lemon fruits was identified.
- Zygomycotina of the genus Rhizopus; one species of R. stolonifer from rotten cucumber fruits was identified.
- Deuteromycotina of the genus Alternaria ; one species of A. alternata from infected tomato fruits was identified.
Enzyme production
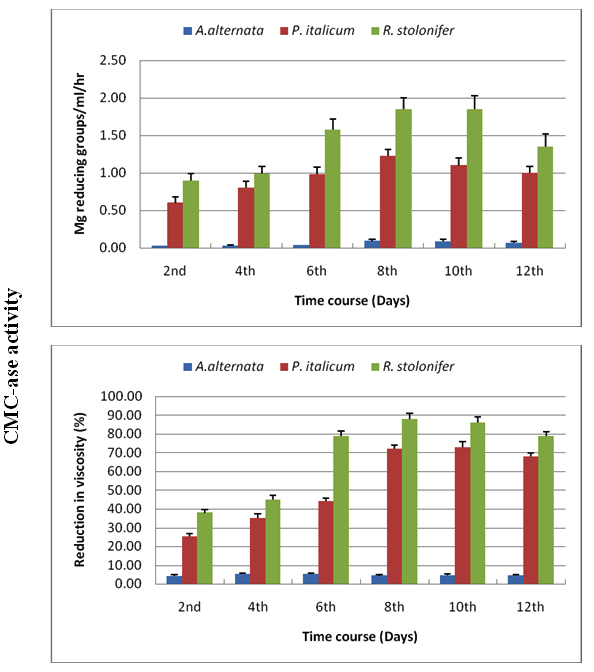
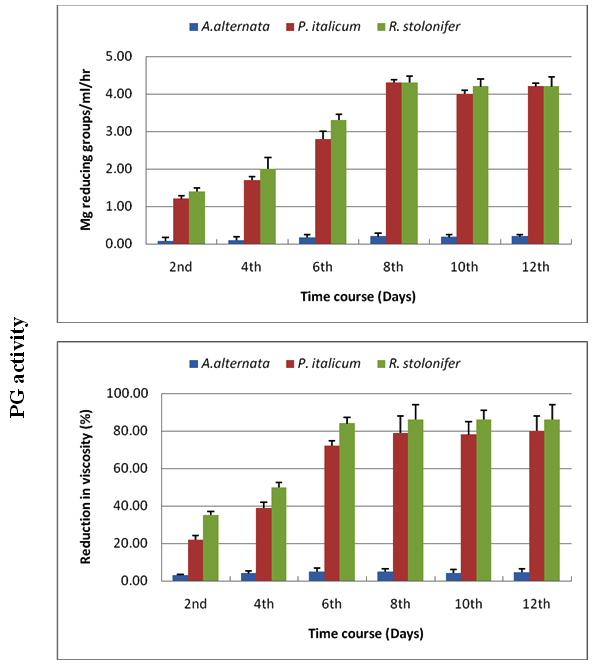
Time course of CMC-ase and PG was done using Czapek Dox medium amended with cellulose for CMC-ase production or pectin for PG production. CMC-ase and PG were produced after 2 days for P. italicum, R. stolonifer whereas very little quantity was detected for A. alternata. Maximum CMC-ase and PG production were seen after 8-10 days of incubation at 28ºC (Figures 1, 2).
Fig. 1. Time course of CMC-ase using Czapek Dox medium amended with cellulose as carbon source for A. alternata, P. italicum and R. stolonifer at 28 ºC in the dark. All the data are the mean of three replications and Bar on each column represents the standard deviation
Fig. 2. Time course of production of PG using Czapek Dox medium amended with pectin as carbon source for A. alternata, P. italicum and R. stolonifer at 28 ºC in the dark. All the data are the mean of three replica-tions and Bar on each column represents the standard deviation
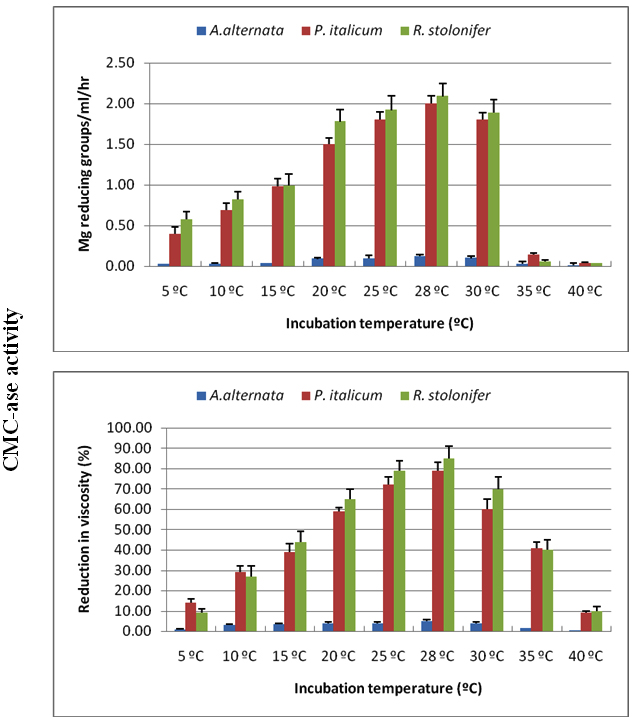
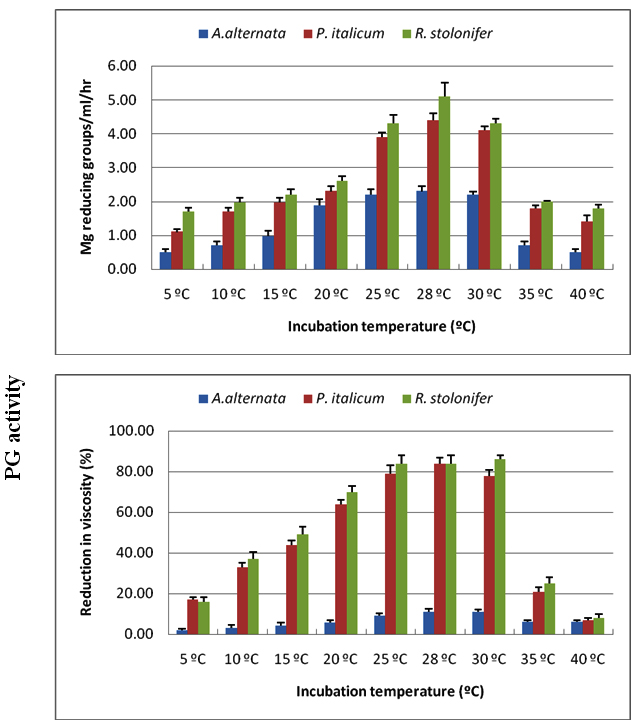
Enzyme production, for the three tested fungi, started at a temperature of 5°C but in small quantities, then the production began to increase steadily until the temperature of 28°C, which produced the highest amount of enzymes. Temperature of 30°C was also suitable for the production of enzymes, but it was slightly less than 28ºC, and then the production began to decline starting at 35ºC to be at the lowest rates at 40ºC (Figures 3, 4).
Fig. 3. Effect of incubation temperature on production of CMC-ase using Czapek Dox medium amended with cellulose as carbon source for A. alternata, P. italicum and R. stolonifer after 8 days incubation in the dark. All the data are the mean of three replications and Bar on each column represents the standard deviation
Fig. 4. Effect of incubation temperature on production of PG using Czapek Dox medium amended with pectin as carbon source for A. alternata, P. italicum and R. stolonifer after 8 days incubation in the dark. All the data are the mean of three replications and Bar on each column represents the standard deviation
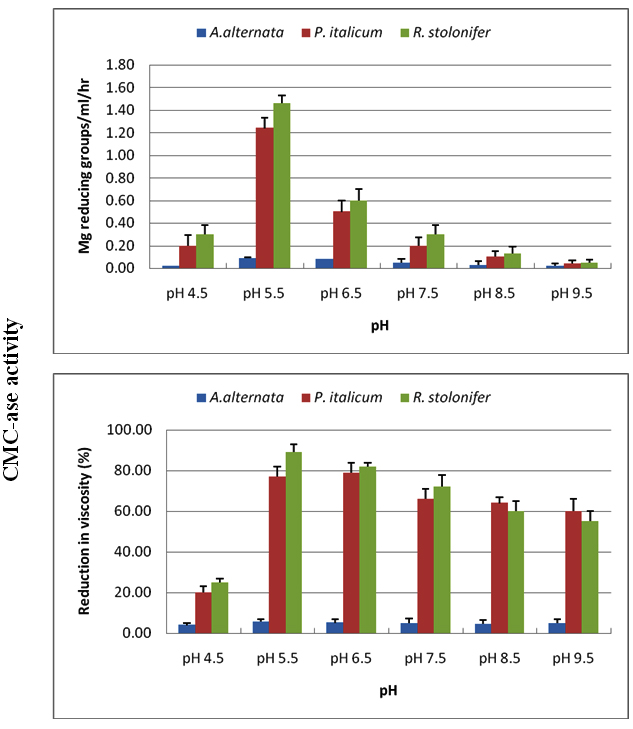
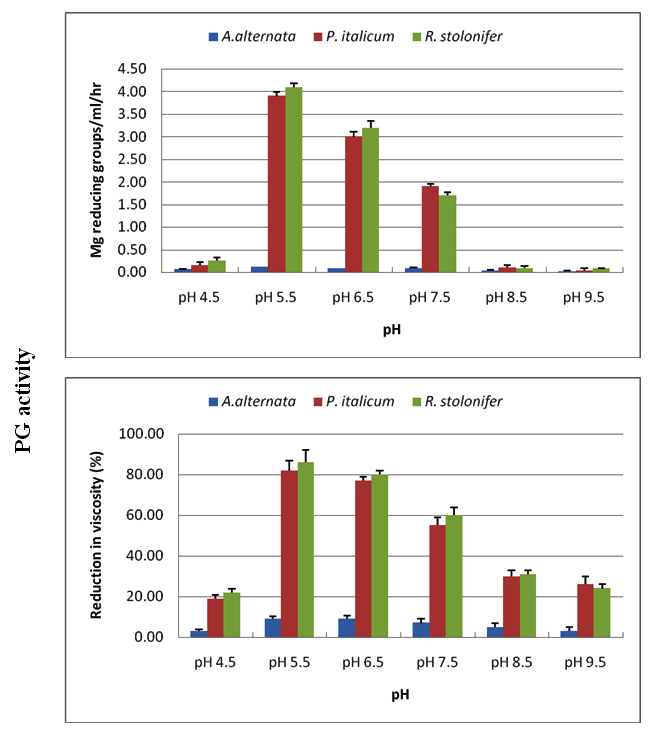
CMC-ase and PG were produced at pH values of 4.5-9.5, with optima between 5.5-6.5 for the three tested fungi. Acidic medium of pH 4.5, and alkaline medium (above pH 6.5) affected negatively on the production of enzymes, and this effect was more evident in the exo CMC-ase and exo PG than in endo enzymes (Figures 5, 6).
Fig. 5. Effect of incubation pH on production of CMC-ase using Czapek Dox medium amended with cel-lulose as carbon source for A. alternata, P. italicum and R. stolonifer after 8 days incubation at 28 ºC in the dark. All the data are the mean of three replications and Bar on each column represents the standard deviation
Fig. 6. Effect of incubation pH on production of PG using Czapek Dox medium amended with Pectin as carbon source for A. alternata, P. italicum and R. stolonifer after 8 days incubation at 28 ºC in the dark. All the data are the mean of three replications and Bar on each column represents the standard deviation
Effect of three essential oils of camphor, mint and a mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate, on mycelium growth and production of conidia and sporangiospores of A. alternata P. italicum and R. stolonifer:
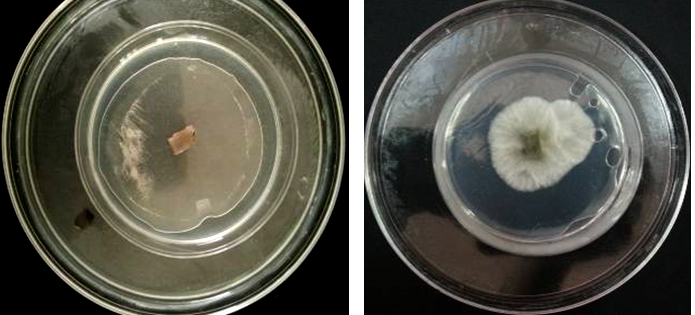
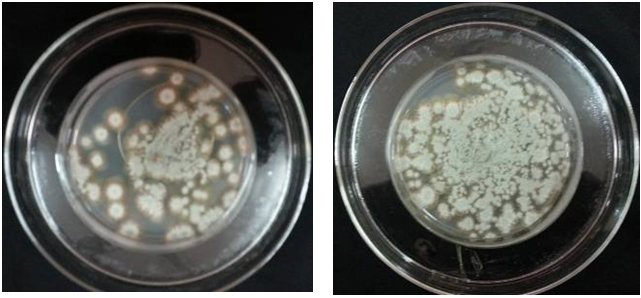
Results showed that the enriched air of the mixture (menthol crystals & Eucalyptus oil & camphor & methyl salicylate) inhibited growth of A. alternata and R. stolonifer by 100%, but inhibited growth of P. italicum by about 50%, compared to the control sample [Figures 7 (A, B) ~ 9 (A, B)].
Fig. 7. (A). Mycelium growth of Alternaria alternate, After adding 5 ml of menthol crystals & Eucalyptus oil & camphor & methyl salicylate and incubated for one week at 27°C;
(B). Mycelium growth of Alternaria alternata, After adding 5 ml of distilled water (in the outer dish) and incubated for one week at 27°C
Fig. 8. (A). Mycelium growth of Penicillium italicum, after adding 5 ml of menthol crystals & Eucalyptus oil & camphor & methyl salicylate (in the outer dish) and incubation for one week at 27°C
(B). Mycelium growth of Penicillium italicum, After adding 5 ml of distilled water (in the outer dish) and incubated it for one week at 27°C
Fig. 9. (A). Mycelial growth of Rhizopus stolonifer, After adding 5 ml of Fes (in the outer dish) and incubation for one week at 27°C
(B). Mycelial growth of Rhizopus stolonifer, After adding 5 ml of distilled distilled water (in the outer dish) and incubated it for one week at 27°C
The suspended air of both camphor and mint oils did not affect mycelium growth and production of spores and conidia of the three tested fungi.
In previous researches, it was found that the studied fungi (A. alternata, P. italicum and R. stolonifer) have a high reputation for decomposition of fruits21,22. The isolated fungi from rotten fruits may represent a serious danger to the health of people, many of which are the product of fungal toxins, causing many cancers, especially liver cancer16.
Enzymes play an important role in infecting fruit and vegetables with fungi and thus causing its corruption and spoilage. Cellulase enzymes cause softening and dissolution of primary cell walls of fruits, which are mainly composed of cellulose and leads to easy entry of penetration begs and haustoria of fungi into parenchymatous cells. Poly galacturonase (PG) play an important role in the decomposition and disintegration of plant cells of fruits as a result of dissolution of the middle lamella in the cell walls of the cells. This, in turn, causes cells to disintegrate and then to decompose and lose their cohesion until they become rotten. Results of this study showed that P. italicum and R. stolonifer have a high ability to produce cellulose (CMC-ases) and PG enzymes starting from the second day of inoculating media and ideally in production on the 8th to 10th day at the temperature of 28°C at pH values between 5.5-6.5. It is worth mentioning that the cell juice of fruits studied here is acidic, which encourages fungi to produce enzymes23.
Consistent with many previous studies. Results, here, did not show any significant activity for the production of CMC-ases and PG in the case of A. alternata compared to P. italicum and R. stolonifer. This may be explained by the fact that the Alternaria fruit rot disease of tomatoes does not lead to the watery decomposition of fruits.
Because of the hot climatic condition and random methods of transport and storage of vegetables and fruit in the study area, we had to look for ways to reduce the rate of corruption of fruits in the outlets sold to reach the consumer. Therefore, we searched for natural sources safe to resist the impact of these fungi. Essential oils are a natural source of resistance to certain fungal diseases24. Data obtained in this study showed that the use of air that loaded with steam oil of the mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate had an inhibitory effect (100%) on two of the three tested fungi. In addition, the mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate inhibited the P. italicum growth by 50%. In contrast, neither camphor nor mint oils had any effect on the same tested fungi.
Consequently, it has been recommend from results of this research, using and packaging air stores and outlets for the sale of vegetables and fruit by any commercial compound containing a mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate oil as a tool that can alleviate and eliminate fungi that may cause rotten of vegetables and fruit in outlets selling those products. Moreover, usage of devices to emit the odor and quench this mixture of menthol crystals & Eucalyptus oil & camphor & methyl salicylate to sterilize the air of stores and outlets for selling vegetables and fruits are recommended. Further studies are needed on the effect of some essential oils on production and activity of cellulases and pectinases produced by molds since this study was focused on the effect of these oils on growth of mycelium of the tested fungi.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Akinro, E.B., Adetuberu, I.A., Efunwole, O.O. and Olakunle, T. P. Isolation and Identification of Fungal Species Associated With the Spoilage of Some Selected Edible Fruits in Iree Town of Boripe Local Government, Osun State, Nigeria. Journal of Research in Pharmaceutical Science 2015; 2: 07-10.
- Amusa, N.A., Kehinde, I. A. and Ashaye, O. A. Biodeterioration of breadfruit in storage and its effects on the nutrient composition. African journal of biotechnology 2002; 1(2): 57-60.
- Awoite, T.M., Olorunfemi, M. F., Ajani, A. O. and Oyelakin, M. O. Studies on Fungi Associated With Post Harvest Spoilage of Pawpaw Carica PAPAYA Fruit. Journal of Pharmacy and Biological Sciences 2013; 4: (6) 1-4. ISSN: 2278-3008.
- Tafinta I.Y, Shehu, K., Abdulganiyyu, H., Rabe, A. M. and Usman, A. Isolation and identification of fungi associated with the spoilage of sweet orange (Citrus sinensis) fruits in Sokoto State. Niger. J. Basic Appl. Sci. 2013; 21(3):193–196.
- Thiyam, B., and Sharma, G. D. Isolation and identification of fungi associated with local fruits of Barak Valley, Assam. Curr. World Environ. 2013; 8(2): 319–322.
- Miedes, E., and Lorences, E. P. Apple (malus domestica) and tomato (Solanum lycopersicum) fruits cell-wall hemicelluloses and xyloglucan degradation during Penicillium expansum. Infection. Journal of Agricultural and Food Chemistry 2004; 52: 7957–7963.
- Alkenz , S., Sassi, A. A., Abugnah, Y. S. and Alryani, M. B. Isolation and identification of fungi associated with some Libyan foods, African Journal of Food Science 2015; 9 (7): 406-410.
- Al-Hindi, R.R., Al-Najada, A. R., and Mohamed, S. A. Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes. Afr. J. Microbiol. Res. 2011; 5(4): 443–448.
- Barth, M., Hankison, T. R., Zhuang, H. and Breidt, F. Microbiological spoilage of fruits and vegetables. In: Sperber W.H, Doyle M.P, editors. Compendium of the Microbiological Spoilage of Foods and Beverages, Food Microbiology and Food Safety. New York: C Springer Science Business Media, LLC. 2009; 135–183.
- Mary A. E., Mulunda, M., Patrick, B. N., Judith, Z. P., Cynthia, A. C. and Micheal, F. D. Isolation of Filamentous Fungi Species Contaminating Some Nigerian Food Commodities. Journal of Food Research 2015; 4: No. 1.
- Elnaghy, M. A., Elkatatny, M. S. T. and Abdelzaher, H. M. A. Role of phenolic in resistance of some onion varieties to maceration by Sclerotium cepivorum. Bull Fac. Sci. Assiut University 1989; 18 (1-D), 39-49.
- Elnaghy, M. A., Elkatatny, M. S. T. and Abdelzaher, H. M. A.. Factors affecting production and activities of polysaccharide degrading enzymes produced on isolated onion cell walls by Sclerotium cepivorum. Bull. Fac. Sci. Assiut Univ. 1989; 18 (1-D), 15-26.
- Elnaghy, M. A., Elkatatny, M. S. T. and Abdelzaher, H. M. A.. Degradation of isolated cell walls of onion varieties by wall degrading enzymes of Sclerotium cepivorum. Bull. Fac. Sci. Assiut Univ., 1989; 18 (1-D), 27-37.
- Srivastava1, D. and Misra, N. Fungal Spoilage of Stored Fruits of Carica papaya L. and Vitis vinifera L. and Fungitoxicity of Plants Extracts. Journal of Plant Science & Research, 2017; 4 (2): 1-7.
- Moustafa, S.M.N. and Abdelzaher, H. M. A. Responsibility of fungi in rotting vegetables and fruits in the outlet markets in Sakaka after the harvest and the possibility of resistance spoilage. 11th Arabian Conference in Plant Protection Sciences, Amman, Jordon, 3-13 November, 2014.
- Baiyewu, R.A., Amusa, N. A., Ayoola, O. A. and Babalola, O. O. Survey of the post-harvest diseases and aflatoxin contamination of marketed pawpaw fruit (Carica papaya) in South Western Nigeria. African journal of agricultural research 2007; 2(4): 178-181.
- Meyer’, R. S., Grant, M. A., Luedecke, L. O. and Leung, H. K. Effects of pH and Water Activity on Microbiological Stability of Salad Dressing, Journal of Food Protection 1989; 52 (7): 477-479.
- Moustafa, S. M. N. and Abdelzaher, H. M. A. Occurrence of Hemolytic Fungi Mounted on Wheat Grains in the main Silo of Sakaka, Saudi Arabia. Journal of pure and applied microbiology 2016; 10 (3): 1817-1824.
- Ainsworth and Bisby. Dictionary of the fungi. 2001; Tenth Edition, prepared by CABI, Book.
- Ichitani, T., Abdelzaher, H. M. A. and Watanabe, S. Pectinolytic and cellulolytic abilities of acquired resistant isolate of Pythium vanterpoolii against metalaxyl. Bull. Univ. Osaka Pref. 1996; 48 (B), 13-17.
- Janisiewicz, W. J. and Korsten, L. Biological control of postharvest diseases of fruits. Annual Review of Phytopathology 2002; 40: 411–441.
- Van Kan, J. A. L. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends in Plant Science 2006; 11: 247–253.
- Anon. pH values of food products. Food Eng. 1962; 34 (3): 98-99.
- Sitara, U., Niaz, I., Naseem, J. and Sultana, N. Antifungal effect of essential oils in vitro growth of pathogenic fungi. Pak. J. Bot. 2008; 40 (1): 409-414.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.