ISSN: 0973-7510
E-ISSN: 2581-690X
Hepatitis B surface antigen (HBsAg) is the important marker in diagnosis and decision making of Hepatitis B infection. Equivocal results are common diagnostic challenges with HBsAg. The study evaluates the role of neutralization assay in equivocal HBsAg results. Serum samples which documented equivocal or low positive COI where subjected to HBsAg neutralization assay. Percentage reduction in false positivity and recommended COI for deciding the assay was calculated. A subset of samples was subjected to molecular confirmation. Analysis of 51 samples showed 87.71% reduction in false positivity by using neutralization assay. 9.17 was the COI which showed highest AUC (0.702). Percentage positivity of molecular analysis of 13 samples was 70% (9/13). The optimum COI for utilizing neutralization assay is high from the study which is probably due to the high mean COI (12.1) of the included samples. Use of follow up HBsAg neutralization assay is recommended in equivocal HbsAg results to confirm the interpretation. However, the cut-off COI for implementation of test may vary for different population and analysers used for testing.
Hepatitis B, Neutralization Assay, HBsAg, Chemiluminescence, Cut-off Index
Hepatitis B is a global disease with high burden. It is also the leading cause of hepatic cirrhosis and hepatocellular carcinoma.1 Hepatitis B is a disease with multiple stages and diagnostic markers with different clinical utilities. Hepatitis B surface antigen (HBsAg) is the first marker to appear and is usually the marker for initial screening. CDC recommends triple marker screening in the recent guidelines which includes HBsAg, anti-HBs antibodies and HBc IgM antibodies.2 Definition and classification of chronic hepatitis B also is dependent on detection of HBsAg.3 HBsAg quantification is possible in some platforms.4,5 Over the years the testing formats have changed for better sensitivity. The assay platforms like chemiluminescence have improved sensitivity and turnaround time significantly.6,7 Highly sensitive assay platforms have also been beneficial in blood banks for screening for HBV.8 The analysers and kits are continuously working on improving detection level.9 However, increased sensitivity mostly comes with disadvantage of increased false positivity.10 In chemiluminescence platforms the positive results with low cut-off index (COI) are usually a diagnostic challenge as those samples test negative in other platforms. These results are differently referred to as weak reactive, equivocal, or indeterminate results. In a previously non-reactive individual this can lead to diagnostic dilemma. Sequential testing using different platforms, quantitative HBsAg estimation are some of the alternatives evaluated in equivocal results.11-13 Other serological markers and HBV DNA are also used in this context. Hepatitis B surface antigen neutralization assay is also useful in such situations. The assay works on the principle of neutralizing the HBsAg in the tested serum sample and then demonstrating a decrease in the resulting signal strength. Though the assay has been available for some years now, it is not commonly used. The existing literature demonstrates advantages of test in different test formats and not the overall test usefulness.14 The assay is not a part of diagnostic algorithm by any guidelines. The test is not indicated in clear positive cases of Hepatitis B, however, even in doubtful positive cases, the exact guideline is not yet established. The present study tries to look at the usefulness of the assay and probable cut-off index below which the assay may be helpful.
The prospective observational study was conducted in the Department of Microbiology, Kasturba Medical College, Manipal, after approval from the institutional ethics committee (IEC:13/2019). Informed consent was not taken as study was approved under exempt category and test was performed on samples received in the laboratory for routine testing.
The tested serum samples were the ones reactive for HBsAg in Cobas e601 and e411 systems using the kit, Elecsys HBsAg II (Roche diagnostics, Gmbh, Germany) with a COI of < 100 in two runs in same equipment, and non-reactive by other rapid test formats. Immunochromatography (Hepacard, J.Mitra & Co. Pvt. Ltd, India) was the rapid test used for all reactive samples by chemiluminescence. HBsAg confirmatory test using Elecsys HBsAg confirmatory kit (Roche Diagnostics, GmbH, Germany) was performed on all the included samples. The test is selected on manual basis. There was no auto instructions programmed in the analyser to select the test for samples with low COI. Other laboratory markers and values of Hepatitis B (HBV) were collected for the samples. The data on viral DNA levels were also collected for samples where viral load was tested. Viral load testing was done using real-time PCR based on Taqman chemistry using the COBAS Taqman HBV test. (Roche Molecular Systems.Inc)
HBsAg neutralisation assay
The assay works on the principle of antigen neutralisation by specific antibodies. Each sample was treated with the confirmatory and control reagent provided by the manufacturer and incubated for 60 minutes at 15-25°C. The volume of the pre-treatment reagent depended on the primary test’s cut-off index. 30ul for COI <7, 150ul for COI between 7 and 30. The sample with COI >30 was diluted 20 times in universal diluent before treatment with control and confirmatory reagent. After an incubation of 60 minutes, the samples were tested for HBsAg by Elecsys HBsAg II. A COI with the confirmatory reagent ≤ 60 % of that with the control reagent indicates a positive result. The results are interpreted as invalid or indeterminate if the COI of control reagent tests <0.81.
Analysis
Categorical and nominal variables are expressed in percentages. Continuous variables are expressed in mean and standard deviation. Analysis was done using EZR software with R commander. The study’s primary outcome was a percentage reduction in equivocal results after the confirmatory tests. The highest COI, which benefits from confirmatory assay, was also calculated using the ROC curve.
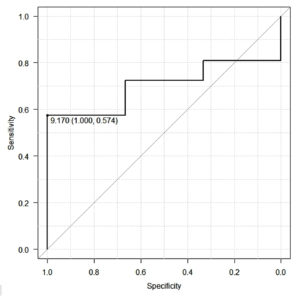
Total samples tested in the study were 57 samples with equivocal and low positive HBsAg results. The mean COI was 12.1+/-10.9. HBsAg confirmatory test was positive in 47 samples, indicating 82.45% of equivocal results were true positives for HBsAg. Three samples (5.26%) were negative by confirmatory assay. 12.28% (7/57) samples tested invalid in confirmatory test. Percentage reduction in equivocal results is 87.71% which includes positive and negative results from confirmatory assay (Figure 1). A COI up to 9.17 showed a maximum area under the curve (AUC) in the receiver operator curve (ROC) analysis indicating upper limit COI which would benefit from neutralization assay. AUC for the COI was 0.702 (0.526, 0.878) (Figure 2).
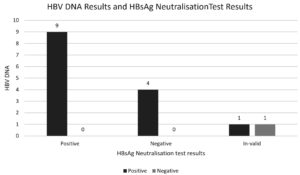
Analysis of molecular confirmation was feasible in 13 samples. Supplementary data shows the details of each sample in terms of molecular and HbsAg confirmatory test. All the samples were positive by HbsAg confirmatory assay. Percentage positivity was 70% (9/13) and percentage of negative results was 30% (4/13) (Figure 3).
The study evaluated the benefits of hepatitis B confirmatory assay. The percentage reduction in equivocal results demonstrated by the study is 87.71%. The percentage is high compared to 59% reported by Shao H et al. and 63% by Punnaswami S et al.10,15 Mean COI recorded is 12+/-10.9 in the present study. High original COI might have influenced the percentage as predicted by the above mentioned literature. In the studies mentioned earlier, the authors have claimed that the positivity of confirmatory assay is 100% when the initial COI is more than four.
The chemiluminescence immunoassay is highly sensitive platform. Though a definitive cut-off is defined by the manufacturer, the samples with low positivity usually test negative in other platforms. Wang et al. compared chemiluminescence results with viral DNA after categorizing the cut-off into three grey zones. That study also suggests a direct correlation between initial cut-off and percentage of negatives.16
We recommend 9.17 as the cut-off below which HBsAg confirmatory assay will be beneficial. This cut-off is comparable with the study by Pasaribu M et al.17 The study was performed using HBsAg Qualitative II (REF 2G22) reagents on the ARCHITECT i2000 analyzer (Abbott Diagnostics). Methodology was like the present study in terms inclusion criteria. The authors report a 100% reduction in false positivity if the initial COI is >10. Other comparable studies recommended lower COI as an indication for HBsAg neutralisation test ranging from 0.8 to 1.08.2,6,10,18 This variation suggests that the cut-off COI may vary depending on the population. It emphasises the necessity to have the cut-off COI customised for each centre.
The other performance indicators also need to be analysed while evaluating an assay. Including the hepatitis B surface antigen neutralisation assay for every positive HBsAg sample would increase the test cost. The cost of using neutralization assay was confirmed to be more compared sequential testing of Hepatitis B surface antigen using different test methodologies.12 In-house neutralization assay using antibodies from vaccinated donors with high antibody titre has been tried. The study documents an agreement of 0.8 kappa between in-house and commercial methods.19 Using neutralization test for confirmation will also increase the TAT of the assay. Frequently encountering invalid results in the confirmatory test is also a concern. Very low COI is the most probable reason, which fails to demonstrate a significant difference between the control and confirmatory reagents results. The present study documented 12.28% of invalid results. Evaluation of HbsAg quantitative assay in a different platform suggest that quantitative estimation of HBsAg can be an alternative to confirmatory assay.11 However, quantitative HBsAg assays may also be equivocal when the value is low positive. HBsAg confirmatory assay is useful in equivocal HBsAg confirmatory results also.20 The indication for conducting the assay should be decided based on clinical background and the suspected stage of the disease. Restricting data to the laboratory is one of the drawbacks of the present study. The HbsAg neutralisation assay is very useful in doubtful results of HBsAg done for acute case definition and to define chronic Hepatitis B.
Diagnostic HBsAg neutralization assay is an underutilized test, which can reduce the diagnostic challenges in newly diagnosed cases with inconclusive HBsAg results. However, the COI where the neutralization assay is highly effective and is yet to be defined globally.
Additional file: Supplementary data
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The study was funded by a University Seed Grant awarded by the Manipal Academy of Higher Education, Manipal, India.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee, Kasturba Medical College, Manipal, India, with reference number IEC:13/2019.
- Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20(8):524-537.
Crossref - Conners EE, Panagiotakopoulos L, Hofmeister GM, et al. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations – United States, 2023. MMWR Recomm Rep. 2023;72(1):1-25.
Crossref - Zhang ZQ, Shi BS, Lu W, Huang D, Wang YB, Feng YL. Quantitative serum HBV markers in predicting phases of natural history of chronic HBV infection. J Virol Methods. 2021;296:114226.
Crossref - Alghamdi A, Aref N, El-Hazmi M, et al. Correlation between Hepatitis B surface antigen titers and HBV DNA levels. Saudi J Gastroenterol. 2013;19(6):252-257.
Crossref - Islam SMRU, Shahera U, Jahan M, Tabassum S. Evaluation and Determination of Quantitative Hepatitis B Surface Antigen Diagnostic Performance in Chronic Hepatitis B Virus-Infected Patients. Cureus. 2023;15(6):e41202.
Crossref - Peng J, Cheng L, Yin B, et al. Development of an economic and efficient strategy to detect HBsAg: Application of “gray-zones” in ELISA and combined use of several detection assays. Clin Chim Acta. 2011;412(23-24):2046-2051.
Crossref - Arcot PJ, Pandey HC, Coshic P, Jain P, Kumar S, Chakroborty S. Comparative evaluation of ADVIA Centaur® XP chemiluminescence system for screening of HBV, HCV, HIV and syphilis in Indian blood donors. Transfus Apher Sci. 2022;61(2):103318.
Crossref - Aluora PO, Muturi MW, Gachara G. Seroprevalence and genotypic characterization of HBV among low risk voluntary blood donors in Nairobi, Kenya. Virol J. 2020;17(1):176.
Crossref - Gupta E, Bhugra A, Samal J, et al. Performance Evaluation of an Improved HBsAg Assay (HBsAg NEXT) for the Detection of HBsAg Levels. J Lab Physicians. 2023;15(4):533-538.
Crossref - Sherly P, Irda H, Asvin N, Uleng B. Determination of reactive HBsAg cut-off that need confirmatory test. Indonesian Journal of Clinical Pathology and Medical Laboratory. 2018;24(3):251-254.
Crossref - Lee J, Lee SY, Cho YG, Kim DS, Park J. Accuracy Validation of the Elecsys HBsAg II Quant Assay and Its Utility in Resolving Equivocal Qualitative HBsAg Results. Medicina (Lithuania). 2023;59(3):443.
Crossref - Tiwari AK, Pabbi S, Aggarwal G, et al. Application of sequential serological testing strategy for detection of Hepatitis B surface antigen (HBsAg) for diagnosing HBV infection. J Virol Methods. 2019;274:113726.
Crossref - Borgaonkar R, Shahapur PR. Serological markers hbsag and hbeag in chronic hepatitis B carriers and their correlation with viral DNA by polymerase chain reaction. J Pure Appl Microbiol. 2019;13(3):1645-1651.
Crossref - Chen D, Kaplan LA. Performance of a new-generation chemiluminescent assay for hepatitis B surface antigen. Clin Chem. 2006;52(8):1592-1598.
Crossref - Shao H, Li Y, Xu WZ, Zhou X. Increased need for testing to confirm initial weakly reactive results for hepatitis B virus surface antigen. Lab Med. 2012;43(4):15-17.
Crossref - Wang M, Yu SH, Han ZZ. The Utility of Grey Zone Testing in Improving Blood Safety. Am J Transl Res, 2021;13(8):9771-9777.
- Pasaribu MM, Wonohutomo JP, Immanuel S, Kumalawati J, Indrasari ND, Yusra Y. Cutoff Value of Qualitative HBsAg for Confirmatory HBsAg Using the Chemiluminescence Microparticle Immunoassay Method. Lab Med. 2022;53(5):475-478.
Crossref - Lee MY, Kang SY, Lee WI, Kim MH. Need for Confirmatory Neutralization Tests for Hepatitis B Surface Antigen Tests in Populations with Intermediate Prevalence. Lab Med. 2021;52(5):485-492.
Crossref - Fletcher GJ, Gnanamony M, David J, Ismail AM, Subramani T, Abraham P. Do we need an “in-house” neutralization assay for confirmation of hepatitis B surface antigen? Answers from a tertiary care hospital in India. J Gastroenterol Hepatol (Australia). 2010;25(5):942-945.
Crossref - Yonezawa H, Tanaka S, Tanaka M, Kobayashi R, Takahashi S. Efficient implementation of hepatitis B surface antigen confirmatory neutralization tests. J Infect Chemother. 2024;30(1):29-33.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.