Scrub typhus, an age old disease, is caused by the intracellular bacterium Orientia tsutsugamushi. It has reemerged in recent years due to factors like climatic changes and human encroachment because of rampant urbanization. The disease is endemic in the area known as the ‘tsutsugamushi triangle’ and has recently spread its fangs into various other continents like South America and Africa. Although the disease is endemic in India, there is a lack of appropriate sero-epidemiology in community settings. It is one of the essential causes of acute undifferentiated fever in tropical locations and, if untreated, can cause mortality ranging from 2-30% of cases. Early diagnosis is an important parameter in administering the non beta-lactam regimen to prevent complications and mortality. Yet, there is a lack of accurate and rapid methods for diagnosis in the early stage of the disease, more so in rural areas where the disease is supposed to be predominant. The gold standard diagnostic test has its problems. Recently, there have been reports of drug resistance to the standard scrub typhus regimen. There is a gap of a decade in the research into this entity. Thus, a new look into the disease, its epidemiology and the challenges in its diagnostic scenario is an apt topic for discussion.
Scrub Typhus, Orientia tsutsugamushi, IgM Capture ELISA, Indirect Immunofluorescence Assay
Scrub typhus was a disease of the pre-antibiotic era, especially during military operations, but even today causes one million cases annually worldwide.1 In the recent years it has reemerged as a significant public health issue due to multiple factors like climatic changes, human activities disturbing the ecological balance, beta-lactam antibiotics overuse and urbanization of rural areas.2 This disease is an underdiagnosed entity due to a lack of typical pathognomonic clinical features coupled with inadequate availability of appropriate laboratory methods. Thus there is limited epidemiological data regarding the disease globally.
Epidemiology of Scrub typhus
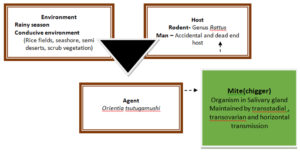
Scrub typhus is caused an intracellular Gram negative small pleomorphic coccobacilli named Orientia tsutsugamushi,3 which was previously under Rickettsia family. It is named as ‘tsutsugamushi’ (dangerous bug), after a jungle mite or chigger, which acts as a reservoir and transmits the disease to man by biting through pores or hair follicles on exposed skin. Human encroachment during deforestation, logging, road building, military operations, rice cultivation, etc., bring us close to infected chiggers, the parasitic stage in the vector Leptotrombidium mite. Climatic change due to global warming also has a role in the reemergence of this disease (Figure 1).4
Figure 1. The epidemiological triad of scrub typhus
The agent O. tsutsugamushi is maintained in its reservoir mites by transstadial and transovarian transmission and transmitted to man by bite of the larvae (chigger) in cases of human encroachment into mite habitat. Man to man transmission is not seen.
Tropical regions have appropriate temperatures and humidity for chigger activity and maintaining the pathogen in transovarian and transstadial transmission in the mites. Researchers have also noted horizontal transmission of Orientia among mites.5 Thus, the disease occurs around the year. However, in temperate zones like in the northern part of Japan, the mite activity is seasonal corresponding to the disease.6
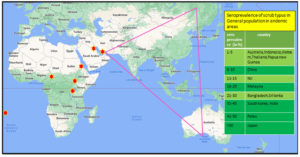
Scrub typhus is endemic to a region denoted as the ‘‘tsutsugamushi triangle’’.South East Asian region is endemic for scrub typhus.7 But in recent times, the disease has surfaced in other non endemic areas like Europe, Chile, Peru, Middle East, African peninsula and sometimes with a different species of the bacteria, like O.chuto seen in the Middle East (Figure 2).8 In India, several states have varying ecological profile, like Haryana, Jammu, and Kashmir, Himachal Pradesh, Uttaranchal in north; Kerala, Tamil Nadu in South; Bihar, West Bengal, Assam in East; Maharashtra in West report this disease.9,10 Sero-prevalence of this infection in India is between 9.3% and 27.9%, and the mortality rate is around 30% among untreated individuals, as noted in passive national surveillance systems.7,11 Among acute onset febrile illness, scrub typhus constitutes about 25.3% of cases in India (Table 1).12
Figure 2. The Tsutsugamushi triangle and other area in the world where the disease has been reported.
Tsutugamushi triangle, an area approximating about 8 million km2 is endemic for scrub typhus. This extends in North from the Russian Far East, to Pakistan in the west, Australia in the south, and the Japan in the east. Seroprevalence of scrub typhus detected in recent studies,63 have been tabulated which ranges from mere 1.1% in Vietnam to 68.4 % in Japan. But apart from this, other area in globe like – Chile, United Arab Emirates, African countries ( Camroon, Kenya, congo, Tanjania) also have reported cases of scrub typhus.
Table (1):
Tabulation of recent studies in India on scrub typhus
Majority of the studies included acute undifferentiated fever cases in their inclusion criteria and were hospital based studies including adult patients
Author and year |
Place |
Time period |
Target population |
No. of patients |
No of cases detected |
No. of deaths |
Test used |
Predominant month |
Age & sex of patients |
|---|---|---|---|---|---|---|---|---|---|
Subbalaxmi et al. [64] |
Andhra Pradesh |
August 2011 to December 2012 |
AUFI Cases >12 years |
NA |
176 |
8 |
Weil felix test (>1:80) ICT |
NA |
41 years; M>F |
Sinha et al. [65] |
Jaipur, Rajasthan |
Oct-2012 to Dec-2012 |
AUFI cases |
170 |
42 |
7 |
IgM ELISA |
NA |
M>F |
Stephen et al. [66] |
Puducherry Tamilnadu |
Sep2012 to March 2013 |
AUFI cases |
45 |
25 |
0 |
ICT test IgM, IgG ELISA (paired) Weil felix (Initial titre1:40 or OXK>320) |
NA |
31 years; M=F |
Krishna, Vasuki et al. [67] |
Chennai Tamilnadu |
Sep 2010 to June 2011 |
Paediatric AUFI |
NA |
52 |
0 |
IgM ELISA |
NA |
|
Jakharia et al. [68] |
Arunachal Pradesh |
NA |
Seroprevalence study in community |
300 |
120 |
0 |
IgG ELISA |
NA |
>40 years; M=F |
Rajendra prasad thakar [69] |
Rajasthan |
July to October 2014 |
AUFI |
290 |
66 |
14 |
IgM ELISA |
NA |
20 -50 year; F>M |
H. Lalrinkima [70] |
Mizoram |
October 2014 to December 2016 |
AUFI |
4081 |
283 |
ICT test |
November -February |
21 – 30 year; M>F |
|
Deepak jain [71] |
Haryana |
July to Nov 2017 |
>14 yrs AUFI |
230 |
39 |
7 |
IgM ELISA |
NA |
39 year; F>M |
Thakur et al. [72] |
Various parts of India |
2013- 2018 |
AUFI in hospitalized patients |
1742 |
210 |
14 |
IgM ELISA andIFA ( Gilliam and Karp strains) |
NA |
|
Laxmi R et al. [73] |
Telangana |
July to October 2018 |
Suspected Scrub typhus |
645 |
89 |
0 |
ICT+ IgM ELISA |
August |
20 TO 50 year; F>M |
Verma et al. [74] |
Lucknow |
Sep 2019 to Jan 2020 |
AUFI >18 yrs |
52 |
NA |
IgM ELISA |
NA |
20 TO 50 year; F>M |
Orientia shows many genetic and antigenic variations resulting from variations in tsa gene, which codes for 56-kDa type specific antigen.1,13 There are around 30 serological types like kato, karp, kuroki, gilliam and kawasaki that as detected by immunoperoxidase reaction14 Litchfield strain is a novel strain detected in Australia.15 The correlation between this antigenic diversity and virulence is still unclear.
Clinical presentation
Fever is the commonest presentation, seen in 95-100% of cases.16,17 Scrub typhus accounts for a significant chunk of “fever of unknown origin” in endemic regions. Even the term “typhus” is derived from Greek terminology ‘Typos’ meaning ‘fever with stupor’ The age group of 50-60 is commonly afflicted while, sex preponderance varies across different countries.3 A papular lesion is formed at the chigger bite site, which becomes larger with time, followed by necrosis and crusting in the centre and finally developing a black eschar, which is a pathognomonic feature of scrub typhus. The presence of eschar is specific (98.9%) for diagnosis of this disease but is limited by sensitivity, which varies between 7%-97%.7 Further, eschar is often absent in the South East Asian population and in endemic areas with less severe illness.18-20 Scrub typhus can present in varied forms ranging in severity from asymptomatic to multi organ failure.21 Common symptoms are myalgia, headache, nausea, vomiting, abdominal pain, cough, generalized lymphadenopathy and skin rash in varying combinations.3,22,23 Owing to its mimicking signs and symptoms, it took almost 30 years to prove the original finding of Coyttarus (1578) that typhoid and typhus were different diseases. Untreated cases may develop several complications generally occurring after the first week of illness. Various complications like acute renal failure, jaundice with rising liver enzymes, pneumonitis and acute respiratory distress syndrome, septic shock, myocarditis, meningoencephalitis and reversible deafness have been noted in prior studies.18,20 Renal involvement can be expected in about 9% of patients. Patients of meningoencephalitis often have CSF changes indistinguishable from viral or tuberculous meningitis. Unusual presentations include conjunctival hyperemia or erosion, gastrointestinal mucosal erosion without any predilecting site and acute abdomen.24,25 Septic shock ensues with further organ damage to liver, lungs, kidneys along with DIC.26,16,27,28 Elevated transaminases, thrombocytopenia and leukocytosis are the biochemical investigations pertinent to diagnosing the disease when used in combination (specificity and positive predictive value for diagnosis – 80%).29
Mortality from this disease varies from 7-30%,30 but much less in children.31 The possible patient factors associated with complicated cases are – age (≥60 years), patients without eschar, WBC counts >10000/mm and serum albumin level ≤3.0 g/dL.31,2 Being a great mimicker, diagnosis in the early stages is challenging yet important for successfully treating scrub typhus. The median case fatality rate is reduced to 1.4% in treated patients from 6% seen in late or untreated ones.11,29,32 Diagnosis is based on clinical suspicion with appropriate lab investigation.
Lab diagnosis of scrub typhus
Serological assays
Serological tests that detect antibodies to against scrub typhus, like Weil Felix test, ELISA, immunofluorescence and immunoperoxidase tests are the commonly performed tests for lab diagnosis. IgM is preferred over IgG detection as it can help diagnose recent infections. But, all there tests have many issues that needs addressing. A ≥ 4-fold increase in antibody titer between two consecutive samples is diagnostic,33 but often not practical. Secondly, a baseline titer (cut-off) is to be established in the geographical setting based on the endemicity of the disease for appropriate reporting, which is often lacking. There is wide variation across India in cut-off values of the various serological tests (Table 2). Then again, most serological tests use an antigen cocktail of Karp, Kato, and Gilliam serotypes. But there are many other antigenic variations apart from these three, differing in different geographical regions of the world.1 For example, in mites collected from a single field in Malaysia, eight different serotypes were found.34 Boryong is the commonest serotype in South Korea in three-fourths of total isolates.35 Similarly, Kawasaki or Kuroki serotypes accounted for >90% of Kyushu island isolates of Japan.36 In India, data on serotype prevalence in different areas is still lacking. Thus, common serotypes must be explored and included as the antigen for serological testing purposes.
Table (2):
Cut off value calculated in different studies from various hospital based studies across India
| Study | Setting | Cut off value | Sensitivity | Specificity |
|---|---|---|---|---|
| ELISA | ||||
| Manjunathachar et al. [87] | Madhya Pradesh | 0.73 (IgM) | 95 | 100 |
| Koraluru et al. [79] | Karnataka | 1.0 (IgM) | 85 | 95 |
| Gupta et al. [88] | New Delhi | 0.87 (IgM) | 100 | 94.12 |
| Gupta A et a.l [89] | Himachal Pradesh | 0.46 (IgM) | 91.7 | 99.5 |
| Gautam et al. [81] | Nepal | 0.5 (IgM) | – | – |
| Karthikeyan et al. [85] | Puducherry | 0.4 | – | – |
| Rawat et al. [90] | Uttarakhand | 0.6 (IgM) 1.6 (IgG) |
96.4 91 |
82.7 75 |
| Verghese et al. [91] | Vellore, South India | 0.8 (IgM) 1.8 (IgG) |
– – |
– – |
| Immunofluorescence assay | ||||
| Fomda et al. [82] | Kashmir | 1:128 (IgM)
1:256 (IgG) |
–
– |
–
– |
| Gupta et al. [88] | New Delhi | 1: 64 | 100 | 93.5 |
| Gupta et al. [88] | New Delhi | 1:512 | 98 | |
| Gautam et al. [81] | Nepal | 1:128 | – | – |
| Rawat et al. [90] | Uttarakhand | 1:512 (IgM) 1:2048 (IgG) |
||
| Verghese et al. [91] | Vellore, South India | 0.251 (IgM) 0.205( IgG) |
– – |
– – |
IFA
IFA, the gold standard test for detection suffers from many pitfalls. For example, in a Korean study, IFA had false negative results in six patients with a typical eschar which was positive for O. tsutsugamushi DNA.37 Further, it is labor intensive, needs resource settings and can have interoperator variations.7
Rapid test
The dot blot immunoassay dipstick is rapid, semi-quantitative, accurate and easy to use inexpensive point of care test that can also be used in rural settings.19,38 Rapid immunochromatographic test is another POC test with higher sensitivity and specificity of 96.8% and 93.3%, respectively when used for detection of IgM.39 Studies considering Bayesian class models show that ICT kits can even have higher specificity than IFA.40-42 ICT kits can be used with another method like LAMP/ PCR assays for improving accuracy (Table 3).
Table (3):
Various methods for laboratory diagnosis of scrub typhus
Indirect diagnostic methods comprise of method which detect antibodies developed against Orientia tsutsugamushi while direct methods detect the organism from the samples either by culture, animal inoculation or the DNA of the bacteria by amplification methods
| INDIRECT DIAGNOSTIC METHODS | ||||
|---|---|---|---|---|
| Test | Principle | Advantages | Issues | |
| Weil Felix | ≥ 4 times rise in titre to proteus OX-K and no reaction to proteus OX-2 or OX-19 Single titre ≥1:160 is also diagnostic (normal is ≤1:40[75] |
Antibodies are detectable after 5 – 10 days following the onset of fever [9] Inexpensive Easy to perform Results are available overnight |
Not a sensitive test When positive, it is specific test.[76] False negative – in UTI by Proteus, Leptospira infections etc. |
|
| ELISA KpKtGm-wc ELISA or KpKtGm r56 ELISA IgM capture ELISA |
56-kDa protein (located on the outer membrane of O.tsutsugamushi highly reactive inducing antibodies[77] Antigen used – whole cell antigen / r56 from the Karp, Kato, and Gilliam strains of O. tsutsugamushi [78] |
Higher sensitivity and specificity than Weil felix 85% sensitive and specificity in comparison to IFA (InBios kit) IgM capture ELISA can capture the acute infection |
Specified Cut off is needed Need paired sera Many serotypes may need constant monitoring |
|
| Indirect immunofluorescence antibody detection test (IFA) | Uses fluorescein linked anti-human reporter antibody to detect the presence of scrub typhus-specifc antibodies (mostly against Karp, Kato, Gilliam) in the serum sample.[33] An 20-fold rise in titre in paired (14 days) sampling is considered positive |
Current Gold standard test for diagnosis | Need Paired sera Specified cutoffs needed Specialized equipments required Costly Other methods offer fair sensitivity and specificity Reexamination not possible |
|
| Indirect immunoperoxidase antibody detection test (IPA) | Fluorescent antibody tagging is substituted by peroxidase tagging | Preparations can be preserved for reexamination All cells infected and uninfected can be visualised Any serotype can be used as an antigen Can measure either IgG or IgM Sophisticated instruments are not necessary |
Subjective readings | |
| ICT | Recombinant antigen mixture of 56-kDa outer-membrane proteins of Karp, Kato and, Gilliam strain is captured for detection of IgG and IgM antibodies to Orientia tsutsugamushi. The serotypes can be changed as per endemicity in different geographical location |
Fairly good sensitivity and specificity which is increased when used with other techniques like LAMP assays or IFA Total antibody(IgG+IgM+ IgA) test has lesser specificity than IgM alone IgM ICT kit is an important ruling in test |
Sensitivity and specificity lesser than other serological techniques All issues as in serological tests |
|
| DIRECT DIAGNOSTIC METHODS | ||||
| PCR | On blood sample targeting 56 kDa tsa, GroEL, 16s RNA and 47 kDa HtrA genes Can be performed as conventional PCR, nested PCR, qualitative or quantitative real time PCR and LAMP assays |
Diagnosis during first week of illness (Rickettsiamia) No paired sera necessary Eschar PCR has better sensitivity than blood/serum |
Genetic diversity may cause false negativity. False negative with previous treatment. Extensive clinical evaluation is pending. |
|
| LAMP | PCR based POC. Targets the groEL gene, the 60 kDa heat shock protein of Orientia tsutsugamushi.[37] The reaction can be quantitatively interpreted in real-time by measuring the turbidity or by fluorescence using intercalating dyes such as SYTO 9. |
Thermo cyclers and other PCR set up for extraction and interpretation are not needed Diagnostic accuracy more than other PCR methods Advantage over serological testing in first week of illness |
Further clinical evaluation needed | |
| Cell culture | Culture in cell lines like HeLa, BHK 21, Vero cells | Improves sensitivity and specificity | BSL-3 facility required 27 days for positivity. |
|
| Animal inoculation | Detection of organism by Giemsa stain in tissues following intraperitoneal inoculation Scrotal reaction following intra peritoneal injection of blood into male guinea pig |
Sensitivity and specificity good | BSL-3 facility Time taking affair |
|
Molecular assays
PCR, either conventional, nested or real-time PCR can be used for diagnosis of scrub typhus.43,44 Q-PCR is faster, has higher sensitivity and specificity and produces quantitative results than other methods.45 Q PCR has been already reported with targets like- 16S rRNA gene (using hydrolysis probes), 60-kDa heat shock GroEL gene and 47-kDa HtrA outer membrane protein gene. 46-48 Q PCR with 16S r RNA as the desired target has the highest sensitivity and accuracy compared to other targets and also when compared with immunofluorescence assay for diagnosis. Specimens from which PCR can be done are eschar, whole blood, clots or buffy coat. Immunohistochemical staining and PCR from eschar material are more sensitive and remains positive even after treatment.37,49 All the PCR assays remain positive only during the period of rickettsemia. Common genetic targets for OT detection are- tsa gene encoding the 56-kDa type-specific antigen; htrA gene coding for 47-kDa periplasmic serine protease48; groEL gene – Hsp60; 16S rRNA.50,51 Although the56-kDa antigen is highly specific,52,53 but variability in sequence can affect the annealing of the primer and reduce test sensitivity.44 Assay targeting the 16S rRNA gene showed a higher sensitivity than 56-kDa gene.51As O. tsutsugamushi genome has a high degree of genetic variations, improving specificity of the detection by using multiple genes approach either by conventional or real-time PCR is the need of the hour.
LAMP assay
LAMP assay with the groEL gene of Orientia tsutsugamushi has been tried.37 LAMP assay has many advantages such as not needing a thermal cycler and visual result reading. But, clinical use warrants further validation. A study has also shown that limit of detection with LAMP assay is 14 copies/μL compared with three copies/ μL for real-time PCR.50
STIC criteria for diagnosis of scrub typhus
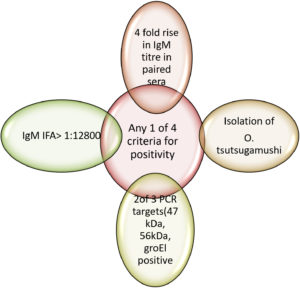
Gold standard or reference test for scrub diagnosis includes IFA or IIP assays, which have many limitations for accurate diagnosis. Bayesian model showed that the IFA IgM assay has sensitivity and specificity of 70.0% and 83.8%, respectively.41 Keeping this in mind, STIC criteria (Figure 3) using a battery of tests with high specificity has been proposed as an alternative reference comparator for accurate diagnosis.54 Table 4 summarizes the commonly available serological and molecular tests for scrub typhus diagnosis.
Table (4):
The sensitivity, specificity, Positive predictive value and Negative predictive value of the common tests available for scrub typhus from various studies conducted from low and middle income country settings
Test for Scrub Typhus |
Type of assay evaluated |
Reference/Gold standard assay |
Reported Sensitivity (95%CI) and specificity (95% CI) |
Reported PPV (95%CI) and NPV (95% CI) |
Study setting |
Reference |
|---|---|---|---|---|---|---|
Scrub Typhus Detect IgM ELISA kits InBios International |
IgM ELISA |
IgM Microimmunofluorescence |
85.3% (78.4–90.7) 95.5% (93.0–97.3) |
87.1%(80.4–92.2) 94.8% (92.2–96.7) |
Tertiary care hospital, Karnataka, India |
Koraluru M, et al. [79] |
Scrub Typhus Detect IgM ELISA kits from InBios International |
IgM ELISA |
Conventional PCR positive for O.tsutsugamushi56kDa type specific antigen (TSA) or 47kDa htrA (high temperature requirement A) qPCR positive |
92.41% (86.8–96) 93.67% (88.7–96.9) |
93.59% (88.5–96.9) 92.50% (87.3–96.1) |
Tertiary care hospital, Tamil Nadu India |
Kannan K et al. [80] |
Scrub Typhus Detect IgM ELISA kits from InBios International |
IgM ELISA |
IgM Immunofluorescence test |
84% (79.73–87.68) 94.82% (93.43-95.99) |
82.12%(78.28–85.42) 95.44% ( 94.27–96.38) |
Hospital setup, Central Nepal |
Gautam R et al. [81] |
IgG ELISA InBioS International, Inc. USA |
IgG ELISA |
IgG IFA |
86.67% (73.21–94.95) 97.86% (95.08–99.30) |
88.64% (76.49–94.93) 97.45% (94.77–98.77) |
Tertiary care hospital, Kashmir |
Fomda et al. [82] |
Conventional PCR |
56 KDa gene |
IgM Microimmunofluorescence |
75.32% (67.8–81.8) 100% (95.4–100) |
100% (96.5–100) 80.20% (73.9–85.5) |
Tertiary care hospital, Tamil Nadu India |
Kannan K et al. [80] |
Real time PCR (47 KDa) |
47 KDa gene |
IgM Microimmunofluorescence |
97.47% (93.8–99.3) 100% (96.5–100) |
100% (96.5–100)
97.53% (93.6–99.3) |
Tertiary care hospital, Tamil Nadu India |
Kannan K et al. [80] |
Real time PCR |
16S rRNA qPCR |
Fourfold increases in IgM or IgG titer on IFA |
91.9% (86.3- 95.7) – |
– – |
Tertiary care hospital , Korea |
Yun et al. [83] |
Nested PCR |
nPCR |
IgM IFA |
29.73% (15.87- 46.98) 99.58% (97.67- 99.99) |
70.46% (9.37- 52.98) 0.71% (0.57 to 0.87) |
Tertiary care hospital, Wardha, Maharastra |
Roy S et al. [84] |
LAMP |
47 kDa gene of O. tsutsugamushi |
IgM IFA |
16.22% (6.19- 32.01) 99.16% (96.99–99.9) |
19.22%(4.03-91.66) 0.84% (0.73 to 0.97) |
Tertiary care hospital, Wardha, Maharastra |
Roy S et al. [84] |
LAMP |
groEl gene |
IgM ELISA |
100% 73% |
– – |
Tertiary care hospital, Puducherry India |
Karthikeyan PA et al. [85] |
Immune med ICT test kit |
IgM IgG |
IgM IFA IgG IFA |
98.6% (96-100) 98.2% (96-99) 97.1% (94-99) 97.7 %(95-99) |
97.2%(97-99) 99.1% (98-100) 96.4% (93-99) 98.2% (93-99) |
Korea |
Kim YJ et al. [86] |
SD Bioline Tsutsugamushi ICT kit |
IgM IgG |
IgM ELISA IgG ELISA |
91.67%(72.96-98.73) 90.48%(69.58-98.55) 85.71% (67.32-95.88) 100% (80.33-100) |
91.67% (72.96-98.73) 90.48% (69.58-98.55) 100% (65.62-100) 80.95% (58.08-94.44) |
Tertiary care set up, Tamil Nadu, India |
Stephen S et al. [66] |
Treatment of scrub typhus
The treatment options for scrub typhus are- doxycycline and tetracycline. Azithromycin, ciprofloxacin and rifampicin are effective alternatives where there is poor response to doxycycline. In pregnant women and children less than 8 years old, azithromycin is the preferred regimen. Severe disease needs to be treated with intravenous chloramphenicol with intravenous tetracycline. A recent multicentric study has concluded that combination therapy of intravenous doxycycline and azithromycin is a better treatment option for severe scrub typhus than any agent alone.55 There is no significant difference in outcome when azithromycin therapy is compared with other antibiotics singly or in combination in paediatric patients as noted in a recent meta-analysis.56 But recently, there have been reports of drug resistance, which needs further pondering.57-59
Prophylaxis of scrub typhus
Different localities have different antigenic variants of O. tsutsugamushi strains showing no to weak cross-protection. Thus, an effective vaccine for scrub typhus must account for multiple strains thriving in the population.60-62 WHO recommends single oral dose of tetracycline, doxycycline or chloramphenicol every 5 days for a total of 35 days for prophylaxis against Orientia infection63 as opposed to CDC which opines that such a prophylactic treatment may only delay the disease and also hinder diagnosis. Other safety measures include avoiding exposure to vegetation by using full-sleeved clothing, mats to sit on the grass, using shoes, cleaning the garments with insect repellant after a possible exposure to get rid of mites, and rodent control.63
Scrub typhus, a disease of wars, has raised its fangs with growing climate change and human activities encroaching on the habitat of the mite reservoir. Despite its long presence, there needs to be more data citing its actual prevalence, serotypes involved and determinants of clinical course, especially in India. One of the important reasons for this is the lack of a diagnostic test with desirable accuracy. Molecular methods are helpful early in the disease and are yet to be widely used for diagnosis. Adopting a rapid, accurate test protocol for clinical diagnosis of scrub typhus is necessary. Further clinical trials and research is needed for evaluating various regimens used for scrub typhus, keeping in mind the evolving drug resistance and its intracellular persistence causing relapses.
ACKNOWLEDGMENTS
We sincerely acknowledge the support of S’O’A University, Kalinga Nagar, Bhubaneswar, Odisha, for their support in carrying out this review.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(suppl 3):S203-S230.
Crossref - Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10(1):1-7.
Crossref - Mahajan SK. Scrub typhus. J Assoc Physicians India. 2005;53(955):954-958.
- Park SW, Ha NY, Ryu B, et al. Urbanization of scrub typhus disease in South Korea. PLOS Negl Trop Dis. 2015;9(5):e0003814.
Crossref - Traub R, Wisseman CL Jr, Jones MR, O’Keefe JJ. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann N Y Acad Sci. 1975;266:91-114.
Crossref - Matsui T, Kramer MH, Mendlein JM, et al. Evaluation of national tsutsugamushi disease surveillance-Japan, 2000. Jpn J Infect Dis. 2002;55(6):197-203.
- Saraswati K, Day NPJ, Mukaka M, Blacksell SD. Scrub typhus point-of-care testing: a systematic review and meta-analysis. PLOS Negl Trop Dis. 2018;12(3):e0006330.
Crossref - Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375(10):954-961.
Crossref - Mahajan SK, Kashyap R, Kanga A, Sharma V, Prasher BS, Pal LS. Relevance of Weil-Felix test in diagnosis of scrub typhus in India. J Assoc Physicians India. 2006; 54:619-621
- Singh P. Scrub typhus, a case report: military and regional significance. Med J Armed Forces India. 2004;60(1):89-90.
Crossref - Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: a systematic review. PLOS Negl Trop Dis. 2017;11(9):e0005838.
Crossref - Devasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: A systematic review. PLOS Negl Trop Dis. 2021;15(7):e0009619.
Crossref - Blacksell SD, Luksameetanasan R, Kalambaheti T, et al. Genetic typing of the 56-kDa type-specific antigen gene of contemporary Orientia tsutsugamushi isolates causing human scrub typhus at two sites in north-eastern and western Thailand. FEMS Immunol Med Microbiol. 2008;52(3):335-342.
Crossref - Enatsu T, Urakami H, Tamura A. Phylogenetic analysis of Orientia tsutsugamushi strains based on the sequence homologies of 56-kDa type-specific antigen genes. FEMS Microbiol Lett. 1999;180(2):163-169.
Crossref - Odorico DM, Graves SR, Currie B, et al. New Orientia tsutsugamushi strain from scrub typhus in Australia. Emerg Infect Dis. 1998;4(4):641-644.
Crossref - Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31(4):240-244
- Jamil M, Lyngrah KG, Lyngdoh M, Hussain M. Clinical manifestations and complications of scrub typhus: A hospital based study from North Eastern India. J Assoc Physicians India. 2014;62(12):19-23
- Cowan GO. Rickettsial infections. In: Manson’s Tropical Diseases. Gordon C (Edi.) 21st Edi. Saunders Elsevier Science (Health Sciences Division); 2003:891-906.
- Watt G. Scrub typhus. In: Warrel DA Cox TM, Firth JD, et al., eds. (Edi) 4th Edi. Oxford Textbook of Medicine. Oxford University Press; 2003:629-631.
- Silpapojakul K. Scrub typhus in the western Pacific region. Ann Acad Med Singapore. 1997;26(6):794-800.
- Walsh DS, Myint KS, Kantipong P, Jongsakul K, Watt G. Orientia tsutsugamushi in peripheral white blood cells of patients with acute scrub typhus. Am J Trop Med Hyg. 2001;65(6):899-901.
Crossref - Banerjee A, Kulkarni S. Orientia tsutsugamushi: The dangerous yet neglected foe from the East. Int J Med Microbiol. 2021;311(1):151467.
Crossref - Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. Scrub typhus: clinical, pathologic, and imaging findings. In: RadioGraphics. 2007;27(1):161-172.
Crossref - Kim SJ, Chung IK, Chung IS, et al. The clinical significance of upper gastrointestinal endoscopy in gastrointestinal vasculitis related to scrub typhus. Endoscopy. 2000;32(12):950-955.
Crossref - Yang CH, Young TG, Peng MY, Hsu GJ. Unusual presentation of acute abdomen in scrub typhus: a report of two cases. Zhonghua Yi Xue Za Zhi (Taipei). 1995;55(5):401-404.
- Cracco C, Delafosse C, Baril L, et al. Multiple organ failure complicating probable scrub typhus. Clin Infect Dis. 2000;31(1):191-192.
Crossref - Thap LC, Supanaranond W, Treeprasertsuk S, Kitvatanachai S, Chinprasatsak S, Phonrat B. Septic shock secondary to scrub typhus: characteristics and complications. Southeast Asian J Trop Med Public Health. 2002;33(4):780-786
- Mathai E, Rolain JM, Verghese GM, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359-364.
Crossref - Varghese GM, Abraham OC, Mathai D, et al. Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. J Infect. 2006;52(1):56-60.
Crossref - Walker D, Raolt D, Dumler JS, et al. In Harrison’s Principles of Internal Medicine Braunwald E, Fauci AS, Kasper DL et al (Edi.) 15th Edi. McGraw-Hill Companies Inc; 2001:1070.
- Dumler JS, Siberry GK. In: Kliegman RM, Behrman Re JHB, Stanton BF, eds. Nelson Textbook of Pediatrics. 18th ed. Saunders, Elsevier; 2007 Scrub Typhus (Orientia Tsutsugamushi). (Part XVI. Section 11. Chapter 226):1295-1296.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLOS Negl Trop Dis. 2015;9(8):e0003971.
Crossref - Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NPJ. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44(3):391-401.
Crossref - Miesse M, Diercks F, Danauskas J. Strain differences among Rickettsia tsutsugamushi. Bacteriol Proc. 1950;M45:90-91.
- Ree HI, Kim TE, Lee IY, Jeon SH, Hwang UW, Chang WH. Determination and geographical distribution of Orientia tsutsugamushi serotypes in Korea by nested polymerase chain reaction. Am J Trop Med Hyg. 2001;65(5):528-534.
Crossref - Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. Mar. 2010;82(3):368-370.
Crossref - Kim DM, Kim HL, Park CY, et al. Clinical usefulness of eschar polymerase chain reaction for the diagnosis of scrub typhus: a prospective study. Clin Infect Dis. 2006;43(10):1296-1300.
Crossref - Chinprasatsak S, Wilairatana P, Looareesuwan S, et al. Evaluation of a newly developed dipstick test for the rapid diagnosis of scrub typhus in febrile patients. Southeast Asian J Trop Med Public Health. 2001;32(1):132-136.
- Blacksell SD, Jenjaroen K, Phetsouvanh R, et al. Accuracy of AccessBio immunoglobulin M and total antibody rapid immunochromatographic assays for the diagnosis of acute scrub typhus infection. Clin Vaccine Immunol. 2010;17(2):263-266.
Crossref - Sankar S, Saravanan N, Rajendiran P, Ramamurthy M, Nandagopal B, Sridharan G. Identifcation of B- and T-cell epitopes on HtrA protein of Orientia tsutsugamushi. J Cell Biochem. 2019;120(4):5869-5879.
Crossref - Lim C, Paris DH, Blacksell SD, et al. How to determine the accuracy of an alternative diagnostic test when it is actually better than the reference tests: A re-evaluation of diagnostic tests for scrub typhus using bayesian LCMs. PLOS ONE. 2015;10(5):e0114930.
Crossref - Lim C, Blacksell SD, Laongnualpanich A, et al. Optimal cutoff titers for indirect immunofuorescence assay for diagnosis of scrub typhus. J Clin Microbiol. 2015b;53(11):3663-3666.
Crossref - Kim DM, Park G, Kim HS, et al. Comparison of conventional, nested, and real-time quantitative PCR for diagnosis of scrub typhus. J Clin Microbiol. 2011;49(2):607-612.
Crossref - Sonthayanon P, Chierakul W, Wuthiekanun V, et al. Rapid diagnosis of scrub typhus in rural Thailand using polymerase chain reaction. Am J Trop Med Hyg. 2006;75(6):1099-1102.
Crossref - Watthanaworawit W, Turner P, Turner C, et al. A prospective evaluation of real-time PCR assays for the detection of Orientia tsutsugamushi and Rickettsia spp for early diagnosis of rickettsial infections during the acute phase of undifferentiated febrile illness. Am J Trop Med Hyg. 2013;89(2):308-310.
Crossref - Saravanan N, Rajendiran P, Sankar S, et al. Detection of scrub typhus by real-time polymerase chain reaction and immunoglobulin M ELISA among patients with acute febrile illness. J Nat Sci Biol Med. 2020;11(1):66.
Crossref - Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, Richards AL. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70(4):351-356.
Crossref - Sonthayanon P, Chierakul W, Wuthiekanun V, et al. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol. 2009;47(2):430-434.
Crossref - Kim DM, Park CJ, Lim SC, Park KH, Jang WJ, Lee SH. Diagnosis of Scrub Typhus by immunohistochemical staining of Orientia tsutsugamushi in cutaneous lesions. Am J Clin Pathol. 2008;130(4):543-551.
Crossref - Paris DH, Blacksell SD, Newton PN, Day NPJ. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop isothermal DNA amplification. Trans R Soc Trop Med Hyg. 2008;102(12):1239-1246.
Crossref - Paris DH, Aukkanit N, Jenjaroen K, Blacksell SD, Day NPJ. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin Microbiol Infect. 2009;15(5):488-495.
Crossref - Silpapojakul K, Varachit B, Silpapojakul K. Paediatric scrub typhus in Thailand: a study of 73 confirmed cases. Trans R Soc Trop Med Hyg. 2004;98(6):354-359.
Crossref - Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura A. Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. 1993;31(6):1637-1640.
Crossref - Paris DH, Blacksell SD, Nawtaisong P, et al. Diagnostic accuracy of a loop-mediated isothermal PCR assay for detection of Orientia tsutsugamushi during acute scrub typhus infection. PLOS Negl Trop Dis. 2011;5(9):e1307.
Crossref - Varghese GM, Dayanand D, Gunasekaran K, et al. Intravenous doxycycline, azithromycin, or both for severe scrub typhus. N Engl J Med. 2023;388(9):792-803.
Crossref - Kabir KI, Satapathy AK, Gullla KM, et al. Macrolides versus other antibiotics in pediatric scrub typhus: A meta-analysis. Indian J Med Microbiol. 2023;46:100460.
Crossref - Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syste Reve. 2002;3:CD002150.
Crossref - Watt G, Chouriyagune C, Ruangweerayud R, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348(9020):86-89.
Crossref - Strickman D, Sheer T, Salata K, et al. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995;39(11):2406-2410.
Crossref - Kuo CC, Huang JL, Shu PY, Lee PL, Kelt DA, Wang HC. Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl. 2012;22(6):1803-1816.
Crossref - Centers for Disease Control and Prevention (US): Chapter 3. Infectious diseases related to travel. In: Rickettsial (Spotted and Typhus Fevers) and Related Infections (Anaplasmosis and Ehrlichiosis). Yellow Book. Centers for Disease Control and Prevention; 2015.
- Sharma R. Scrub typhus: prevention and control. JK Sci. 2010;12.
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLOS Negl Trop Dis. 2017;11(11):e0006062.
Crossref - Subbalaxmi MV, Madisetty MK, Prasad AK, et al. Outbreak of scrub typhus in Andhra Pradesh—experience at a tertiary care hospital. J Assoc Physicians India. 2014;62(6):490-496.
- Sinha P, Gupta S, Dawra R, Rijhawan P. Recent outbreak of scrub typhus in North Western part of India. Indian J Med Microbiol. 2014;32(3):247-250.
Crossref - Stephen S, Sangeetha B, Ambroise S, et al. Outbreak of scrub typhus in Puducherry and Tamil Nadu during cooler months. Indian J Med Res. 2015;142(5):591-597.
Crossref - Krishna MR, Vasuki B, Nagaraju K. Scrub typhus: audit of an outbreak. Indian J Pediatr. 2015;82(6):537-540.
Crossref - Jakharia A, Borkakoty B, Biswas D, Yadav K, Mahanta J. Seroprevalence of scrub typhus infection in Arunachal Pradesh, India. Vector Borne Zoonotic Dis. 2016;16(10):659-663.
Crossref - Takhar RP, Bunkar ML, Arya S, Mirdha N, Mohd A. Scrub typhus: A prospective, observational study during an outbreak in Rajasthan, India. Natl Med J India. 2017;30(2):69-72.
- Lalrinkima H, Lalremruata R, Lalchhandama C, et al. Scrub typhus in Mizoram, India. J Vector Borne Dis. 2017;54(4):369-371.
Crossref - Jain D, Nand N, Giri K, Bhutani J. Scrub typhus infection, not a benign disease: an experience from a tertiary care center in Northern India. Med Pharm Rep. 2019;92(1):36-42.
Crossref - Thakur CK, Chaudhry R, Gupta N, et al. Scrub typhus in patients with acute febrile illness: a 5-year study from India. QJM An Int J Med. 1. 2020;113(6):404-410.
Crossref - Lakshmi RMMVN, Dharma TV, Sudhaharan S, et al. Prevalence of scrub typhus in a tertiary care centre in Telangana, south India. Iran J Microbiol. 2020;12(3):204-208.
- Verma SK, Gupta KK, Arya RK, et al. Clinical and biochemical profile of scrub typhus patients at a tertiary care hospital in Northern India. J Fam Med Prim Care. 2021;10(3):1459-1465.
Crossref - Wallach J. Interpretation of Diagnostic Tests. 8th ed. Lippincott Williams & Wilkins; 2009:950.
- Jacobson RH. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech. 1998;17(2):469-526.
Crossref - Jang WJ, Huh MS, Park KH, Choi MS, Kim IS. Evaluation of an immunoglobulin M capture enzyme-linked immunosorbent assay for diagnosis of Orientia tsutsugamushi infection. Clin Diagn Lab Immunol. 2003;10(3):394-398.
Crossref - Jiang J, Marienau KJ, May LA, et al. Laboratory diagnosis of two scrub typhus outbreaks at camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and western blot assay using outer membrane 56-kd recombinant proteins. Am J Trop Med Hyg. 2003;69(1):60-66.
Crossref - Koraluru M, Bairy I, Varma M, Vidyasagar S. Diagnostic validation of selected serological tests for detecting scrub typhus. Microbiol Immunol. 2015;59(7):371-374.
Crossref - Kannan K, John R, Kundu D, et al. Performance of molecular and serologic tests for the diagnosis of scrub typhus. PLOS Negl Trop Dis. 2020;14(11):e0008747.
Crossref - Gautam R, Parajuli K, Tshokey T, Stenos J, Sherchand JB. Diagnostic evaluation of IgM ELISA and IgM Immunofluorescence assay for the diagnosis of Acute Scrub Typhus in central Nepal. BMC Infect Dis. 2020;20(1):138.
Crossref - Fomda BA, Abdullah N, Mir YB, et al. Comparative evaluation of serological tests used for the diagnosis of rickettsial diseases prevalent in the temperate region of North India. IJMM. Indian J Med Microbiol. 2022;40(2):294-298.
Crossref - Yun NR, Kim CM, Kim DY, Seo JW, Kim DM. Clinical usefulness of 16S ribosomal RNA real-time PCR for the diagnosis of scrub typhus. Sci Rep. 2021;11(1):14299.
Crossref - Roy S, Yadav S, Garg S, Deshmukh PR, Narang R. Evaluation of nested PCR and loop mediated isothermal amplification assay (LAMP) targeting 47 kDa gene of Orientia tsutsugamushi for diagnosis of scrub typhus. IJMM. Indian J Med Microbiol. 2021;39(4):475-478.
Crossref - Karthikeyan PA, Hoti SL, Kanungo R. Evaluation of loop-mediated isothermal amplification assay for detection of scrub typhus in patients with acute febrile illness presenting to a Tertiary Care Center in Puducherry, India. J Lab Phys. 2019;11(1):82-86.
Crossref - Kim YJ, Park S, Premaratna R, et al. Clinical evaluation of rapid diagnostic test kit for scrub typhus with improved performance. J Korean Med Sci. 2016;31(8):1190-1196.
Crossref - Manjunathachar HV, Barde PV, Raut CG, et al. Determination of cut-off of diagnostic ELISA for scrub typhus in endemic setup: central India. J Vector Borne Dis. 2021;58(1):90-93.
Crossref - Gupta N, Chaudhry R, Thakur CK. Determination of cutoff of ELISA and immunofluorescence assay for scrub typhus. J Glob Infect Dis. 2016;8(3):97-99.
Crossref - Gupta A. Determination of Cutoff of IgM ELISA for Diagnosis of scrub typhus in Hilly Northern State of Himachal Pradesh. PSM Microbiol. 2018;3(1):1-3.
- Rawat V, Singh RK, Kumar A, et al. Diagnostic validation of IgM and IgG ELISA and real-time PCR in detecting scrub typhus infection in endemic regions. J Vector Borne Dis. 2018;55(2):165-167.
Crossref - Varghese GM, Rajagopal VM, Trowbridge P, Purushothaman D, Martin SJ. Kinetics of IgM and IgG antibodies after scrub typhus infection and the clinical implications. Int J Infect Dis. 2018;71:53-55.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.