ISSN: 0973-7510
E-ISSN: 2581-690X
The SARS-CoV-2 pandemic demonstrated the importance of proper understanding of the epidemiology of viral infections in the modern cosmopolitan world. In this retrospective study, we analyzed different factors associated with the spread of SARS-CoV-2 in the Sandanski municipality, a small and isolated region of Bulgaria, with a classical continental Mediterranean climate. We collected 418 serum samples from people without symptoms of SARS-CoV-2 infection or other respiratory diseases. The samples were tested for COVID-19 markers with qualitative and quantitative ELISA for anti-N and anti-S antibodies, and a rapid test for (IgM/G) antibodies. The methods were applied to complement each other with the different characteristics each of them has, thus allowing us to perform more accurate and more comprehensive detection of serum antibodies and to analyze the virus spread. We compared the results with the official government data for the SARS-CoV-2 distribution in Bulgaria. Based on the obtained results and available literature data, we discuss the importance of popular diagnostic methods, the role of the climate, the travel of people and pets, psychological stress and the individual reaction of subjects related to the spread of the virus.
COVID-19, Asymptomatic Cases, First Wave, Climate, Serological Tests, WHO, Mass Media
A disease outbreak was reported in Wuhan, China, in 2019, with respiratory symptoms including high fever (77% – 92.8%), cough (69.8% – 81%), shortness of breath (3%), nasal discharge (4.0%), headache (7.2% – 34%), myalgia or fatigue (27.7 – 52%), diarrhea (6.1% – 8%), hemoptysis (3%),1,2 as well as loss of smell and taste3
Epidemiological data show that the first infected people had visited a seafood market in Wuhan, China,4 with the infection rapidly spreading to China, Japan, Russia, Europe, Australia, Latin America and growing from a local outbreak into a pandemic.5-7 The etiological agent of the disease was identified to be a virus that showed 79% – 82% homology with SARS-CoV and 50% with MERS-CoV; therefore, it was named SARS-CoV-2 and was classified in the same genus (Betacoronavirus) of the subfamily Orthocoronavirinae.8-11
The clinical manifestation of viral infections is just ‘the tip of the iceberg’ of virus circulation. The WHO defines the term ‘asymptomatic case’ as a laboratory-confirmed infected person without obvious symptoms.11 In the initial stages, the spread of SARS-CoV-2 occurred through asymptomatic cases,12,13 but their importance as a factor during this period is difficult to determine owing to the vague use of the term ‘asymptomatic’. This was further complicated depending on how thoroughly such subjects were clinically examined. In addition, the distinction between asymptomatic and pre-symptomatic individuals was often overlooked in the definitions of COVID-19 cases.14 Because the data for asymptomatic infections caused by SARS-CoV-2 were incomplete at the beginning of the pandemic, there was insufficient information, as asymptomatic cases can vary within a wide range of 0.3 – 56.5%.15,16 Evidence has shown that the rapid spread of the virus was namely due to asymptomatic cases,17,18 although transmission is less likely to occur through contacts with asymptomatic individuals than with symptomatic ones.19 The amount and infectivity of asymptomatic SARS-CoV-2 infection determine the type of measures that are most effective to prevent virus transmission.19 Asymptomatic SARS-CoV-2 cases posed a major threat early in the pandemic because they remained undetected by the symptom-based control measures.15 In addition, the spread of respiratory and other viruses also depends on geographical and climatic features,20,21 especially during the initial emergence and spread of a virus, when new ecological niches are occupied related to the epidemiological circulation. Globally the seasonal spread of viruses shows a trend from north to south, with temperature being an important factor in this seasonal variation. This is best illustrated by the spread of respiratory transmitted viruses, such as Influenza viruses, Mumps, SARS-CoV-1, respiratory syncytial virus and human rhinovirus, seasonally in countries or regions located at different latitudes and climatic factors. There are various hypotheses, some directly related to temperature and others to temperature and humidity, geographic location, air quality and sociodemographic factors.22-28 At the molecular level, temperature affects the virus entry through the cell membrane and, consequently, its replication. At low temperatures, viruses cannot effectively enter host cells (by fusion) and cannot introduce their genetic material.20,21
An important part of controlling the spread of respiratory viruses is the availability of reliable diagnostic assays. The serological diagnosis of SARS-CoV-2 utilizes assays with different characteristics (antibody types and quantity), based on various principles, such as quick antigen tests based on nanoparticles,29,30 qualitative and quantitative ELISA, immunofluorescence,31-33 electrochemiluminescence assay34 and automated systems.35,36 Different serological tests also have different informativeness depending on the team and the combination of antibodies they prove.
In this context, the present study used three methodologically different serological tests with the aim to analyze the potential role of some known factors for the spread of SARS-CoV-2 in a geographically isolated region, Sandanski municipality in Southwest Bulgaria, trough the three different serological tests. Sandanski (N 41.39778) is the main town with predominantly Mediterranean climate in this region of Bulgaria. It is located near the Struma River, on a major highway; hence, the intensive traffic of people and goods. The town of Sandanski is known for its clean air, since the area is open to the south but is surrounded by mountains to the east and west, and to the north by the Kresna Gorge. Another town in this region is Petrich; its climate is largely influenced by Belasitsa Mountain (and shows less Mediterranean characteristics).
Samples
We tested 418 serum samples from subjects without symptoms of SARS-CoV-2 infection. The samples were obtained randomly at MHAT Southwestern Hospital and SIMD Laboratory, St.Ivan Rilski, Sandanski, and subject demographics – gender and age range – were collected. The samples were collected as part of routine medical examinations. The subjects showed no symptoms of SARS-CoV-2 or other respiratory diseases at the time of sampling. Written informed consent was obtained from the participants before enrolling in the study.
The samples were obtained during March 13th – December 8th, 2020. The period was conditionally divided into three subperiods, as defined in Table 1.
Table (1):
Subperiods, time frame and samples included in the study. The subperiods were defined according to the Decision of the National Assembly on the proposal of the Council of Ministers with Decision 80/13-Mar-2020 and Order No. RD-01-973/25-Oct-2020 of the Blagoevgrad Regional Health Inspectorate
Study periods |
Time frame |
Number of samples |
|---|---|---|
I first – state of quarantine /lockdown |
Mar 13 – Apr 13, 2020 |
26 |
II second – after the state of quarantine/lockdown |
Apr 14 – Aug 7, 2020 |
316 |
III third – epidemic in the municipality |
Oct 26 – Dec 8, 2020 |
76 |
Total |
418 |
The obtained serum samples were stored at -80°C.
Methods
ELISA
We used a semi-quantitative dual recognition (DR) ELISA (INgezim COVID-19 DR, Eurofins, Spain), which detects IgA, IgG and IgM against the N protein of the virus, where the conjugate is a peroxidase-labeled SARS-CoV-2 N protein. We measured the optical density and performed the corresponding calculations using an ELISA reader (Biobase, China).
To detect virus-neutralizing IgG against the S glycoprotein of the virus, we used a quantitative indirect (QI) ELISA (Demeditec, Germany). We calculated the values using a standard curve of 5 standards obtained according to the manufacturer’s instructions. The results are presented as the number of bound antibodies (BAU/ml), and were interpreted as negative (below 9 BAU), doubtful (between 9 and 11 BAU/ml) with testing recommended after 14 days, and positive for contact with SARS-CoV-2 (values above 11 BAU/ml).
The ELISA diagnostic kits used in this study have been validated and registered for in vitro diagnostics in the EU and were applied according to the manufacturers’ instructions.
Rapid test
To differentiate the classes of antibodies in order to determine the time of infection, the samples that were positive in the dual recognition IgM/A/G ELISA and negative in the quantitative IgG ELISA serum were tested with a rapid test for antibodies against SARS-CoV-2.
We used a rapid in vitro diagnostic test based on nanotechnology with gold nano-particles which demonstrates the presence of Ig M and IgG with specificity and sensitivity of, respectively, 98.41/85.19% for Ig M and 96.34/94.33% for IgG (Arton, Canada).
Other data
The study includes data for the pandemic in the study period published on the official website of the United Information Portal (https://coronavirus.bg).
Statistical analysis
Descriptive statistics data are presented as means with standard deviation (±SD). Qualitative variables are presented as relative proportion (%) and confidence intervals (95% CI). Detailed statistical analysis was performed using GraphPad Prism 9 software. To assess differences in proportions between groups, we used Pearson’s chi-squared test and Fisher’s test (when testing small sample sizes). We used t-test to determine whether differences between mean values are significant. Differences were considered statistically significant at the P < 0.05 level in all the tests.
Information about the weather conditions in the studied period was obtained from the National Institute of Meteorology and Hydrology – BAS and https://www.stringmeteo.com/.
The gender distribution of the tested subjects is shown in Figure 1.
Figure 1. The results presented in the figure are based on testing 418 subjects from the municipality of Sandanski, R. Bulgaria, in the period of Mar – Dec 2020, where 49.5% (95% CI: 44.8% – 54.3%, n = 207) were male and 50.5% (95% CI: 45.7% – 55.2%, n = 211) female, from 4 to 96 years, mean (SD) = 58.5±19.2 years
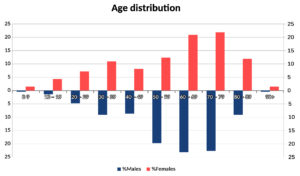
The age distribution of the samples showed that those obtained from people aged 50 to 89 years had the largest share (Figure 2).
Analysis by DR ELISA detected 1 positive sample (collected on Apr 13th) and 2 doubtful/borderline ones (collected on Apr 10th) in the first subperiod. One of the borderline samples (collected on Apr 10th) was tested via a rapid IgM test. The results from the second subperiod showed 29 positive and 37 borderline samples, and from the third one, respectively, 25 positive and 8 doubtful/borderline ones (Table 2).
Table (2):
ELISA results from the retrospective study of 418 serum samples from the municipality of Sandanski in the three subperiods
| Period I | Mar 13, 2020 – Apr 13, 2020 | ||||
|---|---|---|---|---|---|
| Gender | Positive | Borderline | Negative | Total | Age |
| m | 0 | 1 | 11 | 12 | 23 – 85 |
| f | 1 | 1 | 12 | 14 | 15 – 86 |
| Total | 1 | 2 | 23 | 26 | – |
| Period II | Apr 14, 2020 – Aug 7, 2020 | ||||
| Gender | Positive | Borderline | Negative | Total | Age |
| m | 14 | 21 | 120 | 155 | 5 – 93 |
| f | 15 | 16 | 130 | 161 | 5 – 96 |
| Total | 29 | 37 | 250 | 316 | – |
| Period III | Oct 26, 2020 – Dec 8, 2020 | ||||
| Gender | Positive | Borderline | Negative | Total | Age |
| m | 24 | 5 | 13 | 42 | 27 – 79 |
| f | 1 5 | 3 | 16 | 34 | 27 – 87 |
| Total | 39 | 8 | 29 | 76 | – |
| Total | 69 | 47 | 302 | 418 | |
⃰f – female; m – male;
Sixty-nine samples were positive for IgM/A/G antibodies against SARS-CoV-2 (16.5%); (95% CI: 13.3% – 20.4%, n = 69) in the QI ELISA screening. Among the women, 14.7% tested positive (95% CI: 10.5% to 20.1%, n = 31), and among the men 18.4% (95% CI: 13.7% – 24.2%, n = 38), with no statistically significant difference in SARS-CoV-2 antibody status between men and women in this study (Pearson’s χ 2 =1.019, df =1; p = 0.313).
The 69 positive samples were from people aged 27 to 85 years, mean (SD) = 57.1 ±15.8 years, with a non-significant difference (Student’s t-test t=1.54, df = 67; p=0.128) in age between men (Mean (SD) = 54.5 ±16.3 years) and women (Mean (SD) = 60.4 ±14.8 years).
The percentage of positive samples in the three subperiods was as follows: for Period I or Quarantine (Q) – 3.85% (95% CI: 0.197% – 18.9%, n=1; for Period II or Post-quarantine (PQ) – 9.18% (95% CI: 6.47% – 12.9%, n=29); and for Period III or Post summer (S) – 51.4% (95% CI: 40.3% – 62.2%, n= 39. There was no statistically significant difference between the percentage of positive cases in Q and PQ (p=0.715, Relative ratio (RR)=0.419; 95% CI: 0.07 to 2.14), while the difference between S and the other two subperiods was significant (QS to S – p< 0.01, RR=0.075, 95% CI: 0.013 – 0.37; PQ to S – p<0.01, RR=0.179, 95% CI: 0.012 – 0.27).
There was a statistically significant difference between the number of positive cases during Period II as compared to the peak of the wave in Bulgaria (Figure 3). At the peak for the country, the percentage of positive samples was significantly higher (22.58% vs. 9.18%; p = 0.007) (Figure 3).
Figure 3. Comparative analysis of data from Apr 14, 2020 to Aug 7 2020 (PQ) from our survey in Sandanski and the peak of the wave in Bulgaria. PC – positive cases, NC – negative cases
We examined the 69 samples that were positive in DR ELISA by IgG QI ELISA and we found that 16 samples had values between 4.4 and 8.92 BAU (negative), 8 samples had values between 9 and 11 BAU and 45 samples had values ≥ 11 BAU (positive) (Supplementary 1).
We ran the 16 negative samples in a rapid test, and 7 of them gave a positive result as follows: two for IgG (sample numbers: 48 and 103), two for IgG/M (sample numbers: 22 and 265) and three for IgM (sample numbers: 148, 149 and 177) (Supplementary 2). Ten of the samples that were positive in the DR ELISA gave a negative result for IgG against SARS-CoV-2 in the QI ELISA and the rapid test. The sample from Period I that tested positive in the DA ELISA gave a negative result for virus neutralizing antibodies in the QI ELISA.
In this study, we used ELISA to detect IgA, IgG and IgM, because screening for several classes of anti-SARS-CoV-2 immunoglobulins increases the possibility of detecting positive samples when the antibody titers are low. This approach is justified by the study reported by Ma et al.,37 in which the chemiluminescence assay had higher sensitivity with a combination of the three classes of anti-SARS-CoV-2 antibodies, IgA, IgM and IgG.
When the 69 samples that were positive in the DR ELISA were tested with IgG QI ELISA, 16 samples had values between 4.4 and 8.92 BAU, 8 samples scored between 9 and 11 BAU and 45 samples ≥ 11 BAU. The samples that tested positive for three classes of immunoglobulins (DR ELISA) showed variations in the IgG titers, probably corresponding to the development of the immune response depending on the time when each person had contact with the virus. That is why the samples that had values between 9 and 11 BAU in the QI ELISA, i.e. doubtful according to the manufacturer’s instructions, could be considered as positive cases in the initial stage of IgG production. The level of IgG is initially low but increases sharply 2-3 weeks after SARS-CoV-2 infection.37
The negative results in the rapid test for IgM in 10 samples that were positive in the DR ELISA and negative in the quantitative ELISA, could most likely be attributed to the lower sensitivity of the rapid test for detection of this class of antibodies as compared to the IgM/A/G ELISA technique and/or to the lower diagnostic value of IgM.37 The first positive case for IgM antibodies against SARS-CoV-2 with a rapid test was on April 10, 2020, and the first positive case via ELISA on April 13. A positive ELISA and low levels of virus neutralizing antibodies (BAU 6.5) indicates predominantly specific IgM, IgA and IgG antibodies that are not virus neutralizing (according to QI ELISA). This may indicate an initial stage in the production of virus-neutralizing IgG, or 15-25 days after infection with the virus.37 This suggests that SARS-CoV-2 was most likely in circulation during the lockdown (Period I) and possibly even before that.
Due to the retrospective nature of this study, it is not possible for the borderline samples to be validated as being either positive or negative. Therefore, different serological tests with different characteristics, when applied in combination, can provide complementary information that allows more accurate and more comprehensive detection of serum antibodies and analysis of the virus spread.
There are different techniques for diagnosis of asymptomatic SARS-CoV-2 cases 15, 16, 38 and age is not a factor for the development of asymptomatic infections. Asymptomatic cases have been reported in the age range of 2-75 years, or 41 years on average.38
Our study included an almost equal number of subjects of both genders, but the age distribution was not homogeneous. This is relevant to the obtained results, because studies have established a relationship between age and the development of COVID-19, with young people being associated mainly with asymptomatic cases or with mild symptoms.39 Our results show a higher number of asymptomatic cases in people over 50 years (median 54.5 years), which is due to the larger number of people of this age group who were examined. These differences from other reports could most likely be attributed to the larger number of samples in this higher age range
(Figure 2).
SARS-CoV rapidly loses viability at higher temperatures and high relative humidity.22 In vitro experiments with SARS-CoV-2 show that the virus is very stable at 4°C, but is sensitive to heat,40 and loses its infectivity at normal body temperature (37°C). On the basis of the inverse relationship between humidity and the spread of SARS-CoV-2, Baker et al.41 suggest that, although there is a strong negative relationship between climate and the spread of the virus, this effect is not likely to be very large because the percentage of people susceptible to the virus is high.
A study of climatic conditions and the global spread of SARS-CoV-2 shows that weather conditions, such as humidity, radiation, temperature and wind distribution, play a secondary role in the spread of the disease. It also suggests that the spread of SARS-CoV-2 infection is slightly reduced in summer, but not enough to stop the pandemic.42 A study has reported a relationship between average temperature, minimum temperature and air quality for the spread of SARS-CoV-2 in some Latin American cities.6
The first positive cases were registered in Bulgaria on March 8, 2020, in the cities of Gabrovo and Pleven (National Operative Staff briefing, Bulgarian National Radio, March 8, 2020, 00:06 a.m.), and by March 13, there were several cases in Sofia (National Operative Staff- coronavirus.bg). Thus, the virus was certainly circulating in Bulgaria in March. To understand the role of climate in the circulation of the virus (relevant to our study), we need to compare the weather data with those of the persistence of the virus under various conditions. In the municipality of Sandanski, the average length of the day in March 2020 was 8.1 hours, with 16 days of sunny weather and 10 days of rainy weather, 54.2 mm of precipitation (i.e. near average humidity) and temperature of 12.5°C. According to Dabisch et al.,43 the rate of inactivation of the virus is 1.7/min at 10°C and 70% relative humidity, meaning that 90% virus inactivation will occur in about 53 min. Another important factor for the transmission of viruses is the turbulent diffusion of sub-micron aerosol particles,44 where Brownian motion is mainly observed.45 Bioaerosols with a size of less than 100 µm evaporate, and the biological component remains in the air.46 Thus, the majority of viral particles that remain in the air will not settle under the influence of Brownian forces and will be blown away (in case of wind). The sunlight intensity increases in an altitude-dependent manner, and it is one of the leading factors that influence the infection ratio.43 Sunlight can reduce the time for 90% inactivation of the virus to under 53 min. At an average wind speed of 7.7 m/s, the virus can travel a distance of 7 km in one hour, with decreasing infectivity.
The asymptomatic spread of SARS-CoV-2 in Sandanski Municipality in our study followed the same trend as the officially registered cases in Bulgaria (from Jul 6th, 2020) for the corresponding periods. The non-significant difference between asymptomatic cases during and after lockdown can also be explained by the effect that the rise in temperatures, the increase in day length and the decrease in air humidity have on the infection ratio.47 Climatic conditions can probably slow down the spread of SARS-CoV-2, as is the case with other similar viruses, such as the influenza and parainfluenza virus,48 respiratory syncytial and other respiratory viruses.49,50
Such a trend has been observed by Yordanova et al.,51 in samples from the town of Kardzhali (N 41.6500015), with transitional Mediterranean climate in south-eastern Bulgaria. They also report data from June to November and also show low values during the summer (9.67-17.00%) as compared to October and November (38.86-55.65%). These seasonal variations during the first wave and the first year of the SARS-CoV-2 spread are also in agreement with some early suggestions about seasonality of the virus associated with UV radiation.52,53 The share of positive samples on a monthly basis in Kardzhali was higher than that in our study because Yordanova et al.51 included serological tests, PCR and foreigners who entered the country. In addition, there was a different number of sunny weather days in the two southern regions of the country (west and east) in the studied months in 2020.54 This can have affected the positive COVID-19 cases because longer days in the summer are associated with more UV exposure, which, on the one hand, is unfavourable for the virus and its spread and, on the other hand, enhance the human immune system.52,53
In our study, from a local point of view, the data indicate that the climate in March and April – the first two months of the spread of SARS-CoV-2 in the Municipality of Sandanski – most likely had little effect on the entry of the virus in this region. There were asymptomatic seropositive cases of people in northeastern Bulgaria who had traveled abroad during the period of probable contact with the virus.55 However, our results suggest that the virus was likely circulating asymptomatically in Sandanski Municipality before the first diagnosed case imported from Great Britain. Based on all of the above, we hypothesize that the spread of the virus to the Municipality of Sandanski is unlikely to have occurred via the airborne route; rather, the main factor were humans.
Another potential factor for the spread of SARS-CoV-2 could be pets. Cats are the most sensitive and susceptible pets to the virus.56 They can also be considered as a potential factor in its initial spread because they can shed it. There is evidence for the spread of SARS-CoV-2 among cats in Bulgaria, and domestic cats with outdoor access play an important role in this.32 We speculate that, when such cats traveled with their owners from the capital city to the countryside, e.g. to the Sandanski Municipality (particularly before or during lockdown), they might have contributed to the virus spread to some extent,57-59 with possible transmission human↔cat→cat→human.
Damyanova55 reported a review of the number of cases in the countries neigh-boring Bulgaria (as of 20th April 2020): Turkey – 86300, Romania – 8700, Serbia – 6300, Greece – 2200 and North Macedonia with 1200 cases. In that analysis, Bulgaria formed an ‘island’ of non-spread of the virus. This, however, was not analyzed by the author, due to the initial stage of the pandemic and the lack of data from extensive screening in Bulgaria.55 Two years later, we are also unable to analyze and compare our results because of the lack of official data from the Ministry of Health for that period. The earliest data from the official authority, i.e. the Ministry of Health, date back to Jul 6th, 2020; hence, the WHO data for earlier periods could be considered as approximate or speculative. Nonetheless, our results for 3.84% asymptomatic cases in the first period (during the lockdown in Bulgaria) are comparable to the data reported by Damyanova55 for the north-eastern part of the country, where the seropositive cases were 4.8%, of which 78.6% asymptomatic. On the basis of these data from one period, but in two geographically distant regions in opposite parts of Bulgaria with different microclimates, we speculate that the percentage of asymptomatic cases in other parts of the country was probably also within this range.
It is not possible to perform comparative analysis of the symptomatic cases in the Municipality of Sandanski with the asymptomatic cases in the studied period due to the lack of official or unofficial data. It is also not possible to compare our data for the first and second subperiod with these for the country because the official data available for Bulgaria are from Jul 6th, 2020, but our results are from Apr 15th, 2020, and hence, the overlap is rather short and insufficient for analysis.
The asymptomatic spread of the virus in the municipality of Sandanski in the third subperiod showed a trend identical to that of the SARS-CoV-2 cases registered in the country, with non-significant differences. It is important to note that the positive cases registered in the official database of the Republic of Bulgaria also include those of asymptomatic carriers of the virus who were tested on a voluntary basis, e.g. social responsibility, scientific interest, panic in society or other reasons.
The asymptomatic cases reported in different countries and regions vary in a very wide range, from 0.3%15 to 78.6%.55 Our results also showed a wide range of variation, from 3.85%, 9.18% to 51.4%, in the studied subperiods. We speculate that the percentage of asymptomatic cases could have been higher (hence, the percentage of symptomatic ones lower) because some of the variation could be attributed, at least in part, to stress factors that affect the psychological wellbeing of individuals and, consequently, the individual response against the virus. Some factors that have negative psychosocial consequences include media campaigns, lockdown, personal restrictions such as social distancing and all changes associated with COVID-19 that changed everyday life.60 Mass media play an indispensable role in present-day society. They were actively used during the pandemic to provide information updates on the development of the pandemic,61 encourage hygiene practices and health communication together with the WHO.61,62 Providing information updates on the development of the pandemic has been discussed on a positive note by the authors61; however, media reports also had some negative effects, especially for lay people who are not experts in the field. The negative news reports caused mental sufferings, such as depression, anxiety and stress,63 which probably played a major role in increasing the number of COVID-19 cases and, consequently, in reducing the relative share of asymptomatic cases. The underlying mechanism of this influence can be explained as follows: (a) even brief exposure to negative information in the news (15 min) increases the feelings of anxiety and sadness immediately after exposure,64 with mood changes persisting even after engagement in a distractor task.65 This suggests that the affective impact of news consumption extends beyond momentary responses,66 and thus (b) it affects the psyche and, in turn, the health of the individual,67,68 as there is “no health without mental health”.69,70 (c) Asymptomatic SARS-CoV-2 infection is associated with low levels of activation of the complement and interferon,71 increased levels of CD56briCD16- natural killer (NK) cells and upregulation of interferon-gamma in effector CD4+ and CD8+ Т cells and NK cells and stable TCR clonal expansion, especially in effector CD4+ Т cells.72 Psychological stress leads to altered levels of hormones, cytokines and chemokines, resulting in suppressed immune system.73 In COVID-19 these changes involve increased IFN-alpha and type I interferon, which are associated with clinical symptoms and severe infection.72 In this way, an infected asymptomatic individual may develop symptoms and become registered, thus increasing the percentage of symptomatic cases and decreasing the percentage of asymptomatic ones.
The first COVID-19 official data in Bulgaria published on the website of the Ministry of Health are from Jul, 6th, 2020, but the first report of serological data was from the period of March 26th – April 20th, city of Varna,55 which gave reference to WHO data for Bulgaria before July 2020. Given that the WHO data must be based on the official data reported by each country, it is possible that the figures for that initial period are not completely accurate. Thus, our study, together with that of Damyanova,55 provide the first real data on the spread of SARS-CoV-2 before Jun 6th, 2020. This will throw light on the pathways/routes, mechanisms and factors that influenced its initial distribution in Bulgaria and in particular, in the Mediterranean region of the country. The obtained results suggest that, due to the geographical and climatic characteristics of the municipality of Sandanski, it was the human factor that played the main role for the spread of SARS-CoV-2 in the Mediterranean part of the country (municipality of Sandanski). The virus circulated during the lockdown and probably even before that. Retrospective analyses, even in small populations, are important because they deepen our understanding of the primary spread of the virus across countries and continents. In addition, the factors and the underlying mechanisms of the virus spread that were identified in this study are in agreement with the global trend at that time, involving high levels of stress in society and the resulting consequences for the human health.
The use of different serological tests enables a more complete description of the immune response. This, in turn, helps to more accurately determine the time and path of infection and spread of the virus. Our retrospective study confirms the initial seasonal spread53 of SARS-CoV-2 infection, which is still demonstrated today. Asymptomatic case detection depends on the number of clinically healthy people examined (excluding presymptomatic ones). This, in turn, depends on people’s motivation, which is influenced (depends) on various factors, as the leading role in this was played by the media environment.
Additional file: Supplementary 1 and Supplementary 2.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
IS conceptualized the study and applied methodology. LB, GP, and IP collected the samples. IS and AA collected resources. IS, LB, GP, and IP data curation. DB performed software analysis and visualized the study. DB and IS performed formal analysis. IS, PS and YM investigated the study. IS, YM and PS validated the data. IS, GD and GM wrote original draft. IS, NK, AA, DB and RG wrote, reviewed and edited the manuscript. RG performed supervision. IS performed project administration and funding acquisition. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported through a Research Project No. 8255/23.11.2022 with a contract D-209/03.08.2023 by the Medical University of Sofia, Bulgaria.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
This study was conducted according to the guidelines of the Declaration of Helsinki. The samples investigation was approved by the Institutional Review Board of the Medical University of Sofia, Bulgaria, N: 1139 /1904.21.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924.
Crossref - Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;19:368.
Crossref - Stoyanov V, Grigorova Tsv, Petkov D, Yotov I. The loss of smell and taste in patients with COVID-19 from Bulgarian population. Pediatric and Infectious Diseases. 2020;12(1):17-23.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
Crossref - Araujo DB, Machado RR, Amgarten DE, et al. SARS-CoV-2 isolation from the first reported patients in Brazil and establish-ment of a coordinated task network. Mem Inst Oswaldo Cruz. 2020;23:115.
Crossref - Bolano-Ortiz TR, Camargo-Caicedo Y, Puliafito SE, et al. Spread of SARS-CoV-2 through Latin America and the Caribbean region: a look from its economic conditions, climate and air pollution indicators. Environ Res. 2020;191:109938.
Crossref - Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924.
Crossref - Zaki M, Van Boheemen S, Bestebroer M, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
Crossref - de Groot RJ, Baker SC, Baric RS, et al. Commentary: Middle east respiratory syndrome coronavirus (mers-cov): announcement of the coronavirus study group. J Virol. 2013;87(14):7790-7792.
Crossref - Jiang S, Shi Z, Shu Y, et al. A distinct name is needed for the new coronavirus. Lancet. 2020;395(10228):949.
Crossref - WHO Severe Acute Respiratory Syndrome (SARS).www.who.int/ith/diseases/sars/en/. Clinical management of COVID-19. Interim guide. 2020.
- Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410-411.
Crossref - Yan X, Han X, Fan Y, et al. Duration of SARS-CoV-2 viral RNA in asymptomatic carriers. Crit Care. 2020;24(1):1-2.
Crossref - Nikolai LA, Meyer CG, Kremsner PG, Velavan TP. Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. Int J Infect Dis. 2020;100:112-116.
Crossref - Zhou X, Li Y, Li T, Zhang W. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(7):957-959.
Crossref - Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility -King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
Crossref - Ye W, Yuan S, Yuen S, et al. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686-1697.
Crossref - Li C, Ji F, Wang L, et al. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis. 2020;26(7):1626-1628.
Crossref - Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymp-tomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Medicine. 2020;17(9):e1003346.
Crossref - Brunner J, Zugliani C, Mischler R. Fusion activity of influenza virus PR8/34 correlates with a temperature-induced confor-mational change within the hemagglutinin ectodomain detected by photochemical labeling. Biochemistry. 1991;30:2432- 2438.
Crossref - Yunus AS, Jackson TP, Crisafi K, et al. Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation. Virology. 2010;396(2):226-237.
Crossref - Chan KH, Peiris JM, et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690.
Crossref - Park, JE, Son WS, et al. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region. Influenza other Respir Viruses. 2020;4(1):11-18.
Crossref - Zeng W, Zhao H, et al. Association between NO2 cumulative exposure and influenza prevalence in mountainous regions: a case study from southwest China. Environ Res. 2020;189:109926.
Crossref - Mise K, Sumi A, Takatsuka S, Toyoda SI. Associations between Meteorological Factors and Reported Mumps Cases from 1999 to 2020 in Japan. Epidemiologia. 2021;2(2):162-178.
Crossref - Sloan C, Moore ML, Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci. 2011;4(1):48-54.
Crossref - Gardinassi LG, Simas PV, Salomao JB, et al. Seasonality of viral respiratory infections in southeast of Brazil: the influence of temperature and air humidity. Braz J Microbiol. 2012;43:98-108.
Crossref - Tang JW, Loh TP. Correlations between climate factors and incidence-a contributor to RSV seasonality. Rev Med Virol. 2014;24(1):15-34.
Crossref - Saleh AH, Kumar D, Sirakov I, Shafiee P, Arefian M. Application of nano compounds for the prevention, diagnosis, and treatment of SARS-coronavirus: A review. Journal of Composites and Compounds. 2021;3(9):230-246.
Crossref - Venkatesan B, Vajravelu LK, Ravi S, Thulukanam J, Muthamilan OL. Therapeutic and Diagnostic Approaches by using Nanotechnology in SARS-CoV-2 Infections. J Pure Appl Microbiol. 2022;16(4):2324-2336.
Crossref - Michel M, Bouam A, Edouard S, et al. Evaluating ELISA, immunofluorescence, and lateral flow assay for SARS-CoV-2 serologic assays. Front Microbiol. 2020;11:597529.
Crossref - Sirakov I, Rusenova N, Rusenov A, Gergova R, Strateva T. Human ELISA Detects anti-SARS-CoV-2 Antibodies in Cats: Seroprevalence and Risk Factors for Virus Spread in Domestic and Stray Cats in Bulgaria. Vet Sci. 2023;10(1):42.
Crossref - Luo J, Brakel A, Krizsan A, et al. Sensitive and specific serological ELISA for the detection of SARS-CoV-2 infections. Virol J. 2022;19(1):1-10.
Crossref - Banu ST, Vinotha S, Katragadda R, Vanaja R. Seroconversion in COVID-19 Infection and Comparison of Antibody Responses in Symptomatic Versus Asymptomatic Individuals. J Pure Appl Microbiol. 2023;17(1):590-596.
Crossref - Van Elslande J, Decru B, Jonckheere S, et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin Microbiol Infect. 2020;26(11):1557-e1.
Crossref - Kahya HFH, Mahmood MT. Detection of IgG and IgM Levels in Patients with COVID-19 in Mosul Province, Iraq. J Pure Appl Microbiol. 2022;16(1):167-173.
Crossref - Ma H, Zeng W, He H, et al. COVID-19 diagnosis and study of serum SARS-CoV-2 specific IgA, IgM and IgG by chemilumi-nescence immunoanalysis. MedRXiv. 2020;22:2020-2024.
Crossref - Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204.
Crossref - Yang R, Gui X, Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Network Open. 2020;3(5):e2010182.
Crossref - Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10.
Crossref - Baker RE, Yang W, Vecchi GA, et al. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. medRxiv. 2020.
Crossref - Briz-Redon A, Serrano-Aroca A. The effect of climate on the spread of the COVID-19 pandemic: A review of find-ings, and statistical and modeling techniques. Progress in Physical Geography. 2020;44(5):591-604.
Crossref - Dabisch P, Schuit M, Herzog A, et al. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci Technol. 2021;55(2):142-153.
Crossref - Baron PA, Willeke K. second ed. van Nostrand Reinhold; Aerosol Measurement: Principles, Techniques, and Applications. New York: 2001. https://www.osti.gov/etdeweb/biblio/20556104
- Cox CS, Wathes CM. Bioaerosols Handbook. 1st Ed; 1995. CRC Press.
Crossref - Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection?. InIndoor Air 2005: Proceedings of the 10th International Conference on Indoor Air Quality and Climate. Tsinghua University Press. 2005:9-23.
- Schuit M, Ratnesar-Shumate S, Yolitz J, et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J Infect Dis. 2020;222(4):564-571.
Crossref - Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci. 2009;106(9):3243-3248.
Crossref - Baker RE, Mahmud AS, Wagner CE, et al. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat Commun. 2019;10(1):5512.
Crossref - Pitzer VE, Viboud C, Alonso WJ, et al. Environmental drivers of the spatiotemporal dynamics of respiratory syncytial virus in the United States. PLoS Pathogens. 2015;11(1): e1004591.
Crossref - Yordanova V, Kermedchieva R, Emin D, Dimitrova S, Marinova M, Valcheva D, Tcherkezov T. Epidemiological COVID-19 data for Eastern Rodopi, Bulgaria. Acta Microbiologica Bulgarica. 2021:37(4):232-235.
- Karapiperis C, Kouklis P, Papastratos S, et al. A Strong Seasonality Pattern for Covid-19 Incidence Rates Modulated by UV Radiation Levels. Viruses. 2021;13(4):574.
Crossref - Karapiperis C, Kouklis P, Papastratos S, Chasapi A, Danchin A. Preliminary evidence for seasonality of Covid-19 due to ultraviolet radiation [version 1; peer review: 1 approved with reservations, 1 not approved]. F1000Research. 2020;9:658.
Crossref - https://www.stringmeteo.com. Accessed November 2023. https://www.stringmeteo.com/synop/bg_stday.php?year= 2020 &month=1&day=23&city= 15712&int=2&submit=%D0%9F%D0%9E%D0%9A %D0%90%D0%96%D0%98#sel
- Tsaneva-Damyanova D. SARS-CoV-2: seroepidemiological pattern in northeastern Bulgaria. Biotechnol Biotechnol Equip. 2020:34(1):441-446.
Crossref - Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016-1020.
Crossref - Totton SC, Sargeant JM, O’connor AM. How could we conclude cat-to-human transmission of SARS-CoV-2? Zoonoses Public Health. 2021;68(1):67-68.
Crossref - Sila T, Sunghan J, Laochareonsuk W. Suspected Cat-to-Human Transmission of SARS-CoV-2, Thailand, July-September 2021. Emerg Infect Dis. 2022;28(7):1485-1488.
Crossref - Piewbang C, Poonsin, P, Lohabicharn P. SARS-CoV-2 Transmission from Human to Pet and Suspected Transmission from Pet to Human, Thailand. J Clin Microbiol. 2022;60(11):e01058-22.
Crossref - Poursaadati MZ, Hosseinzadeh S, Maarefbanel M, Bolhari J, Khubchandani J. Mental Health System Responsiveness during COVID-19 in People with Pre-Existing Psychiatric Disorders: Experiences from Iran. Epidemiologia. 2023;4(1):74-84.
Crossref - Anwar A, Malik M, Raees V, Anwar A. Role of mass media and public health communications in the COVID-19 pandemic. Cureus. 2020;12(9):e10453.
Crossref - Arriaga P, Esteves F, Pavlova MA, Piçarra N. Coronavirus disease (COVID-19): the impact and role of mass media during the pandemic. Front Psychol. 2021;12:729238.
Crossref - Zakout YM, Alreshidi FS, Elsaid RM, Ahmed HG. The magnitude of COVID-19 related stress, anxiety and depression associated with intense mass media coverage in Saudi Arabia. AIMS Public Health. 2020;7(3):664-678.
Crossref - Johnston WM, Davey GC. The psychological impact of negative TV news bulletins: The catastrophizing of personal worries. Br J Psychol. 1997;88(1):85-91.
Crossref - Szabo A, Hopkinson KL. Negative psychological effects of watching the news in the television: Relaxation or another inter-vention may be needed to buffer them. Int J Behav Med. 2007;14(2):57-62.
Crossref - Kellerman JK, Hamilton JL, Selby EA, Kleiman EM. The mental health impact of daily news exposure during the COVID-19 pandemic: Ecological momentary assessment study. JMIR Mental Health. 2022;9(5):e36966.
Crossref - Jahangir SF, Nawaz N, Khan N. Effects of media (television) on mental health. FWU Journal of Social Sciences. 2014;8(1):97-107.
- Tukaiev S, Radchuk O, Havrylets Y, Vasheka T. 1858 – The Influence Of The Emotionally Negative Tv-news Plots With Real Violence On The Psychological State Of Human. Eur Psychiatry. 2013;28(S1):1-1.
Crossref - Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370(9590):859-877.
Crossref - Kolappa K, Henderson DC, Kishore SP. No physical health without mental health: lessons unlearned? Bull World Health Organ. 2013;91(1):3-a.
Crossref - Zhang J, Lin D, Li K, et al. Transcriptome analysis of peripheral blood mononuclear cells reveals distinct immune response in asymp-tomatic and re-detectable positive COVID-19 patients. Front Immunol. 2021;12:716075.
Crossref - Zhao XN, You Y, Cui XM, et al. Single-cell immune profiling reveals distinct immune response in asymptomatic COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):342.
Crossref - Aich P, Potter AA, Griebel PJ. Modern approaches to understanding stress and disease susceptibility: A review with special emphasis on respiratory disease. Int J Gen Med. 2010 2;19- 32.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.