Dengue fever, caused by the Dengue virus (DENV), has emerged as a mild to lethal human infection. Globally, an estimated 400 million patients have been infected with DENV over the past 10 years, and this number is expected to increase in the coming years. The DENV, possessing a single positive- stranded RNA with five serotypes, is transmitted by mosquitos of the Flaviviridae family—particularly, the Flavivirus genus and Aedes species. The DENV genome encodes three structural and seven non- structural proteins. In the Kingdom of Saudi Arabia (KSA), serotype 2 of the dengue virus (DENV-2) emerged in 1994 and caused a major epidemic in Jeddah, KSA. Dengue outbreaks first appeared in Makkah in 2004, according to the characteristics studied in hospitalized patients. Major factors causing the wide and rapid distribution of the virus include increased urbanization, migration, global commerce, weather variation, inadequate mosquito control, the development of pesticide resistance in mosquitos, irregular use of insecticides, and shifting climatic circumstances. Detection methods currently used for DENV include the detection of viral antigens (Ag) (virus extraction and purification, immunofluorescence test, and NS1 detection assay), serological assays (plaque reduction neutralization titers (PRNT), IgM/IgG immunological assays), and RNA detection using RT-PCR. Low sensitivity, specificity, and accessibility of the detection protocols represent major challenges necessitating the advent of more amenable protocols. The Aedes mosquito is the primary vector for horizontal transmission of DENV. DENV-infected mosquitos infect people, and DENV passes from one human to another through this vector. Once acquired, the virus requires 5–7 days of incubation before the patient exhibits various symptoms of dengue fever; subsequently, uninfected mosquitoes that come in contact with infected patients feed on their blood and become infected. The DENV may also be spread through the mating of male and female Aedes mosquitoes. The reverse transcription loop-mediated isothermal application (RT-LAMP) has emerged as one of the most adaptable viral detection procedures. This method could prove to be an excellent pathogen detection tool because it is cheap, simple, sensitive, cost-effective, accessible, and fast. The method relies on the use of 4–6 primers to recognize eight different loci in the target sequence contained in the DENV clinical isolates with a 100% success rate and a sensitivity of about 93%. We strongly recommend the use of LAMP in detecting spots of virus spread, especially in urban regions where accessibility to detection methods is scarce.
LAMP, DENV, Horizontal Transmission, Pandemic
The prevalence of the dengue virus (DENV), which causes dengue fever as a lethal infection has considerably increased over the past decades. The DENV, a single-stranded (+)-sense RNA virus belonging to the Flaviviridae family, Flavivirus genus, 1 is spread through mosquitos. The virus has four distinct serotypes, named DENV1–4, 2 which can cause a range of diseases. 1,3 DENV-5 is a recently discovered serotype of the sylvatic strain. Dengue fever can be categorized into two types dengue shock syndrome (DSS) and dengue hemorrhagic fever (DHF); both diseases can prove to be fatal. 3
DENV infection can cause different symptoms, such as mild illness and acute flu-like symptoms. Upon progression, it can cause complications that lead to death. High fever develops following incubation durations ranging from 3 to 15 days.4-7 Current methods used for the detection of DENV infection include the detection of viral antigens (Ag) (virus extraction and purification, immunofluorescence test, and NS1 detection assay), serological assays (plaque reduction neutralization titers (PRNT), IgM/IgG immunological assays), and RNA detection using RT-PCR. Two protocols that can be used only in laboratories with advanced instruments and experienced personnel include virus purification and PRNT. The most commonly used diagnostic methods include NS1 Ag detection and serological methods. Circulating NS1 Ag can be detected using enzyme-linked immuno-sorbent technique assay (ELISA) up to nine days after symptoms start. IgM is detectable later in the first infection but persists longer than NS1 and viral RNA. Public hospitals frequently employ IgM ELISA tests; interpretation of the data can be improved through a rise in the titer when testing coupled samples from both severe- and recovering-phase patients. Their possible interaction with different flaviviruses, though (Diagnosis and Serotyping of Dengue Virus Infection at/near Points of Care, 2020).
Even though a range of procedures are presently employed for diagnosing dengue infections, more than one approach meets the optimum criterion for sensitivity, specificity, rapidness of detection, and cost-effectiveness. Therefore, new detection techniques are urgently needed to address this known diagnostic gap.8
The chance of contracting severe dengue fever increases with consequent infections by various serotypes; however, recovery from an initial infection by one of the five serotypes offers permanent immunity against that specific serotype. A deficiency of effective antiviral medicines and vaccines has contributed to the rising DENV infection-related death and morbidity rates in recent years. Healthcare priorities in this situation include rapid clinical therapy for this condition and early diagnosis of infection using sensitive and targeted testing techniques. Thus, the current research goal is to develop a novel mosquito vector DENV detection assay that is simple, sensitive, specific, cost-effective, and quick.
The first incidence of DENV infection in humans has not been determined yet, mainly because the disease is typically asymptomatic and, therefore, cannot be diagnosed. The first instances of dengue fever to be identified and recorded were in Batavia in 1779. One year later, Philadelphia, USA, had a DENV epidemic.9 According to the World Health Organization, about 1.2 million people were infected by the DENV in 1998. Then, a 30-fold rise in DENV infections was recorded after 50 years. In the 18th and 19th centuries, as the global trade industry grew, port towns thrived and became more urbanized, creating the perfect environment for flourishing the main vector, Aedes aegypti. Although rapid urbanization in Southeast Asia increased transmission and hyperendemicity, the virus has not been identified in the Americas since the first incidence of Dengue infection in the human population because the infection is commonly asymptomatic and, therefore, not diagnosed.2 Asia, Africa, and North America all reported the first dengue outbreaks nearly at the same time. Recent data from several sources revealed that there were around 50 million DENV infections in 100 different countries worldwide.10
The virus can spread between humans through an infected mosquito vector bite.11 These mosquitoes also transmit other viruses, such as the West Nile virus. The first major pandemic of the severe and fatal type of DENV infection was that of Dengue hemorrhagic fever (DHF) in Southeast Asia as a direct consequence of this evolving ecosystem.12 Hypovolemia, shock, plasma leakage, and increased vascular permeability are all indications of DHF. The WHO estimates that there were about 4.2 million DENV infections in 2019.
Global epidemiology
Dengue is a highly infectious disease with an estimated 400 million cases globally. Its incidence has considerably increased during the past decade, placing the lives of half of the global population in more than 125 endemic countries in danger). Dengue fever is an acute viral illness with systemic symptoms that has spread both epidemically and endemically, resulting in a variety of clinical presentations ranging from minor fevers to deadly infections.6
The global epidemiological patterns of dengue have shifted due to various variables. One of these variables is demographics, including population increase, economical patterns in developing nations, and land-use trends. Another important aspect is the growth in the number and density of inhabitants caused by migration to urban regions. 13 This might be due to high employment chances, driving more rural locals to migrate to urban centers. Moreover, transportation modernization has led to changes in dengue epidemiology, owing to increased travel of humans, disease vectors, and viruses. Constant public health policy and infrastructure developments also lead to changes in dengue epidemic patterns.13
Approximately 1800 years ago, the earliest known instance of a dengue-like sickness occurred in 265–420 A.D. in the Chinese Chin Dynasty. The condition was fairly identical to that during water poisoning caused by flying insects.14 Furthermore, there were reports of a dengue epidemic in some French regions and Central America throughout the latter half of the seventeenth century. As a result, dengue might have spread widely even before the 18th century. Dengue fever first became a public health problem in Southeast Asia after world war II between the late 40s and early 50s.15 Shifts in the environment and population provided an ideal atmosphere for the propagation of this vector-borne disease. The migration rates during wartime also increased the portability of the mosquito vector.
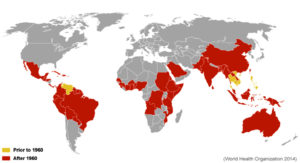
Before the 1970s, only nine nations were impacted by dengue outbreaks, according to the WHO (Dengue and Severe Dengue, n.d.). Currently, more than 100 countries are significantly impacted by dengue epidemics.17 These nations are generally found on the majority of the world’s continents, including South-East Asia, the Pacific Rim region, Arabian nations, the Americas, Europe, Australia, and New Zealand. In addition, the Pacific Rim area suffers around 75% of the worldwide dengue infection effects. Furthermore, the Latin American territory has seen a rise in the severity and rate of dengue outbreaks in recent years (Figure 1).13
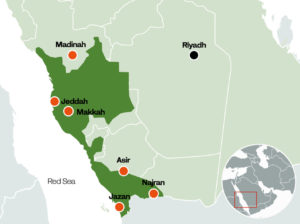
DENV-2 was initially identified in KSA in 1994, when it caused a significant outbreak in Jeddah (Figure 2), with 289 documented and confirmed cases 18 (Figure 3). After that, dengue cases have also been recorded in other KSA regions, including Makkah. When dengue first broke out in Makkah in 2004, hospitalized patients at a particular facility were the first to have their case characteristics recorded. Between 2006 and 2008, 159 patients with DF were hospitalized in Makkah, and 77% of them were infected in the spring and early summer. Afterward, a significant uptick in Makkah’s building activity between 2008 and 2012 resulted in 4,187 incidents of DF being documented, primarily because of the increase in still water.19-21
Figure 2. The Fight against dengue fever in Saudi Arabia, the western region has been the most affected
Additional factors that contribute to the spread of this illness include rising urbanization, migration, international commerce, climate variability, and ineffective vector management. Moreover, uneven pesticide use, the development of resistance in mosquitos against pesticides, and shifting climatic conditions might all be contributing factors as well.22
DENV has spread to new regions due to increased traveling within and across cities and nations.23 Muslims from all over the globe travel to KSA for Umrah and Hajj (pilgrimage). Dengue has been brought into the Umrah and Hajj locations, including three major cities: Makkah, Jeddah, and Al-Madinah, since many of the infected patients come from endemic areas. In addition, internal variables, such as environmental factors, industrialization, and human activities that promote breeding sites, help the spread of the mosquito vector.24,25 All of these cities have seen large dengue epidemics on occasion.22,26 The Saudi Ministry of Health (MOH) documented 3350 instances of DENV infections in the Kingdom of Saudi Arabia in 2009, with a case fatality rate of 4.6 per 1000 (https://ghdx.healthdata.org/record/saudi-arabia-health-statistical-yearbook-2018).
The underestimation of dengue fever cases is one of the concerns for surveillance systems in the KSA.27 This highlights the frightening extent of silent viral transmission and the limitations of the present surveillance methods, which have led to several outbreaks.25
Entomological monitoring can aid in detecting DENV in Aedes aegypti sooner than that in humans.29 DENVs have been identified in Aedes aegypti mosquitos from endemic and epidemic areas using clinical and laboratory screening tests. During outbreaks, such identification assists in determining regions with a greater incidence of DENV distribution, allowing vector control authorities to concentrate their efforts and target the areas where people are at greater risk of getting infected.30,31
DENV morphology
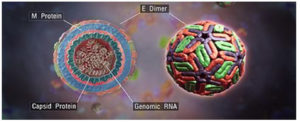
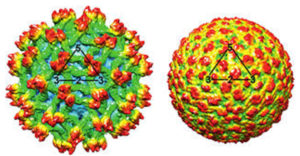
DENV has a diameter of 50 nm and is spherical in structure. Mature virions contain membrane-attached proteins, namely M (membrane) and E (envelope) (Figure 4). When virions develop, the precursor structure seen in intracellular immature virions is converted into the mature form (M).32 DENV virion structures have been studied using X-ray crystallography.33,34 The envelope, E, is a rod-like protein comprising two identical subunits with icosahedral symmetry and a herringbone-like arrangement of the protein dimers (Figure 5).35
Figure 4. The structural component of dengue virus. (Source: https://en.wikipedia.org/wiki/Dengue_virus)
Genomics and proteomics of DENV
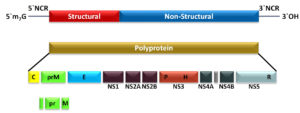
There are five DENVs, namely DENV1–5 2 which have an amino acid sequence similarity of 65–70%.36 The viral genome is a (+)-sense RNA comprising 10.6 to 11 kilobases37 and consists of one open reading frame (ORF) (~ 3400 codons), accompanied by untranslated regions on the 5′- and 3′-ends.38 The ORF is translated into three structural (capsid (C), membrane precursor (prM), and envelope (E)) and seven non-structural proteins (NS1, NS2A-B, NS3, NS4-B, and NS5) Figure 6).
Figure 6. Genomic arrangement and proteolysis of the ORF to produce the viral proteins in members of the genus Flavivirus. (NCR = Non-coding region)33
DENV detection
The efficient and correct identification of dengue fever is critical for clinical management.40 Definitive diagnosis procedures for verifying DENV infection include detecting the virus, viral genetic material, antigens or antibodies, or a mix of these methods. The virus is found in the bloodstream and circulating hemocytes in addition to other sites in the body. Throughout the initial phases of the infection, extraction and purification of the virus and viral genetic material or Ag detection can be employed to diagnose the disease. At the end of the severe phase of infection, serology is the commonly used diagnostic approach. Antibody response to infection varies according to the host’s immunity status. Numerous methods for laboratory diagnosis have been designed to aid in patient care and the prevention of the disease.
Virological diagnosis of DENV
Blood is drawn for viral isolation, especially in the first five to six days after the onset of symptoms, when the condition is severe. The viral RNA and NS1 Ag can also be detected in such samples. Using monoclonal antibodies against each of the five recognized serotypes, an indirect immunofluorescence test is used to identify the isolated viruses.41
Molecular diagnosis of DENV
In this technique, DENV can be detected using specific primers for the C and/or prM gene(s). This region is flanked by a sequence shared by all five DENV serotypes, enabling genomic amplification. In a semi-nested PCR with a subsequent amplification, the serotypes are subsequently identified using serotype-specific primers.42 RT-PCR enables the processing of a large number of samples at once. This molecular test is the most commonly used virological method for DENV detection. Using RT-PCR in the early detection of DENV has been demonstrated to be a useful diagnostic technique, with the benefit of not exhibiting a substantial difference in sensitivity in both active and passive infections.43
Loop-mediated isothermal amplification (LAMP)
Heat-dependent amplification of genomic DENV RNA is another molecular diagnostic technique, requiring only one temperature setting for the amplification procedure. For instance, PCR-testing equipment may not be accessible in rural locations. Since isothermal amplification-based tests require no thermocyclers, they are less expensive and complicated than PCR testing.44 So far, there is no isothermal brand for DENV diagnosis permitted by the CDC.
Reverse transcription loop-mediated isothermal amplification (RT-LAMP), reverse transcription recombinase polymerase amplification (RT-RPA), and nucleic acid sequence-based amplification (NASBA) are some of the platforms being explored for isothermal amplification-based detection of DENV infection.
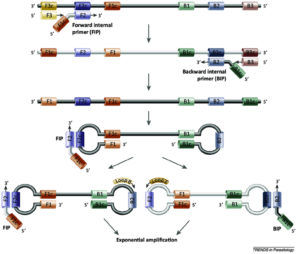
The RT-LAMP procedure, introduced by Notomi et al.,45 consists of three primer pairs: outer pair, inner pair, and loop ones to recognize eight different loci on the target sequence.45,46 This technique, established as a potential detection tool, amplifies the input sequence at a constant temperature, typically between 60 and 65°C, using a water bath or conventional heating block.47
Figure 7. Detection of DENV RNA with (A) the LAMP method; initially, ssRNA is converted to dsDNA. (B) the portable laboratory with minimal chemicals and devices48
The RT-LAMP typically begins by reverse transcribing the RNA structure to DNA (Figure 7A) and building a loop construction that replicates the sequence. The replicated sequence can be seen with the naked eye or even under UV light.50 The efficacy of RT-LAMP was demonstrated in a study by Hu S-f et al.51 The RT-LAMP test they developed had a success rate of 100% and 98.9% in identifying clinical isolates of DENV and affected patients, respectively. The RT-PCR technique exhibited 93% sensitivity for the clinical isolates and 84.2% for patients. The RT-LAMP produced no false positive results. Li et al.52 demonstrated a one-tube-dependent 30-minute reaction approach employing the RT-LAMP primers for the C-prM gene in all five serotypes. With a LOD of 10 RNA segments for each reaction, Lau et al.,53 developed a one-tube RT-LAMP approach that focused on the NCR gene on the 3′-end present in all serotypes and identified DENV presence based on the variation in sample turbidity.
In another study, DENV RNA was amplified using LAMP and analyzed using a portable MinION sequencer to identify the serotype54 RT-LAMP offers numerous benefits, including being quick, inexpensive, isothermal, very accurate, and precise. However, PCR is preferable for multiplexing and viral quantification applications.55 LAMP-based assays often demonstrate binary results with no quantitative data. NASBA is also a popular isothermal amplification technique. The fundamental benefit of this technique is being a one-step isothermal method that targets DENV RNA isolates.55 Silica is used to isolate RNA from blood samples and is subsequently replicated at 41°C without the need for a thermal cycler.56 The process is completed in 30 minutes, and RNA is visualized using an electrochemiluminescence.57
Recently, many traditional NASBA-based DENV detection tests have been developed.58-60 Yrad et al.,61 described a gold nanoparticle-based DENV-1 RNA detection technology. DENV-1 RNA was amplified using NASBA, followed by the formation of a sandwich complex using AuNP and DENV-1 capture probes. In pooled human sera, with a LOD of 1.2 104 pfu/mL, this compound may be visually identified using a lateral flow biosensor in less than 20 minutes. Although NASBA is straightforward, affordable, and boosts the detection specificity of the biosensor utilized, it requires sample pre-treatment and careful management of the viral particles.62
Another innovative isothermal amplification method for identifying pathogens is the RPA test.63 At a steady temperature range of 37–42°C, RPA can identify 1–10 target DNA fragments for each reaction in less than 20 minutes.64 An RPA-based DENV detection test that focuses on highly conserved 3′-UTR segments was developed by Teoh et al.65 This assay could be completed in less than 20 minutes and had a LOD of 10 copies of RNA for each response. To enable the sensitive detection of DENV quickly, a point-of-need-based mobile RPA unit (Figures 7B and 8) has been created and implemented in Thailand and Senegal.48 To cover all serotypes, researchers developed two assays specifically targeting the 3′-UTR sections of DENV 1–4. The portable device provides the tools and materials required for RNA extraction and fluorescence detection. Additionally, a new RT-RPA-based assay with horizontal flowing dipsticks with an identification range of 1–106 copies/L for DENV-1 has been developed.66
The RPA-based assays mentioned above make it abundantly clear that this platform may be used in resource-constrained settings, as it requires only slightly expensive machines for the rapid and precise detection of DENV.
Detection of NS1 antigen
The NS1 protein sequence, comprising six structural subunits, is highly preserved in all five serotypes of DENV 67 The NS1 Ag has been used as a diagnostic marker in the early stages of the infection. ELISA detection of NS1 is as quick and efficient as RT-PCR but does not identify viral serotypes. The sensitivity of the test may vary based on the condition. In the advanced stages of the disease, the NS1 test is more accurate.68
Serological diagnosis
IgM can be detected in serum, blood on filter paper, and saliva but not in urine. Serum specimens are examined at single or varying concentrations. Most of the antigens tested in this technique are derived from the E protein of DENV.69
DENV infection
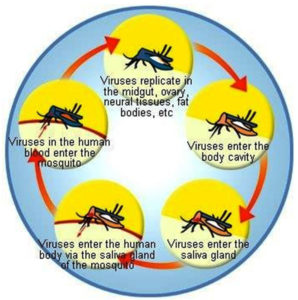
A female mosquito becomes infected upon feeding on humans during the acute and viremic phases of dengue fever. First, DENVs attack the midgut of the mosquito in addition to other organs prior to migrating to the salivary glands. The infection is transmitted to other humans through a bite from an infected mosquito, and it takes between 4 and 7 days for the symptoms to appear and for the infected human to be able to spread the virus to another mosquito. Asymptomatic and symptomatic patients can both spread the virus to mosquitoes 20. The entire time the mosquito is feeding on people, it will transmit the disease.20,70
DENV targets immunological cells to infect the human body. The virus is introduced into the circulation through a mosquito bite and infects the keratinocytes and immature Langerhans cells via receptor-mediated endocytosis.71 When the virus-infected cells reach the lymph nodes, macrophages and monocytes migrate to the site and then get infected by the virus. This results in DENV replication, followed by the spread of the disease through the lymphatic system of the body. As a result, various additional immune cells of the mononuclear lineage, such as the blood-derived monocytes, myeloid dendritic cells, and macrophages in the spleen and liver, are infected as well.72–74
In the later stages of DENV infection, the adaptive immune system is stimulated to release plasma B-cells to produce antibodies and T-cells to precisely target the virus-infected cells 2. Plasma cells produce antibodies to attack the mature viral particles. T-cells act on virus-infected host cells through the release of cytokines. Despite the long-term protection against dengue infection provided by the adaptive immune system, the innate defensive response is still required to help eliminate the DENV. Additionally, a person may be vulnerable to subsequent DENV infection. After the first infection, patients may develop long-lasting immunity against a single serotype; however, they may be vulnerable to infection by the other serotypes.75 When a patient is infected with a serotype other than the one against which the patient has developed immunity, a significantly more severe illness may be experienced. Currently, there is a lack of knowledge on the severe pathophysiology of secondary DENV infections.17
Modes and cycles of DENV transmission Horizontal transmission of DENVDENV, yellow fever, and West Nile viruses, in addition to other arthropod-borne viruses from the Flaviviridae family, are mostly transmitted through mosquitoes. DENV is by far the most prevalent arthropod-borne virus among these viruses. The forms and cycles of DENV mosquito–human transmission can be divided into two categories: horizontal transmission and vertical transmission.
The main route of transmission of DENV infection in humans is horizontal transmission. DENV serotypes all require Aedes mosquitos as the primary vector for transmission. DENV infects humans throughout its sylvatic cycle.76 The endemic DENV has developed from a sylvatic strain that originated in Asia and Africa, employing other mammals as hosts and forest-dwelling Aedes mosquitoes as vectors.77 DENV sylvatic strain is also an ancestor strain. Ancestral transmission refers to non-human transmission, while efficient inter-human transmission necessitates a huge population size of at least 10,000 to 1 million individuals, which did not exist until 4000 years ago, at the advent of urban civilizations.78 Moreover, several evolutionary investigations regarding the sylvatic strain of DENV reported in KSA corroborated this hypothesis. This increases the likelihood of sylvatic DENV-infected Aedes mosquitos infecting people with dengue.79
The horizontal transmission of DENV entails its passage from one individual to another through a mosquito vector. This happens when an Aedes mosquito carrying DENV feeds on an uninfected individual. After the acquisition, DENV requires 5–7 days of incubation before the patient exhibits various dengue fever symptoms.80 Uninfected Aedes mosquitoes that come into contact with the infected person during this time and feed on them then become infected. Thus, the cycle continues spreading the virus to other healthy people (Figure 9).
Transovarial transmission of DENV
Gravid female Aedes mosquitos transmit DENV to progeny or children through transovarial transmission, also known as vertical transmission.81 When DENV enters the mosquito via blood feeding, it infects and replicates in multiple areas of the mosquito’s body, including the mid-gut, ovaries, and neural tissues.82 DENV infects the eggs because the ovaries are infected. As a result, the virus is transferred to the larva and pupae as they develop into adult mosquitos. Natural vertical transmission of DENV in Aedes mosquitos has been documented several times over the last 30 years.83-85 Natural populations of vector mosquitoes have been the subject of several studies over the years, in which immature forms (eggs, larvae, and pupae) of these mosquitoes are recovered from the environment.81-83,86,87 The immature mosquitos are then grown until they reach the adult stage before being tested for DENV. Furthermore, other studies have shown that DENV can be vertically spread in laboratory-infected mosquitos. In many studies, Ae. aegypti or Ae. albopictus mosquitos were intentionally infected through intra-thoracic, oral, or parental injection.88-91 Subsequently, the infected mosquitos were permitted to breed and feed on human blood to reproduce and produce eggs. The offspring were then tested for the presence of the virus in both larvae and adults. DENV vertical transmission serves as a virus maintenance mechanism, allowing all serotypes to survive without a human host or favorable natural environment conditions.92
In addition, multiple laboratory investigations have demonstrated that DENV may survive for up to five generations in mosquitoes and can remain in subsequent generations as well.89,93 This demonstrates how mosquitoes serve as a reservoir for DENV and that vector mosquitoes are crucial to maintaining viruses in the environment. DENV vertically spreads in humans. Pregnant women who have dengue fever during the last stages of pregnancy may pass the virus to their unborn child.94-96 Researchers could extract DENV together with DENV-specific IgM antibodies from fetal blood. Additionally, researchers found evidence that DENV may be transferred vertically through breastfeeding.97 After delivery, DENV was isolated from the breast milk of women. However, further research is required to assess the plausibility of DENV transmission through breast milk.
Sexual transmission and uncommon transmission of DENV
According to research done in the late 1980s, DENV may be spread by mating between male and female mosquitoes.98 All DENV serotypes could be sexually transmitted to female Ae. albopictus from laboratory-infected male Ae. albopictus. If the female mosquitoes were fed on blood two to seven days before breeding, the transmission was significantly accelerated. The infected females would then pass the virus to their offspring.98 Other dengue transmission methods that do not need the vector mosquito have also been reported. These transmission methods include needlestick, transplantation, and transfusion transmission.99 Blood transfusion is certainly a factor in transfusion-related transmission. As a blood pathogen, dengue presumably infects blood recipients, and therefore this is to be expected.100,101
DENV is also transferred through organ transplants, known as “transplantation transmission.” There have been reports of DENV infections in transplant patients’ kidney and bone marrow.102 Since DENV is not included among the infectious agents that must be screened before organ transplants, the probability of DENV transmission seems quite significant.99 Another unusual mechanism of DENV transmission is needle-stick injury, one of the most prevalent incidents in ordinary clinical practices. Numerous infectious diseases, including HIV and HBV, have been linked to infection through needle sticks.99 DENV reportedly transfers through needle-stick injuries, although only a few cases have been documented.103,104
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Rodenhuis-Zybert IA, Wilschut J, Smit JM. Dengue virus life cycle: viral and host factors modulating infectivity. Cellular and molecular life sciences. 2010;67(16):2773-2786.
Crossref - Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nature Reviews Microbiology. 2005;3(1):13-22.
Crossref - Dwivedi VD, Tripathi IP, Tripathi RC, Bharadwaj S, Mishra SK. Genomics, proteomics and evolution of dengue virus. Briefings in functional genomics. 2017 2017;16(4):217-227.
Crossref - Anderson JR, Rico-Hesse R. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. The American journal of tropical medicine and hygiene. 2006;75(5):886.
Crossref - Basurko C, Matheus S, Hildéral H, et al. Estimating the risk of vertical transmission of dengue: a prospective study. The American journal of tropical medicine and hygiene. 2018;98(6):1826.
Crossref - Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504-507.
Crossref - Tuiskunen Bäck A, Lundkvist Å. Dengue viruses-an overview. Infection ecology & epidemiology. 2013;3(1):19839.
Crossref - Gyawali N, Taylor-Robinson AW. Diagnosis of dengue: strengths and limitations of current techniques and prospects for future improvements. Dengue-Immunopathology and Control Strategies London: Intech Open. 2017:55-73.
Crossref - McCallum JE. Military medicine: from ancient times to the 21st century. Abc-Clio; 2008.
- Guzman A, Istúriz RE. Update on the global spread of dengue. International journal of antimicrobial agents. 2010;36:S40-S42.
Crossref - Carrington LB, Simmons CP. Human to mosquito transmission of dengue viruses. Frontiers in immunology. 2014;5:290.
Crossref - Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Wiley Online Library; 2006:3.
Crossref - Ferreira GLC. Global dengue epidemiology trends. Revista do Instituto de Medicina Tropical de São Paulo. 2012;54:5-6.
Crossref - Gubler DJ. Dengue and dengue hemorrhagic fever. Clinical microbiology reviews. 1998;11(3):480-496.
Crossref - Ooi E-E, Gubler DJ. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cadernos de saude publica. 2009;25:S115-S124.
Crossref - Sanyaolu A, Okorie C, Badaru O, et al. Global epidemiology of dengue hemorrhagic fever: an update. J Hum Virol Retrovirol. 2017;5(6):00179.
Crossref - Cheah WK, Ng KS, Marzilawati A-R, Lum LC. A review of dengue research in malaysia. The Medical Journal of Malaysia. 2014;69:59-67.
- Fakeeh M, Zaki AM. Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia, 1994-1999. The American journal of tropical medicine and hygiene. 2001;65(6):764-767.
Crossref - Alwafi OM, McNabb SJN, Memish ZA, et al. Dengue Fever in Makkah, Kingdom of Saudi Arabia, 2008-2012: 2013;
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends in microbiology. 2002;10(2):100-103.
Crossref - Shahina W, Nassara A, Kalkattawia M, Bokharia H. Dengue fever in a tertiary hospital in Makkah, Saudi Arabia. 2009.
- Sami M, Rowaida B, Hafiz A, et al. The epidemiology and incidence of dengue in Makkah, Saudi Arabia, during 2017-2019. Saudi Medical Journal. 2021;42(11):1173-1179.
Crossref - Heymann DL. Control of communicable diseases manual. American Public Health Association; 2008.
- Ali EOM, Babalghith AO, Bahathig AOS, et al. Prevalence of Larval Breeding Sites and Seasonal Variations of Aedes aegypti Mosquitoes (Diptera: Culicidae) in Makkah Al-Mokarramah, Saudi Arabia. International Journal of Environmental Research and Public Health. 2021;18(14):7368.
Crossref - Aziz AT, Al-Shami SA, Mahyoub JA, Hatabbi M, Ahmad AH, Rawi CSM. An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasites & vectors. 2014;7(1):1-4.
Crossref - Fakeeh M, Zaki AM. Dengue in Jeddah, Saudi Arabia, 1994-2002. Saudi Arabia. 2003;27:6.
- Kyle JL, Harris E. Global spread and persistence of dengue. Annual review of microbiology. 2008;62(1):71-92.
Crossref - Al-Nefaie H, Alsultan A, Abusaris R. Temporal and spatial patterns of dengue geographical distribution in Jeddah, Saudi Arabia. Journal of Infection and Public Health. 2022;15(9):1025-1035.
Crossref - Chow VT, Chan YC, Yong R, et al. Monitoring of dengue viruses in field-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. The American journal of tropical medicine and hygiene. 1998;58(5):578-586.
Crossref - Achee NL, Gould F, Perkins TA, et al. A critical assessment of vector control for dengue prevention. PLoS neglected tropical diseases. 2015;9(5):e0003655.
Crossref - Sanchez-Casas RM, Alpuche-Delgado RH, Blitvich BJ, et al. Detection of dengue virus serotype 2 in Aedes aegypti in Quintana Roo, Mexico, 2011. Southwestern Entomologist. 2013;38(1):109-117.
Crossref - Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. Journal of virology. 1997;71(11):8475-8481.
Crossref - Kuhn RJ, Zhang W, Rossmann MG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717-725.
Crossref - Mukhopadhyay S, Kim B-S, Chipman PR, Rossmann MG, Kuhn RJ. Structure of west nile virus. Science. 2003;302(5643):248-248.
Crossref - Yu IM, Zhang W, Holdaway HA, et al. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319(5871):1834-1837.
Crossref - Azhar EI, Hashem AM, El-Kafrawy SA, et al. Complete genome sequencing and phylogenetic analysis of dengue type 1 virus isolated from Jeddah, Saudi Arabia. Virology journal. 2015;12(1):1-11.
Crossref - Shrivastava S, Tiraki D, Diwan A, et al. Co-circulation of all the four dengue virus serotypes and detection of a novel clade of DENV-4 (genotype I) virus in Pune, India during 2016 season. PLoS One. 2018;13(2):e0192672.
Crossref - Wadood A, Mehmood A, Khan H, et al. Epitopes based drug design for dengue virus envelope protein: a computational approach. Computational biology and chemistry. 2017;71:152-160.
Crossref - Simmonds P, Becher P, Bukh J, et al. ICTV virus taxonomy profile: Flaviviridae. The Journal of general virology. 2017;98(1):2.
Crossref - World Health O. Dengue guidelines for diagnosis, treatment, prevention and control : new edition. 2009 2009;(WHO/HTM/NTD/DEN/2009.1)
- Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. The American journal of tropical medicine and hygiene. 1982;31(4):830-836.
Crossref - Lanciotti RS, Calisher CH, Gubler DJ, Chang G-J, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of Clinical Microbiology. 1992;30(3):545-551.
Crossref - Cordeiro MT, Silva AM, Brito CAA, et al. Characterization of a dengue patient cohort in Recife, Brazil. The American journal of tropical medicine and hygiene. 2007;77(6):1128-1134.
Crossref - Kabir MA, Zilouchian H, Caputi M, Asghar W. Advances in HIV diagnosis and monitoring. Critical reviews in biotechnology. 2020;40(5):623-638.
Crossref - Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000;28(12):e63-e63.
Crossref - Sahni AK, Grover N, Sharma A, Khan ID, Kishore J. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) for diagnosis of dengue. medical journal armed forces india. 2013;69(3):246-253.
Crossref - Teoh B-T, Sam S-S, Tan K-K, et al. Detection of dengue viruses using reverse transcription-loop-mediated isothermal amplification. BMC infectious diseases. 2013;13(1):1-9.
Crossref - Abd El Wahed A, Patel P, Faye O, et al. Recombinase polymerase amplification assay for rapid diagnostics of dengue infection. PloS one. 2015;10(6):e0129682.
Crossref - Alhassan A, Li Z, Poole CB, Carlow CK. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends in parasitology. 2015;31(8):391-400.
Crossref - Kim J-G, Baek SH, Kim S, et al. Rapid discriminative detection of dengue viruses via loop mediated isothermal amplification. Talanta. 2018;190:391-396.
Crossref - Hu S-f, Li M, Zhong L-l, et al. Development of reverse-transcription loop-mediated isothermal amplification assay for rapid detection and differentiation of dengue virus serotypes 1-4. BMC microbiology. 2015;15(1):1-15.
Crossref - Li S, Fang M, Zhou B, et al. Simultaneous detection and differentiation of dengue virus serotypes 1-4, Japanese encephalitis virus, and West Nile virus by a combined reverse-transcription loop-mediated isothermal amplification assay. Virology journal. 2011;8(1):1-9.
Crossref - Lau Y-L, Lai M-Y, Teoh B-T, et al. Colorimetric detection of dengue by single tube reverse-transcription-loop-mediated isothermal amplification. PloS one. 2015;10(9):e0138694.
Crossref - Yamagishi J, Runtuwene LR, Hayashida K, et al. Serotyping dengue virus with isothermal amplification and a portable sequencer. Scientific reports. 2017;7(1):1-10.
Crossref - Wong YP, Othman S, Lau YL, Radu S, Chee HY. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. Journal of applied microbiology. 2018;124(3):626-643.
Crossref - Sekaran SD, Soe HJ. Issues in contemporary and potential future molecular diagnostics for dengue. Expert Review of Molecular Diagnostics. 2017;17(3):217-223.
Crossref - Wernecke M, Mullen C. MOLECULAR BIOLOGY| Molecular Biology in Microbiological Analysis. 2014;
Crossref - Jittmittraphap A, Thammapalo S, Ratanasetyuth N, Wongba N, Mammen MP, Jampangern W. Rapid detection of dengue viral RNA in mosquitoes by nucleic acid-sequence based amplification (NASBA). Southeast Asian journal of tropical medicine and public health. 2006;37(6):1117.
- Zaytseva NV, Montagna RA, Baeumner AJ. Microfluidic biosensor for the serotype-specific detection of dengue virus RNA. Analytical chemistry. 2005;77(23):7520-7527.
Crossref - Zaytseva NV, Montagna RA, Lee EM, Baeumner AJ. Multi-analyte single-membrane biosensor for the serotype-specific detection of Dengue virus. Analytical and bioanalytical chemistry. 2004;380(1):46-53.
Crossref - Yrad FM, Castañares JM, Alocilja EC. Visual detection of dengue-1 RNA using gold nanoparticle-based lateral flow biosensor. Diagnostics. 2019;9(3):74.
Crossref - Darwish NT, Sekaran SD, Alias Y, Khor SM. Immunofluorescence-based biosensor for the determination of dengue virus NS1 in clinical samples. Journal of Pharmaceutical and Biomedical Analysis. 2018;149:591-602.
Crossref - Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS biology. 2006;4(7):e204.
Crossref - Lobato IM, O’Sullivan CK. Recombinase polymerase amplification: Basics, applications and recent advances. Trac Trends in analytical chemistry. 2018;98:19-35.
Crossref - Teoh B-T, Sam S-S, Tan K-K, et al. Early detection of dengue virus by use of reverse transcription-recombinase polymerase amplification. Journal of Clinical Microbiology. 2015;53(3):830-837.
Crossref - Xi Y, Xu C-Z, Xie Z-Z, Zhu D-L, Dong J-M. Rapid and visual detection of dengue virus using recombinase polymerase amplification method combined with lateral flow dipstick. Molecular and cellular probes. 2019;46:101413.
Crossref - Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. Journal of Clinical Microbiology. 2000;38(3):1053-1057.
Crossref - Ty Hang V, Minh Nguyet N, The Trung D, et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS neglected tropical diseases. 2009;3(1):e360.
Crossref - Hunsperger EA, Yoksan S, Buchy P, et al. Evaluation of commercially available anti-dengue virus immunoglobulin M tests. Emerging Infectious Diseases. 2009;15(3):436.
Crossref - Siler JF, Hall MW, Hitchins AP. Dengue: its History, Epidemiology. 1926.
- Limon-Flores AY, Perez-Tapia M, Estrada-Garcia I, et al. Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. International journal of experimental pathology. 2005;86(5):323-334.
Crossref - Blackley S, Kou Z, Chen H, et al. Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. Journal of virology. 2007;81(24):13325-13334.
Crossref - Boonnak K, Slike BM, Burgess TH, et al. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. Journal of virology. 2008;82(8):3939-3951.
Crossref - Durbin AP, Vargas MJ, Wanionek K, et al. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008;376(2):429-435.
Crossref - Guzmán MG, Kourı G. Dengue diagnosis, advances and challenges. International journal of infectious diseases. 2004;8(2):69-80.
Crossref - Weaver SC, Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infection, genetics and evolution. 2009;9(4):523-540.
Crossref - Gubler DJ. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem. Dengue and dengue hemorrhagic fever. 1997;
Crossref - Gubler DJ, Kuno G. Dengue and dengue hemorrhagic fever New York. NY: CAB International. 1997;
Crossref - Durbin AP, Mayer SV, Rossi SL, et al. Emergence potential of sylvatic dengue virus type 4 in the urban transmission cycle is restrained by vaccination and homotypic immunity. Virology. 2013;439(1):34-41.
Crossref - Tomashek KM, Lorenzi OD, Andújar-Pérez DA, et al. Clinical and epidemiologic characteristics of dengue and other etiologic agents among patients with acute febrile illness, Puerto Rico, 2012-2015. PLoS neglected tropical diseases. 2017;11(9):e0005859.
Crossref - Martins VEP, Alencar CH, Kamimura MT, et al. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceará, Brazil. PloS one. 2012;7(7):e41386.
Crossref - Akbar MR, Agoes R, Djatie T, Kodyat S. PCR detection of dengue transovarial transmissibility in Aedes aegypti in Bandung, Indonesia. Parasitology and Tropical Medicine Association of Thailand; 2009:84-89.
- Hull B, Tikasingh E, de Souza M, Martinez R. Natural transovarial transmission of dengue 4 virus in Aedes aegypti in Trinidad. The American journal of tropical medicine and hygiene. 1984;33(6):1248-1250.
Crossref - Khin MM, Than KA. Transovarial transmission of dengue 2 virus by Aedes aegypti in nature. The American journal of tropical medicine and hygiene. 1983;32(3):590-594.
Crossref - Rosen L, Shroyer DA, Tesh RB, Freier JE, Lien JC. Transovarial transmission of dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. The American journal of tropical medicine and hygiene. 1983;32(5):1108-1119.
Crossref - Cruz LCdTAd, Serra OP, Leal-Santos FA, Ribeiro ALM, Slhessarenko RD, Santos MAd. Natural transovarial transmission of dengue virus 4 in Aedes aegypti from Cuiabá, State of Mato Grosso, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2015;48:18-25.
Crossref - Gutiérrez-Bugallo G, Rodriguez-Roche R, Díaz G, et al. First record of natural vertical transmission of dengue virus in Aedes aegypti from Cuba. Acta tropica. 2017;174:146-148.
Crossref - Castro MGd, Nogueira RMR, Schatzmayr HG, Miagostovich MP, Lourenço-de-Oliveira R. Dengue virus detection by using reverse transcription-polymerase chain reaction in saliva and progeny of experimentally infected Aedes albopictus from Brazil. Memórias do Instituto Oswaldo Cruz. 2004;99:809-814.
Crossref - Rohani A, Zamree I, Joseph RT, Lee HL. Persistency of transovarial dengue virus in Aedes aegypti (Linn.). Southeast Asian J Trop Med Public Health. 2008;39(5):813-816.
- Mitchell CJ, Miller BR. Vertical transmission of dengue viruses by strains of Aedes albopictus recently introduced into Brazil. Journal of the American Mosquito Control Association. 1990;6(2):251-253.
- Mourya DT, Gokhale MD, Basu A, et al. Horizontal and vertical transmission of dengue virus type 2 in highly and lowly susceptible strains of Aedes aegypti mosquitoes. Acta virologica. 2001;45(2):67-72.
- Hartanti MD, Suryani S, Tirtadjaja IA. Dengue virus transovarial transmission by Aedes aegypti. Universa Medicina. 2010;29(2):65-70.
- Joshi V, Mourya DT, Sharma RC. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. The American journal of tropical medicine and hygiene. 2002 2002;67(2):158-161.
Crossref - Chotigeat U, Khaoluang S, Kanjanapatanakul V, Nisalak A. Vertical transmission of dengue virus. Journal of Infectious Diseases and Antimicrobial Agents. 2000;17(1):33-34.
- Yin X, Zhong X, Pan S. Vertical transmission of dengue infection: the first putative case reported in China. Revista do Instituto de Medicina Tropical de São Paulo. 2016;58
Crossref - Chye JK, Lim CT, Ng KB, Lim JMH, George R, Lam SK. Vertical transmission of dengue. Clinical Infectious Diseases. 1997;25(6):1374-1377.
Crossref - Barthel A, Gourinat A-C, Cazorla C, Joubert C, Dupont-Rouzeyrol M, Descloux E. Breast milk as a possible route of vertical transmission of dengue virus? Clinical Infectious Diseases. 2013;57(3):415-417.
Crossref - Rosen L. Sexual transmission of dengue viruses by Aedes albopictus. The American journal of tropical medicine and hygiene. 1987;37(2):398-402.
Crossref - Wiwanitkit V. Non vector-borne transmission modes of dengue. Journal of Infection in Developing Countries. 2010;4(1)doi:10.3855/jidc.145
Crossref - Seed CR, Kiely P, Hyland CA, Keller AJ. The risk of dengue transmission by blood during a 2004 outbreak in Cairns, Australia. Transfusion. 2009;49(7):1482-1487.
Crossref - Tambyah PA, Koay ESC, Poon MLM, Lin RVTP, Ong BKC. Dengue hemorrhagic fever transmitted by blood transfusion. New England Journal of Medicine. 2008;359(14):1526-1527.
Crossref - Tan FL-S, Loh DLSK, Prabhakaran K. Dengue haemorrhagic fever after living donor renal transplantation. Nephrology Dialysis Transplantation. 2005;20(2):447-448.
Crossref - Nemes Z, Kiss G, Madarassi EP, et al. Nosocomial transmission of dengue. Emerging Infectious Diseases. 2004;10(10):1880.
Crossref - Wagner D, de With K, Huzly D, et al. Nosocomial acquisition of dengue. Emerging Infectious Diseases. 2004;10(10):1872.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.