Quorum sensing (QS) is a type of cell-to-cell communication that is influenced by an increase in signaling molecules known as autoinducers, which is correlated to the increase in the density of microbial communities. In this review, we aim to discuss and provide updates on the different signaling molecules used by Escherichia coli, such as acyl-homoserine lactone (AHL), autoinducer-2 (AI-2), and indole to influence key phenotypes such as antibiotic drug resistance, biofilm formation, and motility during quorum sensing. Based on the literature, E. coli signaling molecules have different functions during cell-to-cell communication such that the increase in AHL and indole was found to cause the modulation of antibiotic resistance and inhibition of biofilm formation and motility. Meanwhile, AI-2 is known to modulate biofilm formation, antibiotic resistance, and motility. On the other hand, in the existing literature, we found that various plants possess phytochemicals that can be used to alter QS and its downstream key phenotypes such as biofilm formation, swimming and swarming motility, and genes related to motility, curli and AI-2 production. However, the exact physiological and molecular mechanisms of these natural compounds are still understudied. Understanding the mechanisms of those phytochemicals during QS are therefore highly recommended to conduct as a necessary step for future scholars to develop drugs that target the actions of QS-signaling molecules and receptors linked to antibiotic resistance, biofilm formation, and motility without putting bacteria under stress, thereby preventing the development of drug resistance.
Autoinducers, Quorum Quenching, Essential Oils, lux genes, Quorum Sensing, sdiA
Bacteria communicate with one another when they experience various stresses from their environment including the presence of antibiotics and competitors. One of the mechanisms used for bacterial cell-to-cell communication during these stress conditions is termed quorum sensing (QS), which involves the production, release, and reception of signaling molecules called autoinducers (AIs). This process is activated by the increase of cell density population and composition of the microbial community in the environment that is correlated to the increase in Ais.1-3 The AIs are used in interspecies and intraspecies communication which act as ligands that will attach to their specific receptor.3,4-6 Once the autoinducer reaches a certain threshold concentration, bacteria within the population or community modify gene expression and adopt coordinated behaviors such as swimming and swarming motility, conjugal plasmid transfer, antibiotic drug resistance, biofilm formation, bioluminescence, secondary metabolite production, symbiosis, and virulence.2,7-10
The aim of this review is to discuss and provide some updates on the different signaling molecules such as AHL, AI-2 and indole that are used by E. coli during quorum sensing in regulating key phenotypes such as antibiotic drug resistance, biofilm formation, and motility. Knowing the physiological roles of these chemical molecules, these can be used as signals for the attenuation of gene expression in antibiotic resistance, biofilm formation, and motility of pathogenic microbes like E. coli. Similarly, this article reported plant metabolites that can alter several phenotypic features during QS in E. coli.
E. coli uses Signaling Molecules during QS to Regulate Biofilm Formation, Motility and Drug Resistance
Similar to other bacteria, E. coli secretes and/or uses signaling molecules to express different phenotypic keys during stress conditions.11 Biofilm formation, motility, and drug resistance are examples of key phenotypes in E. coli that are affected once quorum sensing is activated through the detection of signaling molecules, including autoinducer-2 (AI-2) and indole from the environment during stress conditions, for instance, the presence of antibiotics. However, not all signaling molecules are synthesized by E. coli, like AHL, but rather they use sensors to detect signaling molecule from their environment that is secreted by other bacteria.12
Acyl-homoserine Lactone (AHL)
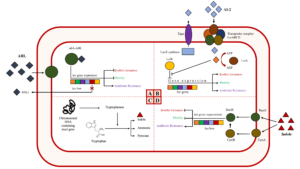
In Gram-negative bacteria, acyl-homoserine lactone (AHL) is one of the essential signaling molecules that is used for QS4. It is made up of a homoserine lactone ring with an attached amide group and variably structured saturated or unsaturated acyl side chain R group, generally between 4 and 18 carbons.1,12,13 This signaling molecule is produced during the exponential growth stage1 and peaks at the stationary growth phase and degrades after this stage.14 Based on the study, it is not synthesized by E. coli due to the lack of AHL synthase encoding gene (luxI) that produces this chemical signal.15-17 However, it can be sensed and intercepted using a receptor-like protein called suppressor of cell division inhibition (sdiA).15,17,18 The sdiA is made up of 240 amino acids and is known to belong in the LuxR family (transcriptional regulator) and is used to induce the genes ftsQAZ responsible for bacterial cell division.15,19. Since E. coli cannot synthesize AHLs, SdiA (a LuxR homolog)19 is used for the eavesdropping of this chemical signal which results in interspecies communication by means of using this protein to sense AHL which is secreted and released by other bacteria. As a result, certain key phenotypes are being controlled like antibiotic resistance but inhibited motility and biofilm formation (Figure 1A).19,20
Figure 1. Action mechanisms of AHL, AI-2, and indole during quorum sensing in E. coli. A: As AHL binds to SdiA, it will cause inhibition of motility and biofilm formation but promotes antibiotic resistance. B: AI-2 is phosphorylated by LsrK after passing through the LsrABCD transporter complex. The phosphorylated AI-2 will subsequently bind to the repressor protein, LsrR, causing biofilm formation, motility, and antibiotic resistance genes to be triggered and expressed. C: Indole synthesis. The tnaA gene from the chromosomal DNA expresses tryptophanase which serves as a catabolic enzyme for the catabolism of tryptophan into indole, ammonia, and pyruvate. D: As indole (intracellular signal) binds to the sensor kinases, BaeS and CpxA, then the two cognate receptors, BaeR and CpxR, receive signals. The BaeR can independently regulate the gene expression for the inhibition of bacterial biofilm formation and motility and development of antibiotic resistance while CpxR serves as a modulator of the activity of BaeR.
Lee et al21 demonstrated that the exogenous AHL can be sensed by the E. coli through SdiA to regulate the gene expression for biofilm formation. They found out that deletion of the gene responsible for the expression of SdiA through protein engineering affects biofilm formation. It was observed that the formation of biofilm in mutant strains increase seven-fold after the additions of N-octanoyl-DL-homoserine lactone and N-(3-oxododecatanoyl)-L-homoserine lactone compared to the wildtype strain. This result was supported and elaborated by Culler and his colleagues wherein a thicker extracellular matrix was observed in the mutant strain (E. coli HFCO1) without sdiA as compared to the wild-type (E. coli ONT:H25) and complemented strain (E. coli HFCO2) with a thinner biofilm. The transcription of genes (bcsA, csgA, csgD, fliC, and fimA) related to biofilm formation is also reduced in the wild-type upon addition of AHLs (3O-C6-DL-HSL and C8-DL-HSL) compared to the mutant strain with a significant increase in the gene expression, particularly in csgD and csgA.19 This suggests that SdiA is a stress sensor that is inhibited to promote biofilm formation during stress conditions.19,21
Motility is one of the important factors during initial adhesion, a first step involved in biofilm formation. It requires the presence of flagella which helps E. coli to move into the surface area. However, as the process approaches the initial development of biofilm, the formation of flagella is repressed to make the E. coli sessile through adhesion to the surface.22 To determine the role of sdiA in E. coli motility, various studies were previously conducted. Using qRT-PCR, it was found that gene-related to motility has a 2-fold increase in the mutant (E. coli HFCO1) with the deleted gene for sdiA compared to the wild-type (E. coli ONT:H25) and complemented strain (E. coli HFCO2).19 It indicates that the deletion of gene encoded by sdiA leads to the increase in flagellar activity responsible for motility. This is similar to the finding obtained by Sharma et al. as they produced an enterohemorrhagic E. coli (EHEC) mutant strain lacking sdiA. Using soft-agar motility plates, they observed that the mutant strain motility was increased by about 30% compared to the parental strain suggesting that SdiA has a negative effect on EHEC motility. The deletion of the sdiA gene in the mutant strain causes an increase in the gene expression of fliC, which encodes for motility.23 Therefore, the deletion of sdiA is an advantage to E. coli since it enhances the flagellar activity of this bacteria, which is required during the initial adhesion of biofilm formation.
Aside from biofilm formation and motility, the sdiA is also involved in antibiotic resistance. Antimicrobial resistance has emerged as a global concern to public health systems across the world. Members of the Enterobacteriaceae family, notably E. coli, are among the bacteria that pose the biggest threat to human health due to antibiotic resistance.24 This mechanism is made possible due to the expression of various efflux pumps. Based on the previous report, SdiA is a positive regulator that manages the cell’s internal environment by eliminating antimicrobial drugs.25-27 For instance, the acrAB, acrD, acrEF are examples of efflux pumps that belong to the resistance-nodulation-division (RND) family transporters which are responsible for the extrusion of antimicrobial agents and serve as a vital determinant of multidrug resistance in Gram-negative bacteria like E. coli.26 The study revealed that the overexpression of acrD, together with acrAB and/or acrEF, could increase the resistance level of E. coli LomEF and E. coli CazE11, which are previously susceptible strains to lomefloxacin (fluoroquinolone) and ceftazidime (β-lactam), respectively.27 The role of acrAB for bacterial drug resistance conforms with the result obtained by Rahmati and colleagues.28 They found that the overexpression of sdiA positively affects the expression of acrAB transporter which results in an increase in resistance level. However, the sdiA mutant strain (lack of sdiA gene) is hypersensitive to drugs due to decreased levels of AcrB protein. Furthermore, Chetri et al.29 demonstrated the role of efflux pumps in E. coli during carbapenem stress. Based on the results, the highest overexpression of AcrA, AcrB, AcrD, and AcrR genes was observed against ertapenem, imipenem, ertapenem, and meropenem, respectively. Hence, it suggests that sdiA plays an important role in the multidrug resistance of bacteria through positive regulations of these genes for the extrusion of antibiotics. On the contrary, since sdiA is a positive regulator of acrAB protein when the gene for the expression of sdiA is deleted, the AHL will not be sensed hence the E. coli will become sensitive to antibiotics.
Autoinducer-2
Autoinducer-2 is a borate diester-derived organic anion30 that was first discovered to produce and induce the bioluminescence activity of Vibrio harveyi. Based on the study, LuxS is a synthase that produces AI-231,32 that is capable of being detected by both Gram-positive and Gram-negative bacterial species receptors during quorum sensing. Thus, this universal signaling molecule can be used for intraspecies and interspecies communication.30,33-35 In E. coli, AI-2 peaks at the mid-to-late exponential phase and rapidly decreases during entry into the stationary phase.6 It is encoded by the LuxS gene7,31 and is transported out of the cell via a transporter called quorum sensing-A (Tqsa).36 During QS, when the AI-2 reaches a particular threshold level, it passes through the complex transmembrane protein (LsrACBD) and is then phosphorylated by LsrK (a cognate signal kinase). The phosphorylated AI-2 will then bind to LsrR (a repressor protein) and eventually affects the gene expression of various density-dependent key phenotypes37 such as antibiotic resistance, biofilm formation, and motility (Figure 1B).38-40
AI-2 production is beneficial for the survival of E. coli once exposed to environmental pressure, for instance, exposure to antibiotics. When an E. coli ECDCM1 (an isolate from a dairy cow that suffered from mastitis) is treated with ampicillin, oxacillin, penicillin, and an exogenous AI-2, the survival increases compared to control strain that is exposed to antibiotics alone. This result indicates that AI-2 is used by E. coli to upregulate genes for drug resistance through the LuxS receptor. It also implies that the long-term exposure of the bacteria to antibiotics may force them to develop a better survival strategy.39 Moreover, an experiment conducted by Kaur et al.41 demonstrated the role of AI-2 in antibiotic resistance. They found out that after the complementation of the luxS gene to the E. coli with deleted gene encoding AI-2, a reduction in MIC was observed and this can be explained by the downregulation of efflux pump genes. Aside from this gene, IsrR also plays a vital role in antibiotic resistance. The deletion of this gene in avian pathogenic E. coli (APEC) upregulates the mdtH which encodes for the mdtH transporter, an efflux pump belonging to the major facilitator superfamily (MFS) family which is known as one of the classifications of multidrug resistance transporters. Once the gene is upregulated, it can cause an increase in resistance to quinolone and tetracycline. However, the overexpression of the lsrR can cause an increase in susceptibility to these classes of antibiotics. Therefore, these results indicate the lsrR affects the activity of mdtH by direct binding to its promoter region which can cause its ability to either increase or decrease antibiotic resistance.42
AI-2 controls the group behavior of E. coli like motility and biofilm formation. The swimming motility of E. coli is mediated by the flagella. This process is involved during the initial cell attachment of biofilm formation, as well as in the virulence of E. coli. To determine the role of AI-2 in the motility of E. coli, a motility assay was performed. Based on the study, the addition of 1 uM concentration of AI-2 did not induce the response of EHEC (Enterohemorrhagic E. coli). However, with the addition of 100 uM of AI-2, the swimming motility of the EHEC evoked a 1.3-fold increase.43 The expression of this phenotypic key could help enhance the biofilm formation of E. coli. Any increase in swimming motility could potentially promote the formation of biofilm.42 This result corroborated and further explained with the findings of Gonzales Barrios and his colleagues that AI-2 is directly involved in biofilm formation. The deletion of the luxS affects the biofilm formation of E. coli BW25113 ΔluxS (a mutant strain). It was found out that in the absence of AI-2, a 50% reduction in biofilm was observed in this strain compared to the wildtype. However, after the complementation of luxS, a restoration of bacterial biofilm was observed. This result confirms that AI-2 is synthesized by the luxS genes. To determine how AI-2 stimulates biofilm formation in E. coli, the addition of AI-2 was performed and probed if this can affect the transcription of genes for motility. Based on their findings, AI-2 positively induces qseB which results in the transcription of other genes related to motility such as flhD, fliA, fliC, and motA.38 Hence, the inhibition of the AI-2 formation is essential to mask antibiotic resistance, biofilm formation, and motility of bacteria.
Indole
Indole is a heterocyclic aromatic compound that is composed of a pyrrole ring fused with a benzene ring.44 It is produced in large quantities by 85 species of bacteria including both Gram-positive and Gram-negative bacteria45 and is known to be exported by the AcrEF pump.46 This molecule is a catabolic product formed from tryptophan with the help of an enzyme called tryptophanase, which is encoded by the tnaA gene. As tnaA encodes tryptophanase, indole signaling enhances the production of indole itself. Along with this, pyruvate and succinate are also produced which are encoded by astD and gabT genes, respectively (Figure 1C).46,47 During exponential growth of E. coli, the production of indole is low but as the stationary phase approaches, synthesis increases.48 In E. coli, the production of indole has gained attention due to its effects on the expression of bacterial phenotypic keys such as antibiotic resistance, motility, and biofilm formation (Figure 1D).43,49
Antibiotic drug resistance of E. coli is also affected by indole production. This quorum sensing-like signal molecule was found responsible for the formation of persister cells (cells that undergo a dormant state when antibiotic is present) through transporter activation.47 Based on the previous study, highly resistant bacteria produced indole to help other less resistant bacteria within the population to survive when there is a presence of antibiotics. Upon checking the transcriptional profile, it was found that multi-drug efflux pumps such as mdtEs are upregulated due to the production of indole.50 The formation of a persister cell when an antibiotic is present is supported by the recent finding observed in Zarkan and his colleagues. The group demonstrated that indole pulse signaling (5 mM) is responsible for the formation of persister cells, specifically when fluoroquinolones antibiotics are applied, rather than persistent signaling (0.5 mM). As they applied pulse and persistent signals, a significant difference was observed between mutant E. coli (without gene encoding tryptophanase) treated with and without 5 mM pulse of indole. Aside from that, the bacterial activity of nalidixic acid, levofloxacin, and moxifloxacin is reduced as shown by the increase in the number of persister cells upon the external addition of pulse signal. Hence, it can be concluded that pulse dependent mechanism of the persister cells helps to overcome the antibiotics, particularly the fluoroquinolone, which is known to target the GyrA subunit of DNA gyrase, a topoisomerase enzyme that catalyzes supercoiling during replication and transcription, especially in Gram negative bacteria. Moreover, indole can also play a vital role in the antibiotic resistance of other bacterial species.49 When Vega and colleagues assessed the interspecific role of indole between Salmonella typhimurium and E. coli, it was found that despite the inability of S. typhimurium to produce indole, it can tolerate the presence of ciprofloxacin via interception of indole released by commensal E. coli which can probably result to the enhancement of antibiotic resistance of S. typhimurium in host intestine. With this evidence, it is possible to conclude that indole is a signaling molecule that can be used for interspecies communication.51
In terms of biofilm formation and motility (chemotaxis and swarming) in E. coli, the role of produced indole plays an opposite role wherein indole inhibits biofilm formation and motility while AI-2 induces the formation of biofilm as well as the motility.52 It means that the influence of these two signaling molecules has opposite effects on the phenotypic expression of bacterial behaviors. Bansal et al.43 showed that indole has the ability to attenuate the expression of biofilm formation in enterohemorrhagic E. coli (EHEC). Based on their laboratory assay, indole has the capability to inhibit the phenotypic expression of chemotaxis, motility, biofilm formation, colonization of epithelial cells, and the expression of genes related to virulence in EHEC. Thus, this result indicates that indole is an important signal for the pathogenesis of this strain.
Medicinal Plants as Source of Quorum Sensing Inhibitors
Pharmaceutical industries have been pushed to develop new therapeutic agents in recent years due to a paucity of novel antimicrobial agents and the continued increase of antibiotic-resistant bacteria as a result of the abuse and overuse of antibiotics in the treatment of bacterial diseases.53 Since the discovery of the ability of bacteria to produce autoinducers for bacterial cell-to-cell communication, researchers have used anti-quorum sensing assays to explore for inhibitors to interrupt such bacterial means of communication.54 Quorum sensing inhibitors (QSI) are a promising alternative to traditional antibiotics that have little or no effect on drug-resistant bacteria. The interruption of QS is considered a non-invasive approach since it can alter bacterial pathogenicity without putting the bacteria under selection pressure.5,55 Today, several inhibitors targeting the QS detection system of bacterial pathogens have been recognized by the Food and Drug Administration for use as anti-virulence drugs. QSIs are a novel type of antimicrobial agent that can be used in medicine, biotechnology, and agriculture.56
Nowadays, plants have been exploited to search for natural products which can be harnessed for human benefits, particulary in the field of medical science. Phytochemicals are the richest reservoir of therapeutic substances that can be isolated and studied from plants against pathogenic multi-drug resistant (MDR) bacteria.57,58 However, despite the fact that many medicinal plants have antibacterial properties, only a few therapeutic plants have been reported to target QS. QSIs, from medicinal plants, are a promising technique for new antibacterial drugs in the face of rising microbial resistance to current antibiotics and a drop in novel antibiotic discovery.59 Hence, the anti-quorum sensing activities of different phytochemicals isolated from various plant sources were presented in Table 1.
Table (1):
Different bioactive constituents that act as inhibitors against E. coli quorum sensing activities.
Plant Source |
Identified Bioactive Compounds |
Bioactivities |
References |
|---|---|---|---|
Black cardamom |
Essential oils (limonene, α-pinene, linalool acetate, α-Terpinyl acetate, hexadecanol acetate, sabinene, methyl linoleate, β-Pinene, α-Terpineol, n-Hexadecanoic acid, nerolidol, g-Terpinene, Fatty acids (C18), 1,8-Cineole, long chain hydrocarbons) |
Inhibition of biofilm formation in E. coli O157:H7 ranges from 47-85% in 0.03-0.5% concentration Inhibition of violacein production ranges from 35-100% in 0.5-1% concentration |
[60] |
Green or true cardamom (Elletaria cardamomum) |
Essential oils |
Inhibition of biofilm formation of Escherichia coli O157:H7 ranges from 64-86% in 0.015-0.125% (v/v) concentration |
[61] |
Pomegranate (Punica granatum) |
Tannin-rich fraction from pomegranate rind (TFPR) |
Inhibition of biofilm formation and motility of E. coli. Downregulation of curli and motility genes in 0.039–0.156 mg/mL. |
[64] |
Myrtle (Myrtus communis) |
Linalool |
Exhibited anti-quorum sensing activity using C. violaceum assay. Inhibition of biofilm formation in UPEC ranges from 31–84%. Inhibition of swarming motility of UPEC in a dose-dependent manner. |
[63] |
Lippia origanoides |
Thymol-carvacrol-chemotype (II) oil & thymol-carvacrol-chemotype (I) oil |
Sub-MIC of essential oils strongly inhibited the biofilm formation of E. coli O33 and E. coli O157:H7 at 75% and 73%, respectively. Inhibition of violacein pigment production at 88% and 70% using Thymol-carvacrol-chemotype (II) and thymol-carvacrol chemotype (I), respectively. |
[57] |
Thyme (Thymus vulgaris) |
Thymol-carvacrol-chemotype (I) oil |
Inhibition of biofilm formation in E. coli O33 and E. coli O157:H7. |
[57] |
Cymbopogon martini |
Thymol-carvacrol-chemotype (I) oil |
Inhibition of biofilm formation in E. coli O33 and E. coli O157:H7. |
[57] |
Cardamon (Elettaria cardamomum) |
Thymol-carvacrol chemotype (I) |
Reduction of 65% in violacein production |
[57] |
Beet (Beta vulgaris) |
– |
Inhibition of violacein pigment production of C. violaceum at 80% in 98.35 mg mL−1 |
[62] |
Leek (Allium porrum) |
– |
Inhibition of violacein pigment production of C. violaceum by 80% at a concentration of 53.75 mg/mL concentration. Inhibition of biofilm formation in E. coli O157:H7 ATCC 25158 at 268.75 mg/mL. |
[62] |
Origanum compactum |
Essential oils (α-Thujene, α-Pinene, 1-Octen-3-ol, 3-Octanone, β-Myrcene, α -Terpinene, P-Cymene, D-Limonene, 1,8-Cineole, g-Terpinene, Linalool, Terpinen-4-ol, α -Terpineo, thymol, carvacrol, β-Caryophyllene, Caryophyllene oxide) |
Anti-quorum sensing through inhibition of biofilm formation |
[53] |
– |
D-limonene |
Inhibition of biofilm formation in E. coli through the suppression of curli and extracellular polymeric substance (EPS) Decreased swimming and swarming ability of E. coli. Repression of the expression of curli related genes and AI-2 importer genes in E. coli. |
[65] |
Essential oils from plant are considered as a good candidates to disrupt bacterial cell-to-cell communication. Abdullah et al.60 revealed that the Black Cardamom essential oils exhibited anti-biofilm formation against E. coli O157:H7 by 47-85% at 0.03- 0.5% treatment concentration. This result is congruent to the outcome obtained using Green Cardamom essential oils in which the biofilm of E. coli was inhibited by 64-86% at a range of 0.015-0.125% (v/v). at 0.03- 0.5% treatment concentration Likewise, these two species of Cardamom are known to inhibit violacein production of C. violaceum using their essential oil extracts.60,61 Similarly, essential oils from Lippia origanoides, Thymus vulgaris, and Cymbopogon martini such as thymol-carvacrol-chemotype (II) oil & thymol-carvacrol-chemotype (I) found to inhibit biofilm formation of E. coli O33 and E. coli O157:H7. Aside from these, the essential oils isolated from the L. origanoidesi inhibit the violacein pigment production using Chromobacterium violaceum assay. This result conforms to the thymol-carvacrol chemotype (I) isolated from Elettaria cardamomum with a 65% violacein inhibition rate.57 Similarly, an 80% reduction rate was found on the production of violacein using ethanolic extracts of dehydrated beet leaves (EEDBL) and ethanolic extract of dehydrated leek leaves (EEDLL) at a concentration of 98.35 and 268 mg/mL, respectively. However, only the EEDLL exhibit biofilm formation inhibition at a dose of 268.75 mg/mL.62 Furthermore, linalool from M. communis was also found to inhibit swarming motility and exhibit anti-QS activity and anti-biofilm formation against uropathogenic E. coli (UPEC).63 Similarly, Yang et al.64 reported that the tannin-rich fraction from the Pomengranate rind (TFPR) of P. granatum inhibited the biofilm formation and motility of E. coli. Based on their analysis, the expression of genes related to curli (csgB and csgD) and motility (fimA, fimH, flhD, motB, qseB, and qseC) were found downregulated. Apart from these bioactive constituents, although the source is not isolated from plants, Wang et al.65 reported that D-Limonene, also known as 4-isopropenyl-1-methylcyclohexene (C10H16), a monocyclic monoterpene, is a promising QSI because of its ability to inhibit biofilm formation and suppress the curli and extracellular polymeric substance, an important component for biofilm formation. Likewise, the study revealed that D-limonene helps to decrease E. coli swimming and swarming activity and was found to repress the expression of curli-related genes and AI-2 importer genes. D-Limonene is found to exist in various citrus plants including lemon, orange, and grape.66
Using natural products as anti-QS is considered a promising and effective strategy for novel antimicrobial development.65 The bioactive compounds derived from plants have the potential to alter bacterial cell-to-cell interactions by inhibiting violacein synthesis, forming biofilms, and downregulating many associated genes related to motility, curli, and AI-2 production. Several investigations have found that hampering the QS mechanism did not affect bacterial growth. Hence, even if this biological method is designed to disrupt bacterial communication without imposing selection pressure on bacteria, MDR E. coli strains are less likely to emerge.32,65,67 Therefore, it is suggested as a new approach for drug discovery against MDR pathogenic bacteria.68
E. coli is a well-known Gram-negative bacteria that communicate with other cells using signaling molecules to trigger the expression of certain genes for different phenotypic keys such as biofilm formation, motility, and antibiotic drug resistance. Previous papers highlighted that E. coli signaling molecules have different functions during cell-to-cell communication such that the increase in AHL and indole was found to cause the modulation of antibiotic resistance and inhibition of biofilm formation, and motility. Meanwhile, AI-2 is known to promote biofilm formation, antibiotic resistance and motility. Although E. coli is incapable to synthesize AHL, it intercepts the produced AHLs by other species. Hence, E. coli can communicate interspecifically. Moreover, this paper provides a review of plant metabolites that possess bioactivities including the disruption of QS. Today, a vast variety of plants have been exploited as a source of bioactive components in medical science in the search for QSI. It is considered one of the promising and best-known prospects as an alternative to ineffective antibiotics to address the problem of antibiotic resistance. Various studies have proven that plant metabolites can be used to alter QS and its downstream effects such as biofilm formation, swimming and swarming motility, and genes related to motility, curli, and AI-2 production. However, although the utilization of QSI exhibited a reduced selective pressure on bacteria, the exact physiological and molecular mechanisms of those phytochemicals are still understudied and it requires a thorough understanding to provide effective natural antimicrobial agents to disrupt cell-to-cell communication against pathogenic E. coli in the future studies. Once the exact mechanism is established, we may be able to formulate drugs that block quorum sensing via degradation of related signaling molecules and receptors. As a result of a good grasp on the mechanisms, we may be able to help minimize the threat of E. coli antibiotic resistance.
ACKNOWLEDGMENTS
KSC is grateful to the Philippine DOST Science Education Institute-Accelerated Science and Technology Human Resource Development Program (DOST SEI-ASTHRDP) for his master’s degree scholarship.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Zohar B-A, Kolodkin-Gal I. Quorum sensing in Escherichia coli: Interkingdom, inter- and intraspecies dialogues, and a suicide-inducing peptide. Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight. 2014:85-99.
Crossref - Ha JH, Hauk P, Cho K, et al. Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci Adv. 2018;4(6):aar7063.
Crossref - Venkatramanan M, Ganesh PS, Senthil R, et al. Inhibition of quorum sensing and biofilm formation in Chromobacterium violaceum by fruit extracts of Passiflora edulis. ACS Omega. 2020;5(40):25605-25616.
Crossref - Dong W, Zhu J, Guo X, et al. Characterization of AiiK, an AHL lactonase, from Kurthia huakui LAM0618T and its application in quorum quenching on Pseudomonas aeruginosa PAO1. Sci Rep. 2018;8(1):6013.
Crossref - Chen J, Wang B, Lu Y, et al. Quorum sensing inhibitors from marine microorganisms and their synthetic derivatives. Mar Drugs. 2019;17(2):80.
Crossref - Li J, Attila C, Wang L, Wood TK, Valdes JJ, Bentley WE. Quorum sensing in Escherichia coli is signaled by AI-2/LSRR: Effects on small RNA and biofilm architecture. J Bacteriol. 2007;189(16):6011-6020.
Crossref - Brameyer S, Plener L, Muller A, Klingl A, Wanner G, Jung K. Outer membrane vesicles facilitate trafficking of the hydrophobic signaling molecule CAI-1 between Vibrio harveyi cells. J Bacteriol. 2018;200(15):e00740-17.
Crossref - Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14(9):576-588.
Crossref - Diggle SP, Williams P. Quorum sensing. Brenner’s Encyclopedia of Genetics, 2nd edn. 2013:25-27.
Crossref - Sharifi A, Mohammadzadeh A, Salehi TZ, Mahmoodi P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J Appl Microbiol. 2018;124(2):379-388.
Crossref - Chung HJ, Bang W, Drake MA. Stress response of Escherichia coli. Compr Rev Food Sci Food Saf. 2006;5(3):52-64.
Crossref - Soares JA, Ahmer BMM. Detection of acyl-homoserine lactones by Escherichia and Salmonella. Curr Opin Microbiol. 2011;14(2):188-193.
Crossref - Chan K-G, Atkinson S, Mathee K, et al. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 2011;11(1):51.
Crossref - Liao L, Schaefer AL, Coutinho BG, Brown PJB, Greenberg EP. An aryl-homoserine lactone quorum-sensing signal produced by a dimorphic prosthecate bacterium. Proc Natl Acad Sci USA. 2018;115(29):7587-7592.
Crossref - Houdt R, Aertsen A, Moons P, Vanoirbeek K, Michiels CW. N-acyl-L-homoserine lactone signal interception by Escherichia coli. FEMS Microbiol Lett. 2006;256(1):83-89.
Crossref - Smith JL, Fratamico PM, Gunther NW. Shiga toxin-producing Escherichia coli. Adv Appl Microbiol. 2014;86:145-197.
Crossref - Kemp CA, McCullough DK, Bialonska D, Johnson PJT. Effect of bromination on the quorum sensing-inhibiting properties of indole-3-carboxaldehydes in Chromobacterium violaceum AHL system. Microbiol Res. 2021;12(2):376-382.
Crossref - Jamuna BA, Ravishankar RV. Effect of small chain N-acyl homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J Food Sci Technol. 2016;53(9):3609-3614.
Crossref - Culler H, Couto S, Higa J, et al. Role of sdiA on biofilm formation by atypical enteropathogenic Escherichia coli. Genes. 2018;9(5):253.
Crossref - Cheng C, Yan X, Liu B, et al. sdiA, a quorum sensing transcriptional regulator, enhanced the drug resistance of Cronobacter sakazakii and suppressed its motility, adhesion and biofilm formation. bioRxiv. 2022.
Crossref - Lee J, Maeda T, Hong SH, Wood TK. Reconfiguring the quorum-sensing regulator sdiA of Escherichia coli to control biofilm formation via indole and N-acylhomoserine lactones. Appl Environ Microbiol. 2009;75(6):1703-1716.
Crossref - Sharma G, Sharma S, Sharma P, et al. Escherichia coli biofilm: Development and therapeutic strategies. J Appl Microbiol. 2016;121(2):309-319.
Crossref - Sharma VK, Bearson SMD, Bearson BL. Evaluation of the effects of sdiA, a luxR homologue, on adherence and motility of Escherichia coli O157:H7. Microbiology (Reading). 2010;156(Pt 5):1303-1312.
Crossref - Galindo-Mendez M. Antimicrobial resistance in Escherichia coli. E coli Infections – Importance of Early Diagnosis and Efficient Treatment. 2020.
Crossref - Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453(2):254-267.
Crossref - Fernando D, Kumar A. Resistance-nodulation-division multidrug efflux pumps in Gram-negative bacteria: Role in virulence. Antibiotics. 2013;2(1):163-181.
Crossref - Tavio MM, Aquili VD, Poveda JB, Antunes NT, Sanchez-Cespedes J, Vila J. Quorum-sensing regulator sdiA and marA overexpression is involved in in vitro-selected multidrug resistance of Escherichia coli. J Antimicrob Chemother. 2010;65(6):1178-1186.
Crossref - Rahmati S, Yang S, Davidson AL, Zechiedrich EL. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator sdiA. Mol Microbiol. 2002;43(3):677-685.
Crossref - Chetri S, Bhowmik D, Paul D, et al. AcrAB-TolC efflux pump system plays a role in carbapenem non-susceptibility in Escherichia coli. BMC Microbiol. 2019;19(1):210.
Crossref - National Center for Biotechnology Information. Autoinducer-2. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/Autoinducer-2. Accessed January 17, 2022.
- Martin-Rodriguez AJ, Ticona JC, Jimenez IA, Flores N, Fernandez JJ, Bazzocchi IL. Flavonoids from Piper delineatum modulate quorum-sensing-regulated phenotypes in Vibrio harveyi. Phytochemistry. 2015;117:98-106.
Crossref - Wang Y, Liu B, Grenier D, Yi L. Regulatory Mechanisms of the LuxS/AI-2 System and Bacterial Resistance. Antimicrob Agents Chemother. 2019;63(10):e01186-19.
Crossref - Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2013;37(2):156-181.
Crossref - Liu Y, Hu H, Luo F. Roles of autoinducer-2 mediated quorum sensing in wastewater treatment. Water Science and Technology. 2021;84(4):793-809.
Crossref - Laganenka L, Sourjik V. Autoinducer 2-dependent Escherichia coli biofilm formation is enhanced in a dual-species coculture. Appl Environ Microbiol. 2018;84(5):E02638.
Crossref - Rettner RE, Saier Jr. MH. The autoinducer-2 exporter superfamily. J Mol Microbiol and Biotechnol. 2010;18(4):195-205.
Crossref - Quan Y, Meng F, Ma X, et al. Regulation of bacteria population behaviors by AI-2 “consumer cells” and “supplier cells”. BMC Microbiol. 2017;17(1):198.
Crossref - Barrios AFG, Zuo R, Hashimoto Y, Yang L, Bentley WE, Wood TK. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J Bacteriol. 2006;188(1):305-316.
Crossref - Xue T, Yu L, Shang F, et al. Short communication: The role of autoinducer 2 (AI-2) on antibiotic resistance regulation in an Escherichia coli strain isolated from a dairy cow with mastitis. J Dairy Sci. 2016;99(6):4693-4698.
Crossref - Zhang L, Li S, Liu X, et al. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat Commun. 2020b;11(1):5371.
Crossref - Kaur A, Capalash N, Sharma P. Expression of Meiothermus ruber luxS in E. coli alters the antibiotic susceptibility and biofilm formation. Appl Microbiol Biotechnol. 2020;104(10):4457-4469.
Crossref - Yu T, Ma M, Sun Y, et al. The effect of sublethal concentrations of benzalkonium chloride on the Luxs/AI-2 quorum sensing system, biofilm formation and motility of Escherichia coli. Int J Food Microbiol. 2021;353:109313.
Crossref - Bansal T, Jesudhasan P, Pillai S, Wood TK, Jayaraman A. Temporal regulation of enterohemorrhagic Escherichia coli virulence mediated by autoinducer-2. Appl Microbiol and Biotechnol. 2008;78(5):811-819.
Crossref - Pino-Rios R, Sola M. The relative stability of indole isomers is a consequence of the Glidewell-Lloyd Rule. J Phys Chem A. 2020;125(1):230-234.
Crossref - Lee J-H, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34(4):426-444.
Crossref - Horinouchi S, Ueda K, Nakayama J, Ikeda T. Cell-to-cell communications among microorganisms. Comprehensive Natural Products II. 2010:283-337.
Crossref - Kim J, Park W. Indole: A signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J Microbiol. 2015;53(7):421-428.
Crossref - Gaimster H, Cama J, Hernandez-Ainsa S, Keyser UF, Summers DK. The Indole Pulse: A new perspective on indole signaling in Escherichia coli. PLoS ONE. 2014;9(4).
Crossref - Zarkan A, Matuszewska M, Trigg SB, et al. Inhibition of indole production increases the activity of quinolone antibiotics against E. coli persisters. Sci Rep. 2020;10(1).
Crossref - Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467(7311):82-85.
Crossref - Vega NM, Allison KR, Samuels AN, Klempner MS, Collins JJ. Salmonella typhimurium intercepts Escherichia coli signaling to enhance antibiotic tolerance. Proc Natl Acad Sci. 2013;110(35):14420-14425.
Crossref - Song S, Wood TK. The primary physiological roles of Autoinducer-2 in Escherichia coli are chemotaxis and biofilm formation. Microorganisms. 2021;9(2):386.
Crossref - Bouyahya A, Chamkhi I, Balahbib A, et al. Mechanisms, anti-quorum-sensing actions, and clinical trials of medicinal plant bioactive compounds against bacteria: A comprehensive review. Molecules. 2022;27(5):1484.
Crossref - Krzyzek P. Challenges and limitations of anti-quorum sensing therapies. Front Microbiol. 2019;10:2473.
Crossref - Santos CA, Lima EMF, Franco BDGM, Pinto UM. Exploring phenolic compounds as quorum sensing inhibitors in foodborne bacteria. Front Microbiol. 2021;12:735931.
Crossref - Escobar-Mucino E, Arenas-Hernandez MM, Luna-Guevara ML. Mechanisms of inhibition of quorum sensing as an alternative for the control of E. coli and Salmonella. Microorganisms. 2022;10(5):884.
Crossref - Caceres M, Hidalgo W, Stashenko E, Torres R, Ortiz C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics. 2020;9(4):147.
Crossref - Subramanian K, Selvaraj H, Sampath Renuga P, Aruni W. Anti-quorum sensing in pathogenic microbes using plant-based bioactive phytochemicals. Adv Mater Sci. 2022.
Crossref - Yarmolinsky L, Bronstein M, Gorelick J. Inhibition of bacterial quorum sensing by plant extracts. Isr J Plant Sci. 2015;62(4):294-297.
Crossref - Abdullah, Algburi A, Asghar A, et al. Black cardamom essential oil prevents Escherichia coli O157:H7 and Salmonella typhimurium JSG 1748 biofilm formation through inhibition of quorum sensing. J Food Sci Technol. 2021a;58(8):3183-3191.
Crossref - Abdullah, Asghar A, Algburi A, et al. Anti-biofilm potential of Elletaria cardamomum essential oil against Escherichia coli O157:H7 and Salmonella typhimurium JSG 1748. Front Microbiol. 2021b;12:620227.
Crossref - Pellegrini MC, Ponce AG. Beet (Beta vulgaris) and Leek (Allium porrum) leaves as a source of bioactive compounds with anti-quorum sensing and anti-biofilm activity. Waste and Biomass Valori. 2020;11(8):4305-13.
Crossref - Alyousef AA, Husain FM, Arshad M, et al. Myrtus communis and its bioactive phytoconstituent, linalool, interferes with quorum sensing regulated virulence functions and biofilm of uropathogenic bacteria: In vitro and in silico insights. J King Saud Univ Sci. 2021;33(7):101588.
Crossref - Yang Q, Wang L, Gao J, et al. Tannin-rich fraction from pomegranate rind inhibits quorum sensing in Chromobacterium violaceum and biofilm formation in Escherichia coli. Foodborne Pathog Dis. 2016;13(1):28-35.
Crossref - Wang R, Vega P, Xu Y, Chen CY, Irudayaraj J. Exploring the anti-quorum sensing activity of a d-limonene nanoemulsion for Escherichia coli O157:H7. J Biomed Mater Res A. 2018;106(7):1979-1986.
Crossref - Anandakumar P, Kamaraj S, Vanitha MK. D-limonene: A multifunctional compound with potent therapeutic effects. J Food Biochem. 2021;45(1):e13566.
Crossref - Rajkumari J, Borkotoky S, Reddy D, et al. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: Insights from in vitro, in vivo and in silico studies. Microbiol Res. 2019;226:19-26.
Crossref - Vivas R, Barbosa AA, Dolabela SS, Jain S. Multidrug-resistant bacteria and alternative methods to control them: an overview. Microb Drug Resist. 2019;25(6):890-908.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.