ISSN: 0973-7510
E-ISSN: 2581-690X
L-asparaginase (E.C.3.5.1.1) is an enzyme responsible for hydrolysis of L-asparagine into aspartic acid and ammonia, and has its significant applications in the therapeutics and food technology. It was produced by the marine Aspergillus terreus and precipitated by 65% ammonium sulphate, followed by purification using gel filtration on Sephadex G-100 and DEAE-cellulose ion exchange chromatography, which yielded 11.96 fold purification. The molecular weight of the purified L-asparaginase was approximately 85 kDa, determined by a sodium dodecyl sulphate polyacrylamide gel electrophoresis. L-asparaginase showed high affinity for L-asparagine with a Km of 31.5 mM and Vmax of 500 U/ml. The optimum pH and temperature of the purified enzyme were 5.8 and 40 oC, respectively. The L-asparaginase enzyme was stable from pH 4 to 5.8 and stable up to 70 oC. The effect of activators and inhibitors was studied providing that CdCl2, Pb Cl2, and Hg Cl2 strongly inhibited the enzyme activity, while Na Cl highly enhanced activity. Anticancer activity of the purified L-asparaginase was detected against HCT-116, Hep-G2 and MCF-7 cell lines with IC50 ranged from 3.79-12.6 µg/ml.
L-asparaginase, Aspergillus terreus, purification, characterization, anticancer activity.
In recent years, L-asparaginase (L-asparagine amidohydrolase, 3.5.1.1) has gained more attention as chemotherapeutic agent with different applications in cancer treatment 1,2. It is mainly produced by microorganisms 3,4 . Different species of fungi as Fusarium, Aspergillus, and Penicillium 5 are the major producers. L-asparaginases from E. coli and Erwinia carotovora are the most available for medical use, but most of these treatments have low stability 6. L-asparaginase production with different characteristics and fewer side effects were reported from eukaryotic microorganisms, which are advantageous for its application7 . In the last years, fungal genera such as Fusarium, Aspergillus, Penicillium have been known as source of L -asparaginase 8-10. Moreover, fungi isolated from marine environments, are recommended as L -asparaginase producers 11. Different studies reported the interesting potentiality of Aspergillus species to produce significant amounts of L-asparaginase 12-14 .
Therefore, the aim of the current study is to purify L-asparaginase from filamentous fungus Aspergillus terreus isolated from marine environment and to investigate its properties and anticancer activity for future medical applications.
Strain and chemicals
The fungus A. terreus used in the current investigation, was isolated from Red sea, Egypt15. The substrate L-asparagine, DEAE-cellulose and Sephadex G-100 were procured from Sigma (Sigma-Aldrich, USA). All the chemicals were of analytical reagent grade.
Production of L-asparaginase by A. terreus
The maintained culture of A. terreus on potato dextrose agar (PDA) slants was used for inoculum preparation and production of L-asparaginase using modified Czapek,s-Dox medium15. 3 ml spore suspension (106/ml) was used to inoculate 50 ml of the broth medium, followed by culture incubation at 35°C under static condition for 5 days.
Assay of L-asparaginase activity
Estimation of the liberated ammonia during catalysis of asparagine by L-asparaginase using Nessler’s reagent was used as indication of L-asparaginase activity. The reaction mixture contained 0.5 ml of crude sample, 0.04 M L-asparagine and 0.05 M acetate buffer (pH 5.4), which incubated for 10 min at 35°C. 0.5 ml of 1.5 M TCA (trichloroacetic acid) solution was added to stop the reaction. The coupled liberated ammonia with Nessler’s reagent was determined spectrophotometry at 500 nm. The amount of L-asparaginase that caused liberation of one micromole of ammonia using the assay conditions was recorded as international unit of L-asparaginase 16
Protein determination
Estimation of protein was carried out according to the method of Lowry et al. (1951)17.
Partial purification of L-asparaginase
The purification steps were performed at 4oC. The supernatant containing the extracellular enzyme was collected after centrifugation at 6,000 rpm for 20min and treated with solid ammonium sulphate saturation ( 35, 50, 65, 75 and 85% ) with continuous overnight stirring and precipitation of proteins. 50 mM Tris-HCl buffer (pH 7.5) was used to dissolve the precipitates. The enzyme solution was dialyzed against the same buffer for 24h with several changes to remove the salt then assay of enzyme activity was done as previously mentioned16,18.
Gel filtration chromatography
Ammonium sulfate fraction resulted after dialysis was applied to Sephadex G-100 column (2.5 X 45 cm) that was pre-equilibrated with 50 mM Tris –HCl buffer, pH 7.5. The protein elution was done with the same buffer at a flow rate of 3 ml/min 19. The collected fractions of L-asparaginase were pooled at 4°C, followed by determination of protein at 750 nm and L-asparaginase activity.
Ion exchange chromatography
The pooled fractions from gel filtration column exhibited the highest specific activity were exposed to an anion exchange chromatography using DEAE cellulose column (1.5×15cm) pre-equilibrated with the Tris-HCl buffer (50 mM; pH 7.5). The first step was washing of the unbound proteins with free-NaCl Tris-HCl buffer 50mM; pH 7.5. The second step was gradient elution using Tris-HCl buffer containing NaCl (1M) at flow rate of 1 ml/min followed by collection and lyophilization.
Characterization of L-asparaginase
Determination of molecular weight of the purified enzyme
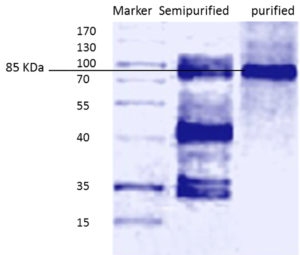
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied to determine the molecular weight of the purified L-asparaginase as was described by Laemmli (1970)20.
Substrate specificity
Various substrates included L-asparagine, L-glutamine, L-aspartic acid and L-glutamic acid were prepared in 50 mM acetate buffer pH 6.0. Each substrate was added separately to the enzyme and incubated for 30 min at 35°C , then activity of the enzyme was estimated each time.
Effect of different substrate concentrations
Asparagine concentration used in the reaction mixture was varied in a range of 10-100 mM. The reaction was done at 35°C and pH 6.0 for 20 min.
Determination of kinetic constants
Lineweaver and Burk (1934)21 method was used to estimate the kinetic parameters. The Michaelis–Menten constant (Km) and maximal velocity (Vmax) were estimated using the Michaelis–Menten equation:
V1= Vmax [S] / Km + [S]
Where V1 is the reaction velocity, [S] is the substrate concentration, Km is the substrate concentration at half-maximal velocity, and Vmax is the maximal velocity.
Effect of pH on L-asparaginase activity and stability
Different ranges of pH were prepared using 50 mM acetate buffer (3.6–5.4), 50 mM phosphate buffer (5.6–8.0) and 50 mM sodium carbonate buffer (9.2–10.7). The activity of the purified L-asparaginase was tested in the range between pH 4 and pH 10. pH stability was studied by pre incubation of the purified enzyme in buffer ( pH 3.6 – pH 10.7) in absence of the substrate for 12 h at room temperature. The residual activity was determined at time intervals under optimum conditions. 100% enzyme activity (at optimum pH) was considered as control.
Effect of temperature on L-asparaginase activity and stability
Activity of the purified L-asparaginase was estimated at various temperatures ranging from 25°C to 60°C. Stability of the purified enzyme at different temperature (40°C to 70°C) was studied by incubating the enzyme for 15, 30 and 60 min separately for each tested temperature. The residual activity was determined in each case using standard conditions and compared with control.
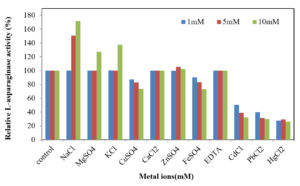
Effect of metal ions, inhibitors and surfactants on L-asparaginase activity
Pb2+, Zn2+, Na+, K+, Fe2+, Cu2+, Mg2+, Ca2+, Hg2+, Cd2+ and EDTA were tested for their effect on L-asparaginase activity. Each solution at (1, 5, 10mM) concentration was mixed separately and incubated with the purified enzyme for 2h and the residual activity of the enzyme was recorded and compared with control (absence of surfactants or metal ions or inhibitors).
Anticancer activity of the purified L-asparaginase
To study the anticancer activity of the purified L-asparaginase produced by A. terreus against three cell lines namely, Hep-G2 (human Hepatocellular carcinoma), MCF-7 (human mammary gland adenocarcinoma) and HCT-116 (colon carcinoma), different steps of lyophilization, cytotoxicity test (measured by MTT assay) and effect of the median inhibitory dose (IC50) were carried out 22,23.
Precipitation of crude enzyme with ammonium sulphate
The first step of enzyme purification occurred by fractional precipitation with ammonium sulphate (35, 50, 65, 75, 85%). It was observed that 65% ammonium sulfate fraction exhibited the highest L-asparaginase activity and recovery of protein. An increase in the enzyme activity from 27.96 to 76.76 U/mg protein with 2.75 fold purification was observed after precipitation with ammonium sulphate (65% precipitate fraction) and dialysis, Similar report was for L-asparaginase from Pseudonocardia endophytica VUK-10 24. Different previous studies recommended ammonium sulphate for L-asparginase precipitation 25, 26 .
The 65% fractional precipitation using ammonium sulfate was followed by gel filtration. Results showing the profiles of L-asparaginase elution and protein from the Sephadex G-100 column are presented in Figure 1A and indicated the presence of four peaks. The highest L-asparaginase specific activity (152.92 U/mg protein) was observed in the second peak.
Fig. 1. Gel filtration in sephadex-G100 of the semi purified L-asparaginase obtained from A. terreus culture (A) and Ion-exchange chromatography on DEAE-cellulose of the major active L-asparaginase component obtained from gel filtration (B)
Ion exchange chromatography
Fractions with the highest activity (numbers 14-18) from the Sephadex G-100 column were further purified using DEAE- column chromatography (Figure 1B). 1.96-fold increase in specific activity was obtained. A summary of L-asparaginase purification from A. terreus is illustrated in Table 1. Purification of fungal L-asparaginase from Mucor hiemalis was achieved with 4.59 purification fold, 18.46% recovery and a specific activity of 69 U/mg protein27 and from Penicillium brevicompactum NRC 829 with151.12 fold, 39.90% recovery and specific activity of 574.24 U/mg protein28 .
Table (1):
Purification steps of L- asparaginase enzyme.
Purification step |
Total protein (mg) |
Total activity (U) |
Specific L- asparaginase activity (U/mg protein) |
Purification (Fold) |
Yield (%) |
|---|---|---|---|---|---|
Culture filtrate |
1080 |
30190 |
27.96 |
1 |
100 |
Ammonium sulphate fraction (65%) |
146.88 |
11275.2 |
76.76 |
2.75 |
37.35 |
Sephadex G-100 |
28.38 |
4340 |
152.92 |
5.47 |
14.38 |
DEAE-cellulose |
12.66 |
4296 |
339.34 |
11.96 |
14.22 |
Characterization of purified L-asparaginase produced by A.terreus
Molecular weight of L-asparaginase by SDS–PAGE
Standard molecular weight markers (molecular mass range: 15- 170 kDa) were used for comparing the molecular weight of the purified L-asparaginase. Figure 2 represents SDS–PAGE of the purified L-asparaginase , showing only one protein band at approximately 85 KDa which is comparable to that purified from Streptomyces noursei 29, which had a molecular weight of 85 KDa. Different studies reported different molecular weights of L-asparaginase purified from different microorganisms 25, 30-32 .
Substrate specificity
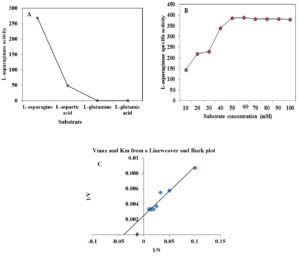
The substrate specificity is an important in the application of the enzymes as diagnostic tools. Among different substrates tested, the enzyme showed high specificity (268.5 U/mg protein) towards L-asparagine, low specificity (48.51) for L-aspartic acid, while absence of activity was detected using L-glutamic acid and L-glutamine (Figure 3 A). These results are close to the L-asparaginase obtained from Fusarium culmorum ASP-87 33 .
Fig. 3. Effect of substrate type (A), substrate concentration on L-asparaginase activity produced by A. terreus (B) and Lineweaver and Burk plot of L-asparaginase (C).
Effect of different substrate concentration
The effect of different concentrations of L-asparagine (ranged from 10 to 100 mM) on the purified L-asparaginase activity was detected. The increase in the substrate concentration (10 to 60 mM) caused an increase in the activity of L-asparaginase followed decrease in the enzyme activity. The data represented in Figure 3B revealed that the optimum substrate concentration for the purified enzyme was 60 mM, which gave the highest L-asparaginase activity (388.77 U/mg protein). Further increase of substrate concentration yielded slightly lower enzyme activity. Lineweaver and Burk (1934)21 method was applied to estimate both Km and Vmax values of the purified enzyme showing Km of 31.5 mM toward L-asparagine and Vmax of 500 U/ml (Figure 3C). Km value (2.56 µM) was reported for L-asparaginase from Trichoderma viride PERS 34. Another study by Singh et al. (2017)35 reported that Km and Vmax for L-asparaginase were found to be 6.09 mM and 88.12µM/min, respectively.
Effect of pH on enzyme activity and stability
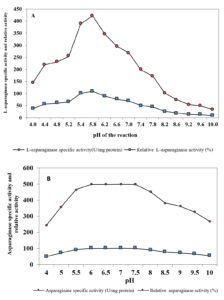
Activity and stability of purified enzyme is affected by pH, because it has an effect on the ionic form of the active site. L-asparaginase activity was estimated at different values of pH under standard conditions. Results in Figure 4A showed that increase in initial pH of the reaction caused an increase in L-asparaginase activity with the highest activity (109.09%) at pH 5.8 , followed by decreased in the activity. The obtained results are in good agreement with Abass Ahmed et al. (2015)36 who demonstrated that pH 6.0 was the favorable for maximum L-asparaginase activity produced by marine Aspergillus sp. ALAA-2000. pH 5.0 to 9.0 were also reported to be optimum for L-asparaginase activity 37. The maximum L-asparaginase activity produced from various fungi was recorded at pH 8.0 33,34, 38 . Results in Figure 4 B showed the stability of L-asparaginase over the tested pH range (4-10).
Fig. 4. Effect of pH of the reaction on L-asparaginase activity (A) and pH stability of L-asparaginase (B)
Effect of temperature on enzyme activity and stability
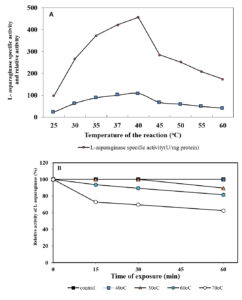
Evaluation of L-asparaginase activity was carried out at a range of temperature (25°C – 60°C). It was observed that the favorable temperature for the maximum L-asparaginase activity (456.65 U/mg protein) was 40°C (Figure 5A). However, decrease in the activity was observed with increase in temperature. The results in the current study are parallel with Loureiro et al., (2012)39 and Jalgaonwala and Mahajan (2014)38 who concluded that the optimum temperature for the highest L-asparaginase activity of Aspergillus terreus and Eurotium sp.was observed at 40oC. The same was reported by many workers for Streptomyces griseoluteus40, Aspergillus nidulans 41 and Fusarium culmorum ASP-87 33. On the contrary Elshafi et al. (2012)28, Monica et al. (2013)27 and Lincoln et al. (2015)34 showed that the optimum temperature for L-asparaginase activity produced by Trichoderma viride, Penicillium brevicompactum NRC 829 and Mucor hiemalis was 37°C. The current results indicated the thermo stability of the purified L-asparaginase in absence of substrate. At 50°C, it retained 89.54% of its activity, while it retained 62.47% and 81.60% at 70 and 60 °C, respectively after 60 min (Figure 5B). The present finding obtained for L-asparaginse produced by marine Aspergillus terreus was found to be more stable than L-asparaginase produced from Aspergillus sp. ALAA-2000 and Fusarium culmorum ASP-87 36 ,33. The half-life time (T1/2) was 2826.90 min at 50 °C, while being 88.41 min at 70 °C. Similar results were recorded with Streptomyces noursei 29(Dharmaraj, 2011) and Streptomyces fradiae NEAE-82 42. Kumar and Selvam (2011)43 reported the highest L-asparaginase activity from Streptomyces radiopugnans when the enzyme was pre-incubated at 40 °C for one hour.
Fig. 5. Effect of temperature of the reaction on L-asparaginase activity (A) and thermal stability of L-asparaginase (B)
Effect of different inhibitors and activators on L-asparaginase activity
Among the tested metals (Figure 6), MgSO4 and KCl (1.0 and 5.0 mM) had no effect on activity of the enzyme. However, at 10.0 mM they caused increase in the activity by 27.33% and 37.16%, respectively. Dias et al. (2016) 44 reported that the purified l-asparaginase produced by Aspergillus oryzae CCT 3940 was activated in the presence of MgSO4 and MnSO4, at a concentration of 5 mmol/l. It was reported that activated effect of Mg2+ may be due to substrate activation, direct bound to the enzyme-substrate complex, causing rapid release of the reaction products 45. The enzyme activity was increased to 50.75 and 71.84%, respectively during the presence of NaCl at 5 and 10 mM. The presence of ZnSO4 and CaCl2 (1.0, 5.0 and 10.0 mM) had weak effect on the enzyme activity. A slight decrease in L-asparaginase activity was observed in case of FeSO4 and CuSO4 at concentration of 10mM. The highest reduction was 32.19, 30.33 and 26.19% caused by CdCl2, PbCl2 and HgCl2 (at 10 mM), respectively. In parallel study, Ali (2009) 46 showed that, Pb2+ and Hg2+ caused partial inhibition of asparaginase produced by Vigna unguiculata. A study by El-Naggar et al. (2018)47 showed activated effect of asparaginase purified from Streptomyces bollosae NEAE-115 by 145.15, 143.04 and 121.52%, respectively when the enzyme pre incubated with Mn2+, Co2+ and Tween 80, the enzyme activity enhanced in the presence of Mg2+ (111.81%) and reached maximal activity (145.15 and 143.04 ) in the presence of Mn2+ and Co2+ . However, the presence of Zn2+ and Ca2+ caused 14.8 and 16%, reduction in asparaginase activity. Our results indicated that EDTA did not affect the enzyme activity as was reported by Abbas Ahmed et al. (2015) 36 and Kumar and Selvam (2011)43 who observed the activation of the L-asparaginase produced by Streptomyces radiopugnans when EDTA was added. Contrary, Lincoln et al. (2015)34 reported 88% inhibition of L-asparaginase activity by EDTA. Moreover, inhibition of L-asparaginase activity produced by Streptomyces bollosae NEAE-115 was detected in the presence of EDTA, reducing the activity by 37.55% 47.
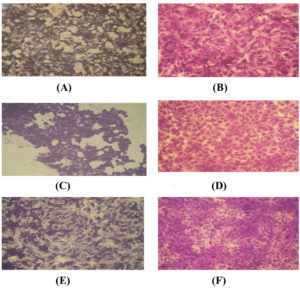
Cytotoxicity and anticancer activities of the purified L-asparaginase
The cytotoxicity of purified L-asparaginase was tested on human HCT-116, Hep-G2 and MCF-7 tumor cells. The obtained results concluded that the inhibition of cell viability with L-asparaginase was dose dependent and different cytotoxic activity against HCT-116, Hep-G2 and MCF-7 was observed with IC50 ranged from 3.79-12.6 µg/ml. The anti-proliferative activity of L-asparaginase on cancer cells was estimated on HCT-116, Hep-G2 and MCF-7 cells. The obtained data indicated that, the activity of asparaginase against Hep-G2 was superior to that with either HCT-116 or MCF-7 cells (Figure 7). Our results agree with Moharib (2018)48 who reported that cytotoxic activity of L-asparaginase was tested on HELLA, MCF7, HEPG2 and HCT-116 showing that L-aspargenase had higher growth inhibition against Hep-G2 and HCT-116 than Hella and MCF7 carcinoma cell lines. El-Naggar et al. (2016)42 studied the activity of L-asparaginase against CACO 2, HeP 2, HePG 2 cancer cells and reported that IC50 was 2 – 4 U/ml with the highest activity against CACO 2. Anticancer activity of L-asparaginase was reported in different studies 49-51. Anticancer activity of L-asparaginase may be attributed to proliferation inhibition of the cancer cell lines that could arrest the cell cycle and generate apoptosis as was reported by Moharib (2018)48.
Fig. 7. Photographs illustrating the effect of the purified enzyme (A) on the growth inhibition of HCT-116 tumor cell line compared with control (B); the difference between the effect of the purified L-asparaginase enzyme (C) on the growth inhibition of Hep-G2 tumor cell line compared with control (D); the difference between the effect of purified L-asperaginase (E) on the growth inhibition of MCF-7 tumor cell line compared with control (F).
- Appel IM, van Kessel-Bakvis C, Stigter R, Pieters R. Influence of two different regimens of concomitant treatment with asparaginase and dexamethasone on hemostasis in child hood acute lymphoblastic leukemia. Leukemia, 2007; 21(11): 2377–2380.
- Patro KR, Gupta N. Extraction, purification and characterization of L-asparaginase from Penicillium sp. by submerged fermentation. Int. J. Biotechnol. Mol. Biol. Res, 2012; 3(3): 30-34.
- Deshpande N, Choubey P, Agashe M. Studies on optimization of growth parameters for l-asparaginase production by Streptomyces ginsengisoli. Sci. World. J, 2014, 1–6.
- Lopes AM, Oliveira-Nascimento L, de Ribeiro A., et al. Therapeutic l-asparaginase: upstream, downstream and beyond. Crit. Rev. Biotechnol, 2017; 37(1): 82-99.
- Sarquis MI, de M Oliveira EMM, Santos AS, da Costa GL. Production of l-asparaginase by filamentous fungi. Mem. Inst. Oswaldo. Cruz, 2004; 99(5): 489-492.
- Zuo S, Zhang T, Jiang B, Mu W. Recent research progress on microbial l-asparaginases. Appl. Microbiol. Biotechnol, 2014; 99(3): 1069–1079.
- Sreenivasulu V, Jayaveera K, Rao PM. Solid-state fermentation for the production of l-asparaginase by Aspergillus Sp. Res. J. Pharmacogn. Phytochem, 2009; 1(1): 21–25.
- Mishra A. Production of l-asparaginase, an anticancer agent, from Aspergillus niger using agricultural waste in solid state fermentation. Appl. Biochem. Biotechnol, 2006; 135(1): 33–42.
- Dange V, Peshwe S. Purification and biochemicalcharacterization of l-asparaginase from Aspergillus niger and evaluation of its antineoplastic activity. Int J Sci Res, 2015; 4(2): 564–569.
- Hosamani R, Kaliwal BB, Thippeswamy S. Isolation, identification and optimization studies for l-asparaginase production from fungal isolates of marine sediments. Int. J. Pharm. Bio. Sci, 2017; 8(3): 114 – 121.
- Thirunavukkarasu N, Suryanarayanan TS, Murali TS,Ravishankar JP, Gummadi SN. l-asparaginase from marinederived fungal endophytes of seaweeds. Mycosphere.2011; 2(2):147–155.
- Gurunathan B, Sahadevan R. Optimization of cultureconditions and bench-scale production of l-asparaginase bysubmerged fermentation of Aspergillus terreus MTCC 1782. J. Microbiol. Biotechnol, 2012; 22(7): 923–929.
- Anjum Zia MAZ, Bashir R, Ahmed I, Iftikhar T. Production of l-asparaginase from Aspergillus niger using agro wastes by-products in submerged fermentation process. J Teknol, 2013; 62(2): 47-51.
- Saeed H, Ali H, Soudan H, Embaby A, El-Sharkawy A, Farag A, Hussein A, Ataya F. Molecular cloning, structural modeling and production of recombinant Aspergillus terreus. asparaginase in Escherichia coli. Int. J. Biol. Macromol, 2018; 106: 1041-1051.
- Farag AM, Hassan SW, El-Shenawy MA. Optimization of production of anti-tumor l-asparaginase by free and mmobilized marine Aspergillus terreus. Egypt. J. Aquat. Res, 2015; 41(4): 295-302.
- Imada A, Igarasi S, Nakahama K, Isona M. Asparaginase and glutaminase activities of microorganisms. J. Gen App. Microb,1973; 76: 85-99.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin-phenol reagent. J. Biol. Chem., 48: 17–25.
- Kalyanasundaram J, Nagamuthu B, Srinivasan A, Pachayappan Muthukumarasamy S. Production, purification and characterization of extracellular L-asparaginase from salt marsh fungal endophytes. World. J. Pharam. Pharmaceut. Sci. 2015; 4(03): 663-677.
- Kumar R, Singh KA, Singh VK, Jagannadham MV. Biochemical characterization of a peroxidase isolated from caribbean plant: Euphorbia cotinifolia. Process. Biochem, 2011; 46: 1350–1357.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970; 227: 680-685.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J. Am. Chem Soc, 1934; 56: 658-66.
- Baghbani-Arani F, Movagharnia R, Sharifian A, Salehi S, Shandiz SAS. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J. Photochem Photobiol. B: Biol, 2017; 173: 640-649.
- El-Sersy NA, Abdelwahab AE, Abouelkhiir SS, Abou-Zeid DM, Sabry SA. A. Antibacterial and anticancer activity of µ-poly-L-lysine (µ-PL) produced by a marine Bacillus subtilis sp. J. Basic. Microbiol, 2012; 52: 1-10.
- Mangamuri U, MuvvaV, Poda S. Purification and characterization of L-asparaginase by Pseudomonas endophytica VUK-10 isolated from Nizampatnam mangrove ecosystem. Int. J .Pharm. Pharm. Sci, 2016; 8 (3): 281-285.
- Amena S, Vishalakshi N, Prabhakar M, Dayanand A, Lingappa K. Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Brazil J Microbiol, 2002; 41(1): 173–178.
- Gervais D, NAllison N, Jennings A, Jone S, Marks T. Validation of a 30-yearold process for the manufacture of L-asparaginase from Erwinia chrysanthemi. Bioprocess. Biosyst. Eng, 2013; 36: 453–60.
- Monica T, Lynette L, Niyonzima FN, Sunil SM. Isolation, purification and characterization of fungal extracellular L-asapraginase from Mucor hiemalis. J Biocat Biotrans. 2013; 2: 1-9.
- Elshafei AM, Hassan MM, Abouzeid MA, Mahmoud DA, Elghonemy DH . Purification, characterization and antitumor activity of l-asparaginase from Penicillium brevicompactumNRC829. Brazilian. J. Microbiol .Res. 2012; 2 (3): 158-174.
- Dharmaraj S. Study of L-asparaginase production by Streptomyces noursei MTCC 10469, isolated from marine sponge Callyspongia diffusa. Iran. J. Biotechnol. 2011; 9: 102–108.
- Kavitha A, Vijayalakshmi M. Optimization and purification of L-asparaginase produced by Streptomyces tendae TK-VL_333. Z. Naturforsch, 2010; 65: 528–531.
- Huang L, Liu Y, Sun Q, Yan Jiang Z . Biochemical Characterization of a Novel l-Asparaginase with low Glutaminase activity from Rhizomucor mieheiand its application in food safety and leukemia treatment. App. Environ. Microbiol, 2014; 80(5): 1561–1569.
- Dutta S, Ghosh S, Pramanik S. L-Asparaginase and L-glutaminase from Aspergillus fumigatus WL002: production and some physicochemical properties. Appl. Biochem. Microbiol, 2015; 51(4): 425–431.
- Meghavarnam A K, Janakiraman S. Purification and characterization of therapeutic enzyme L-Asparaginase from a tropical soil fungal isolate Fusarium culmorum ASP-87. MOJ Proteomics. Bioinform, 2015; 2(6): 00064.
- Lincoln GL, Niyonzima FN, More S S. Purification and properties of a fungal L-asparaginase from Trichoderma vidide Pers. J Microbiol. Biotech. Food. Sci, 2015; 4 (4): 310-316.
- Singh A, Verma N, Kumar K . Screening and identification of medicinal plants for L-asparaginase production. Int. J. Recent. Sci .Res, 2017; 8(11): 22029-2203.
- Abbas Ahmed MM, Nageh AboDahab F, Taher Taha M, Fareed Hassan, SM. Production, purification and characterization of L-Asparaginase from marine endophytic Aspergillus sp. ALAA-2000 under submerged and solid state fermentation. J Microb Biochem Technol, 2015; 7: 165-172.
- Ohshima M, Yamamoto T, Soda K. Further characterization of glutaminase isozymes from Pseudomonas aeruginosa. Agri Biologi Chem, 1976; 40: 2251–2256.
- Jalgaonwala RE, Mahajan RT. Production of anticancer enzyme asparaginase from endophytic Eurotium Sp. isolated from rhizomes of Curcuma longa. Euro. J. Exp. Biol, 2014; 4(3): 36-43.
- Loureiro B, Borges S, Andrade F, Tone G, Said S. Purification and biochemical characterization of native and pegylated form of L-asparaginase from Aspergillus terreus and evaluation of its antiproliferative activity. Adv. Microbiol, 2012; 2: 138–45.
- Kamala Kumari PV, Girija Shankar G, Prabhakar T, Satya Lakshmi S. Purification and characterization of L-asparagianse from Streptomyces griseoluteus WS3/1. Int. J. Pharma. Sci. Rev. Res, 2013; 23(2): 198-202.
- Archana RJ, Raja RP. Production, purification and characterization of L-asparaginase from Aspergillus nidulans by solid substrate fermentation. Eur J Biotechnol Biosci, 2014; 2(4): 51-58.
- El-Naggar NE, Deraz SF, Soliman HM, El-Deeb NM, El-Ewasy SM. Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Sci Rep, 2016; 1-16.
- Kumar S, Selvam K. Isolation and purification of high efficiency L-asparaginase by quantitative preparative continuous-elution SDS-PAGE electrophoresis. J. Microb. Biochem. Tech, 2011; 3(5): 073- 083.
- Dias,, F. F. G., Gois Ruiz, A. L.T. , Torre, A. D., Sato. H. H. Purification, characterization and antiproliferative activity of l-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity. Asian Pac. J. Trop.Biomed, 2016; 6(9): 785-794.
- Knape MJ, Ahuja GL, Bertinetti D, Burghardt NCG, Zimmermann B, Taylor SS, Herberg FW. Divalent metal ions Mg2+ and Ca2+ have distinct effects on protein kinase a activity and regulation. ACS Chem Biol. 2015; 10(10): 2303 –15.
- Ali E M. Purification and characterization of Vigna unguiculata cultivar asparaginase. J. Biol. Res. Thessaloniki. 2009; 11: 29–36.
- El-Naggar N E, Sahar F, Deraz2, El-Ewasy SM, Suddek G M. Purification, characterization and immunogenicity assessment of glutaminase free L-asparaginase from S. brollosae NEAE-115. 2018; BMC Pharmacol. Toxicol. 19: 51.
- Moharib SA. Anticancer activity of L-Asparaginase produced from Vigna uUnguiculata. World Sci. Res. 2018; 5: 1-12.
- Dai ZJ , Gao Z F, Li Z Z, Ji, H F, Kang HT, Guan Y, Diao BF, Wang, and Wang X. In vitro and in vivo antitumor activity of scutellaria barbate extract on murine liver cancer. Molecules. 2011; 16: 4389–4400.
- Moharib SA. Anticancer and antioxidant effects of fructooligosaccharide (FOS) on chemically induced colon cancer in rats. Electronic J Polish Agri. Univ. 2016; 19: 1-18.
- Qeshmi FL, Homaei A, Fernandes P, Javadpour S. Marine microbial L-asparaginase: Biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol. Res, 2018; 208: 99-112.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.